Abstract
A combinatorial library of lipidoids was constructed and studied for in vitro gene delivery. The library of lipidoids was synthesized by reacting commercially available amines with lipophilic acrylates, acrylamides, or epoxides. Lipidoids derived from amine 86 (N,N-Bis(2-hydroxyethyl)ethylene diamine) and amine 87 (N-(3-aminopropyl)diethaneamine) showed high efficiency in DNA delivery, some with a higher transfection efficiency than Lipofectamine 2000, a commonly used commercial gold standard for in vitro gene delivery. The structure-activity relationship between the lipidoids was further studied with respect to small variations in chemical structures and the resulting efficiency in DNA delivery in vitro. Since these lipidoids are easy to synthesize and do not require a co-lipid for efficient DNA delivery, they could offer an inexpensive but effective alternative to other commonly used commercial gene delivery carriers.
Keywords: DNA delivery, lipidoid, cationic lipid nanoparticles, gene therapy, combinatorial library
Introduction
Recently, a combinatorial library of lipid-like materials termed “lipidoids” was developed, and their utility as carriers for the delivery of siRNA and antisense oligonucleotides was described.1–5 These lipidoids were prepared by reacting commercially available amines with lipophilic acrylates, acrylamides, or epoxide. The lipidoids have several advantages as a potential new class of nucleic acid delivery reagents: (i) the chemistry used to synthesize lipidoids is simple and economical, (ii) a library of structural diversity was already developed, (iii) a correlation between structure and function of delivery systems could be constructed from the large data sets accumulated from screening the library of lipidoids.
Utilizing lipidoids for DNA delivery, however, has not been previously reported. Lipidoids are structurally similar to many reported cationic lipids widely used for non-viral delivery of DNA.6–9 In this paper, we evaluated the potential of lipidoids to facilitate DNA delivery in vitro. We demonstrated the capability of lipidoids synthesized from hydroxyl terminated diamines to deliver DNA efficiently into HeLa cells. We also studied structure-activity relationships of the lipidoids with regards to facilitating DNA delivery by designing a series of lipidoids with the same head and tail groups and differing linkers. We found that the lipidoids synthesized through amine and acrylamide addition reactions resulted in the most efficient DNA delivery compared to other lipidoids in the library. However, lipidoids synthesized with an ester linker were not stable and degraded completely in an hour via hydrolysis, hence losing the ability to facilitate DNA delivery.
Results and Discussion
Lipidoids were prepared by heating commercially available amines with lipophilic acrylamides, acrylates, or epoxides without solvent or catalysts, according to our previous reported methods.2,4 The simplicity of these reactions allowed us to build a structurally diverse library of lipidoids by varying the types of amines, and the lengths and types (acrylamide/acrylate/epoxide) of tails.2,4 The resulting crude products were directly used for in vitro delivery of DNA.
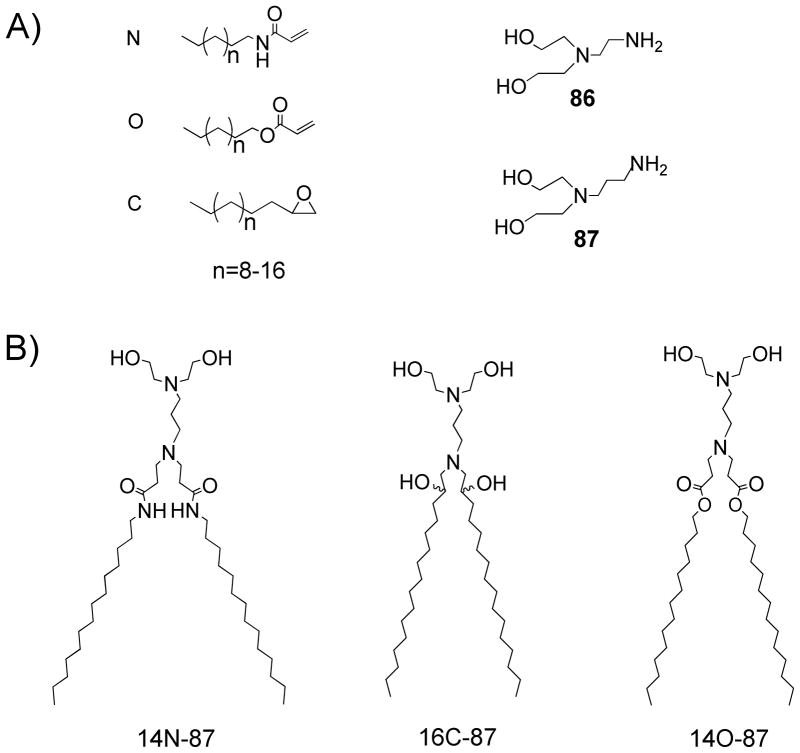
In order to facilitate high-throughput library screening, plasmid DNA encoding β-galactosidase (β-gal) was employed as the reporter gene. The β-gal enzymatic assay was carried out to measure the level of β-gal expression. HeLa cells, in their exponential growth phase, were seeded at 10,000 cells per well into 96-well plates to ~80% confluency the day before transfection. The library of crude lipidoids was initially screened by delivering lipidoid/DNA complex to the HeLa cells in 96-well plates. The screening results showed most lipidoids were not effective in facilitating DNA delivery, with transfection efficiencies lower than the leading transfection reagent, Lipofectamine 2000® (data not shown). However, we observed the crude lipidoids derived from amine 86 (N,N-Bis(2-hydroxyethyl)ethylene diamine) and amine 87 (N-(3-aminopropyl)diethaneamine) (Scheme 1, A) generally showed relatively high efficiency in DNA delivery, sometimes equal or higher than Lipofectamine. This observation led us to synthesize a refined library of purified lipidoids from the reaction of amines (86 and 87) and different types and lengths of tails (acrylamide, acrylate and epoxide), shown in scheme 1A.
Scheme 1.
Combinatorial synthesis of lipidoids for DNA delivery. (A) Alkyl-acrylamide, alkyl-acrylate, alkyl-epoxide, and amine molecules used for the library synthesis; (B) Chemical structures of the selected lipidoids with high DNA delivery efficiency. Lipidoids are named as follows: (carbon numbers of tail) (acrylate, acrylamide, or epoxide)-(amine number)
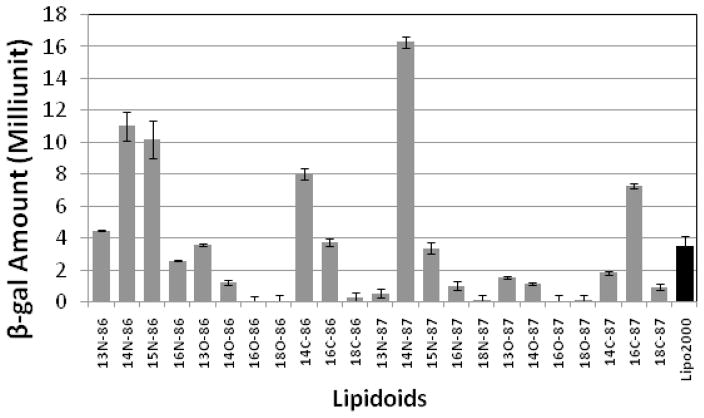
The lipidoids with different tail lengths were purified from the crude reaction mixtures by column chromatography and tested for their ability to deliver β-gal DNA to HeLa cells. The screening results showed that most of the pure lipidoids in the refined library supported DNA delivery into the HeLa cell with expression of β-gal (Figure 1); however the efficiency varied depending on the lipidoid structure. Lipidoids derived from amine 86 and 87 with two medium-length tails (e.g., C14 or C15 for acrylamide and acrylate, respectively, and C14 or C16 for epoxide) displayed high transfection efficiencies, in some cases higher than Lipofectamine 2000®. We observed that the linkers between tail and head groups of the lipidoids affect the efficiency in facilitating DNA delivery. The lipidoids with amide linkers displayed substantially higher delivery efficiency than those with ester and hydroxyl groups. The delivery efficiency also varied with the amine molecules utilized for lipidoids synthesis, for example, the acrylamide and epoxide conjugated with amine 87 (14N-87 and 16C-87) delivered DNA more efficiently than those conjugated with amine 86 (14N-86 and 16C-86), while for the lipidoids synthesized from acrylate, the reverse results were observed (Figure 1).
Figure 1.
Initial in vitro screening of lipidoids for DNA delivery. Lipidoids were screened by delivering plasmid DNA encoding β-gal into HeLa cells. Relative β-gal expression level was determined by assaying the enzyme activity according to reported methods.
To further investigate the structure-activity relationship of these lipidoids for DNA delivery, we selected lipidoids prepared from the reaction of amine 87 with acrylamide (14N-87), epoxide (16C-87), and acrylate (14O-87) (Scheme 1B), which displayed high delivery efficiency. DNA was then complexed with these lipidoids (immediately dissolved in sodium acetate buffer (50 mM, pH = 5.5) before use) in different weight ratios for delivery. The results showed the lipidoid to DNA ratios (w/w) affected the delivery efficiency and the resulting β-gal activity. These lipidoids have a Mw around 680 Da and two positive charges; a single base pair of DNA has a Mw around 660 Da and two negative charges. The weight/weight (w/w) ratio of the lipidoid/DNA has a similar value to the molar ratio and positive/negative (P/N) charge ratio. For all lipidoids selected, β-gal enzyme activity was detectable at the lipidoid/DNA ratio of 1:1 (Figure 2). The delivery efficiency was enhanced by an increasing ratio. For 14N-87 and 14O-87, the transfection efficiency increased to the highest level at a lipidoid/DNA ratio of 5:1 and slowly declined by further increasing the ratio. Lipidoid 16C-87, on the other hand, enhanced its transfection efficiency until the lipidoid/DNA ratio increased to 15:1. At the dose level used in these experiments, no obvious cytotoxicity was observed from the delivery reagents used. Under the same conditions, the 14N-87 showed higher tranfection efficiency compared with 14O-87 and 16C-87.
Figure 2.
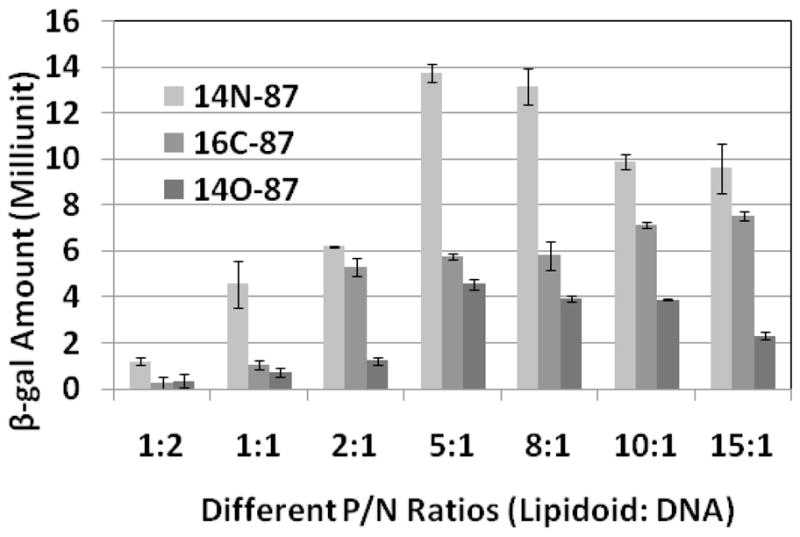
The relationship of positive to negative charge (P/N) ratios of lipidoids/DNA and DNA delivery efficiency. Lipidoids 14C-87, 16C-97, and 14O-87 were selected according to initial screening results. The efficiency was monitored as the β-gal expression level.
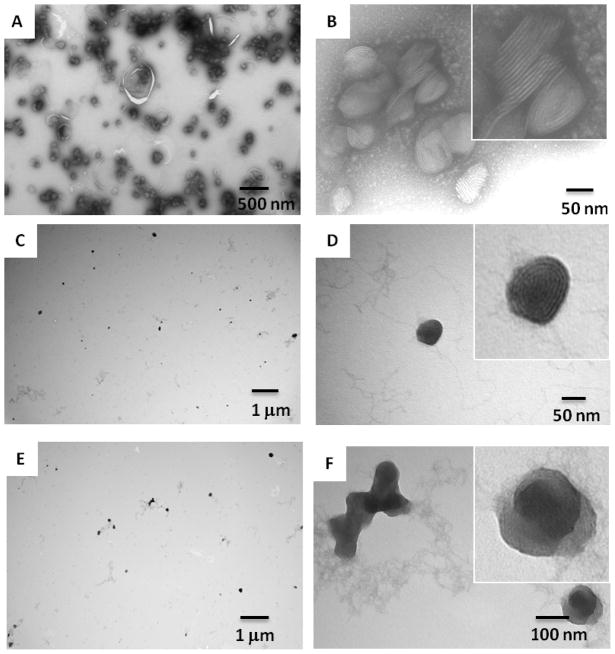
It is known that the transfection efficiency of a given lipid-DNA complex depends on its structure and physicochemical properties.10 We used negative-staining transmission electron microscopy (TEM) to study the size and morphology of the lipidoid-DNA complexes and to illustrate a structure-function correlation of lipidoids with biological activity. The 14N-87 was able to efficiently self-assemble with DNA through electrostatic interactions and form condensed multilamellar structure nanocomplexes (~100 nm) with DNA intercalated between the lipid bilayers (Figure 3). In contrast, 14O-87 and 16C-87 tended to form loose “spaghetti and meatball-like structures”.10 The higher transfection efficiency of DNA delivery by 14N-87 may be attributed to its higher efficiency in nanoparticle formation compared with 14O-87 and 16C-87.
Figure 3.
Negative staining TEM images of lipidoids/DNA (w/w = 5:1) nanoparticles. A and B: 14N-87/DNA complex; C and D: 16C-87/DNA complexes; E and F: 14O-87 /DNA complexes.
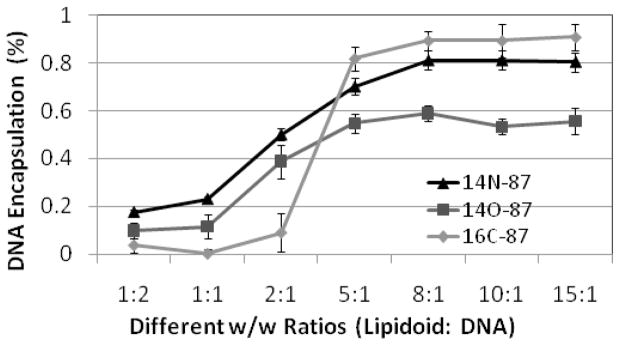
We next determined the efficiency of DNA encapsulation by different lipidoids at different P/N ratio using PicoGreen assay (Figure 4). At a low P/N ratio (<5), the DNA encapsulation percentage was low for all three lipidoids, which led to the observed low transfection efficiency (Figure 3). All three lipidoids showed increased DNA encapsulation with an increase of the P/N ratio. 14N-87 and 16C-87 had a maxium percentage of encapsulation of 80–90%, while 14O-87 reached 60%, which may explain why DNA delivery by 14N-87 and 16C-87 were more efficient than 14O-87. Although lipidoids 14N-87 and 16C-87 displayed similar binding abilities with DNA in ratios above 5:1, 14N-87 was generally more efficient in mediating DNA delivery than 16C-87. This was probably due to 16C-87 and DNA forming a large amount of flocculent nanostructures, while 14N-87 and DNA formed condensed individual multilamellar nanoparticles (Figure 3).
Figure 4.
The Picogreen assay of the lipidoids (14N-87, 16C-87 and 14O-87)/β-gal encoding DNA complexes in different ratios.
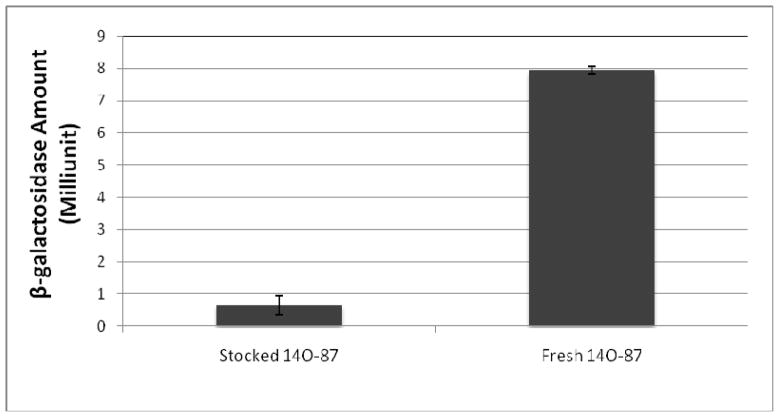
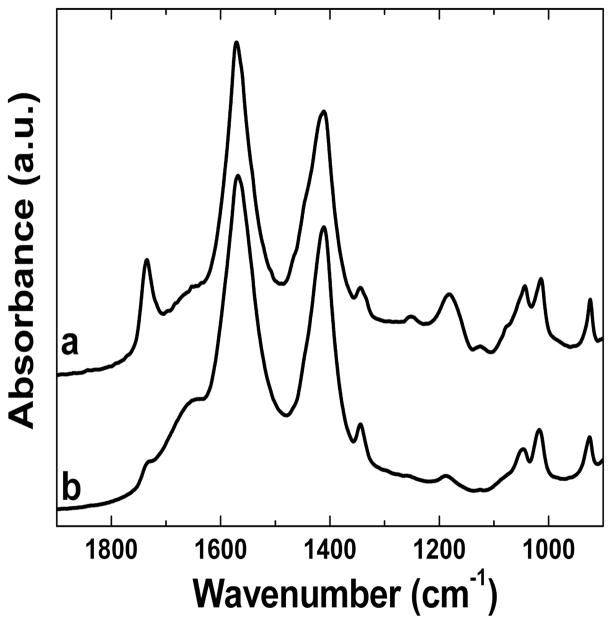
We noticed that 14O-87 lost their capability in DNA delivery when the as-prepared solution was stored for a certain period of time (>2hrs), a phenomenon not observed with the other two lipidoids, 14N-87 and 16C-87. No successful DNA delivery was observed using the stored 14O-87 compared with the freshly prepared sample (Figure 5). We observed that the stored 14O-87 and DNA are not able to assemble to form nanoparticles. We hypothesize that the loss of ability to mediate DNA delivery is ascribed to the hydrolysis of the ester linker in 14O-87. This was confirmed by FTIR analysis of fresh and stored 14O-87 solutions (Figure 6). The peak at 1720 cm−1 assigned to the ester bond in 14O-87 weakened after 1 h in a sodium acetate solution and a new peak at 1680 cm−1 appeared. It has been observed that the rapid degradation of amine-containing polyesters is attributed to the intramolecular nucleophilc assistance by the pendant amine groups.11–13 In the case of 14O-87, we speculate that the rapid hydrolysis attributed to the nucleophilic attack of the free hydroxyl group of the molecule itself or nearby molecules.14
Figure 5.
Different β-gal expression level delivered by fresh and stocked 14O-87/DNA complex. The delivery efficiency was converted from the β-gal activity assay. Lipidoid/DNA with a ratio of 5:1 was used in the experiment.
Figure 6.
Normalized FT-IR spectra of fresh 14O-87(a) and stocked (b) solution. 14O-87 was dissolved in sodium acetate buffer solution (50 mM, pH = 5.5).
Conclusions
We report the evaluation of a library of lipidoids for in vitro DNA delivery through a facile combinatorial approach. The mild reactions between amines and acrylamides, acrylates, or epoxides enabled the construction of a library to screen for efficient gene delivery carriers. The initial screening for DNA delivery indicated that lipidoids formed through amine and acrylamide addition reactions generated higher delivery efficiencies than acrylate- and epoxide-derived counterparts. The rapid hydrolysis of lipidoids with ester linkages decreased efficiency in DNA delivery. The structure-activity relationships demonstrated provide insight for designing new lipidoids for gene delivery. These lipidoids are easy to synthesize and do not require a co-lipid for efficient DNA delivery. We believe these systems could offer an inexpensive and effective alternative to other commonly used commercial gene delivery agents.
Experimental Section
Synthesis of Lipidoids
Lipidoid library synthesis was performed and characterized as previously described.2,4 Amines and 1-aminoalkanes were purchased from Sigma-Aldrich (St. Louis, MO) and TCI America (Portland, OR). Acrylamides were synthesized by the drop-wise addition of acryloyl chloride to a solution of the appropriate 1-aminoalkane in dichloromethane in an ice-bath under an inert atmosphere. Lipidoids were synthesized by conjugation addition of amines to acrylamides, acrylates, or epoxides. These reactions were performed in 5-mL Teflon-lined glass screw-top vials. For lipioids prepared from acrylamides, the number of equivalents of acrylamide was equal to maximum number of conjugate additions possible for each amine (2 for primary amines). The reactions were performed on a 200 mg scale (of amine). The mixture was stirred at 90°C for 7 days. After cooling, the lipid mixtures were used without purification unless otherwise specified. Representative library members were characterized by thin layer chromatography, IR, NMR, and mass spectroscopy.2,4
In Vitro DNA Transfection
HeLa cells were obtained from ATCC (Manassas, VA) and cultured in phenol red-free DMEM supplemented with 10% fetal bovine serum and 100 units/ml of penicillin/streptomycin at 37°C and 5% CO2. All cell culture reagents were purchased from Invitrogen Corporation (Carlsbad, CA) unless otherwise noted. pCMV β-gal DNA was also purchase from Elim Biopharmaceuticals, Inc (Hayward, CA). To facilitate screening throughput, lipidoid-DNA complexes were formed by simple mixing of lipidoid-DNA solutions in 50 mM sodium acetate buffer solution pH 5.5 in microtiter plates. For transfection in 96-well plates, HeLa cells were seeded (10,000 cells per well) into each well of an opaque white 96-well plate and allowed to attach overnight. Cells were transfected with 200 ng of DNA (per well) complexed with lipidoids at fixed lipidoid/DNA weight ratio of 5:1. The lipidoids were added to the DNA solutions, and then incubated for 10 min at room temperature to allow for complex formation. The lipidoid/DNA solution was immediately added to cells in each well. After transfection, cells were incubated for 24 hrs at 37°C and 5% CO2 before analyzing for β-gal protein expression. Control experiments were also performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA), as described by the vendor. Transfection was performed in quadruplicate.
β-gal Activity of Cell Culture
β-gal activity assay is described by the developed protocol15 with slight modification. Briefly, after the transfection over 24 hours, all growth medium in the 96-well plate was removed by vacuum aspiration. 100 μL PBS was added into each well to wash the cell for 5 min (×2) on ice. 50 μl triton (Sigma-Aldrich, St. Louis, MO) solution (0.5% in PBS) was added into each well right after removal of PB. The plate was then left on the ice for 10 min. Fifty μL of o-nitrophenyl-β-D-galactoside (ONPG) (Sigma-Aldrich, St. Louis, MO) solution (4 mg/mL in z-buffer)15 was added into each well by multi-channel pipette. The plate was incubated for 15 min at 37°C. The concentration of β-gal was determined by measuring the absorbance at 409 nm.
DNA binding Assay
PicoGreen assay was performed as previously described.16 Briefly, in 96-well plate, 50 μL/well of lipidoid solutions at 1 mg/mL in NaOAc buffer were added to 50 μL/well of DNA (60 μg/mL in NaOAc buffer). The solutions were mixed vigorously and allowed to sit undisturbed for 5 min to allow for lipidoid-DNA complex formation. Then, 100 μL/well of PicoGreen working solution (Invitrogen,Carlsbad, CA) was added. PicoGreen working solution was prepared by diluting 52.8 μL of the purchased stock into 9.9 mL NaOAc buffer. After 5 min, 30 μL/well of lipidoid-DNA-PicoGreen solution was added to 200 μL/well of DMEM media in a black 96-well polystyrene plate. The plate fluorescence was then measured on a SpectraMax® M2 Multi-Mode Microplate Reader (Molecular Devices, Inc. California, USA) at excitation of 485 nm and emission of 535 nm. The relative fluorescence (RF) was calculated by the following relationship:
where Fsample is the fluorescence of the lipidoid-DNA-PicoGreen sample, Fblank is the fluorescence of a sample with lipidoid or DNA (only PicoGreen), and FDNA is the fluorescence of a sample with DNA-PicoGreen without lipidoid. The DNA encapsulation is one minus RF.
Transmission Electron Microscopy (TEM)
Lipidoid/DNA complexes were prepared with the same protocols as in vitro experiments (5:1 weight ratio of total lipidoid to DNA) Droplets of the sample (5μl) were applied to hydrophilized carbon-covered copper grids (300 meshes) for 30 min. The sample was subsequently rinsed with contrasting material (1% uranyl acetate at pH 4.5). The remaining stain solution was removed with a filter paper and air-dried. TEM microstructure was determined using a Tecnai FEG TEM (FEI Tecnai 12 Spirit Bio-twin, FEI Company, Hillsboro, OR) operating at 80 kV.
Fourier Transform Infrared Spectroscopy (FTIR)
The structural characteristics were observed using FTIR as previously reported by Hu et al.17–18 Lipidoid/DNA nanoparticles were prepared as described in TEM section. Before the measurement, the lipidoid/DNA solution was dropped onto the detector stage, blowing with the air gun at room temperature until all the dissolvent evaporated. Normalization of the curve was established through the protocol described in Hu’s paper.17–18
Acknowledgments
This research was supported by Tufts University. D. G. A and R. L acknowledge the grants from NIH under NIH R01-EB000233 and RO1-CA132091. Q. X. also acknowledges Tufts FRAC award. We want to thank Nicki Watson at Whitehead Institute, MIT for the help on TEM imaging.
References
- 1.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, Jayaprakash KN, Jayaraman M, Rajeev KG, Manoharan M, Koteliansky V, Rohl I, Leshchiner ES, Langer R, Anderson DG. Development of lipidoid–siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17(5):872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Jayaprakash KN, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DWY, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho SW, Hartle L, Son SM, Yang F, Goldberg M, Xu Q, Langer R, Anderson DG. Delivery of small interfering RNA for inhibition of endothelial cell apoptosis by hypoxia and serum deprivation. Biochem Bioph Res Commun. 2008;376(1):158–163. doi: 10.1016/j.bbrc.2008.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DWY, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead KA, Sahay G, Li GZ, Love KT, Alabi CA, Ma M, Zurenko C, Querbes W, Langer RS, Anderson DG. Synergistic Silencing: Combinations of Lipid-like Materials for Efficacious siRNA Delivery. Mol Ther. 2010;19 (9):1688–1694. doi: 10.1038/mt.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler CJ, Felgner PL, Tsai YJ, Marshall J, Sukhu L, Doh SG, Hartikka J, Nietupski J, Manthorpe M, Nichols M, Plewe M, Liang X, Norman J, Smith A, Cheng SH. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc Natl Acad Sci U S A. 1996;93(21):11454–11459. doi: 10.1073/pnas.93.21.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byk G, Dubertret C, Escriou V, Frederic M, Jaslin G, Rangara R, Pitard B, Crouzet J, Wils P, Schwartz B, Scherman D. Synthesis, activity, and structure-activity relationship studies of novel cationic lipids for DNA transfer. J Med Chem. 1998;41(2):224–235. doi: 10.1021/jm9704964. [DOI] [PubMed] [Google Scholar]
- 8.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 9.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesnoy S, Huang L. Structure and function of lipid-DNA complexes for gene delivery. Annu Rev Biophys Biomol Struct. 2000;29:27–47. doi: 10.1146/annurev.biophys.29.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Lim Y, Choi YH, Park J. A self-destroying polycationic polymer: biodegradable poly(4-hydroxy-l-proline ester) J Am Chem Soc. 1999;121(24):5633–5639. [Google Scholar]
- 12.Lim Y, Kim C, Kim K, Kim SW, Park J. Development of a safe gene delivery system using biodegradable polymer, poly[α-(4-aminobutyl)-l-glycolic acid] J Am Chem Soc. 2000;122(27):6524–6525. [Google Scholar]
- 13.Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–10768. [Google Scholar]
- 14.Capon B, McDowell TS, Raftery WV. Hydroxy-group participation in ester hydrolysis. J Chem Soc, Perkin Trans. 1973;2:1118–1125. [Google Scholar]
- 15.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor; New York: 1972. pp. 352–355. [Google Scholar]
- 16.Green JJ, Zugates GT, Tedford NC, Huang YH, Griffith LG, Lauffenburger DA, Sawicki JA, Langer R, Anderson DG. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv Mater. 2007;19(19):2836–2842. [Google Scholar]
- 17.Hu X, Kaplan D, Cebe P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules. 2006;39(18):6161–6170. [Google Scholar]
- 18.Hu X, Kaplan D, Cebe P. Dynamic protein-water relationships during β-sheet formation. Macromolecules. 2008;41(11):3939–3948. [Google Scholar]