Abstract
Current vaccines primarily work by inducing protective antibodies. However, in many infections like HIV, malaria and tuberculosis as well as cancers there remains a need for durable and protective T-cell immunity. Here, we summarize our efforts to develop a safe T-cell based protein vaccine that exploits the pivotal role of dendritic cells (DC) in initiating adaptive immunity. Focusing on HIV, gag-p24 protein antigen is introduced into a monoclonal antibody (mAb) that efficiently and specifically targets the DEC-205 antigen uptake receptor on DC. When administered together with synthetic double stranded RNA, polyriboinosinic:polyribocytidylic acid (poly IC) or its analogue poly ICLC (poly IC stabilized with carboxymethylcellulose and poly-L-lysine), as adjuvant, HIV gag-p24 within anti-DEC-205 mAb is highly immunogenic in mice, rhesus macaques, and in ongoing research, healthy human volunteers. Human subjects form both T and B cell responses to DC-targeted protein. Thus, DC-targeted protein vaccines are a potential new vaccine platform, either alone or in combination with highly attenuated viral vectors, to induce integrated immune responses against microbial or cancer antigens, with improved ease of manufacturing and clinical use.
Keywords: dendritic cells, protein vaccine, adjuvant, T cells, DEC-205, cross-presentation
Introduction
A need for T cell-based vaccines
The immunity induced by vaccines is responsible for major medical successes. Yet medicine has a challenging road ahead to expand the scope and efficacy of vaccines. Successful current vaccines induce protective antibodies from B cells, but there is a lack of vaccines that work by inducing protective T cells. Vaccine-induced T cells should help to resist global infections -- like HIV-1/AIDS, tuberculosis, malaria, Leishmaniasis -- and cancers. In addition, a more recently recognized class of T cells, called regulatory or suppressor T cells (T reg), can turn off unwanted immune reactions like allergy, transplant rejection, autoimmunity, chronic inflammation, and atherosclerosis [1]. Here we would like to summarize a protein-based vaccine platform to induce T cell immunity that we feel has potential applicability to the broad range of medical challenges that T cells can deal with.
Our pursuit of protein vaccines has two tracks. In one, we are trying to uncover principles to induce antigen-specific T cell immunity in vivo, so that the protein vaccine can direct an appropriate immune response for the infection, cancer and so forth. Our focus is to understand how the intricate DC system initiates and controls immunity in the intact animal. DC biology has made it possible to direct antigen specific immunization and memory in vivo, the essence of vaccination. In the other track, we aim to test findings derived from animal models as quickly as possible in proof of concept studies in human subjects. This sets standards for where we are and what we need to know.
Dendritic cells
DC are a dedicated lineage of white blood cells that initiates and controls immunity, as well as tolerance. They are positioned at most sites in the body, especially body surfaces, to capture antigens. Further, DC are able to migrate to the T cell areas of lymphoid organs to select clones of antigen reactive T cells and initiate immunity.
Importantly, DC are able to respond to a spectrum of stimuli by extensive differentiation or maturation, to become immune stimulating cells. As we will discuss later, there are different types or subsets of DC, but all are able with appropriate maturation to enhance immunity. This maturation must accompany antigen uptake, processing and presentation in order for DC to immunize; otherwise DC can induce tolerance or silencing of antigen-specific T cells. But how can these features of DC be harnessed to develop new protein vaccines?
Two types of receptors that are being exploited to improve T cell-based protein vaccines
Protein vaccines have the potential to be more readily manufactured, safe, and less expensive than other types of vaccines. However, proteins are often poorly immunogenic for T cells, even when administered repeatedly in high doses. The ability of scientists to harness DC in vivo and thereby render protein vaccines more immunogenic changed with two advances in immune biology beginning in 1995.
First, in a search for a better molecular understanding of DC by the Steinman and Nussenzweig labs, a commonly used marker for DC was identified as the first receptor on DC in vivo that could mediate antigen uptake, processing and presentation, called DEC-205 (“DEC”) or CD205 [2]. As will be discussed below, this includes processing of proteins onto MHC I products, which is called “cross-presentation”. The latter is critical if the protein vaccine is to elicit not just CD4+ helper T cells but also CD8+ killer or cytotoxic T cells, which are often valuable for resistance to infections and cancers. Many similar innate receptors for antigen uptake were then quickly found following the discovery of DEC, and some also bring about cross-presentation. So now there was a path -- proteins could be targeted to DEC or other DC uptake receptors -- to greatly enhance antigen presentation to both CD4+ and CD8+ T cells in the intact animal and human.
Second, the first of another class of innate receptors was identified, particularly the toll like receptors (TLR) of flies [3] that signal cells and trigger maturation of DC. This TLR discovery by Jules Hoffmann was followed by the identification of mammalian TLR [4–7]. Importantly for the vaccine theme, numerous TLR were identified and shown to be triggered by a corresponding family of defined microbial components and mimics, particularly by Shizuo Akira [8, 9]. These pathways signal cells that a pathogen is on the scene. The literature emphasizes the capacity of TLR to signal cytokine and chemokine production by most cells. In the case of DC, however, there was now a potential to signal their full maturation with defined compounds.
To summarize this introduction, by targeting antigen to DC uptake receptors, and by triggering DC signaling receptors, the stage is set to try to overcome two previously huge hurdles to protein vaccines: to ensure adequate capture and processing of vaccine proteins by DC for presentation to CD4+ and CD8+ T cells, and to give the DC information that an infection or other challenge is at hand.
Preclinical findings in mice for new DC-targeted protein vaccines
Targeting protein to DC improves the efficacy of immunization
Our first goal was to be able to induce an integrated and strong response by CD4+ and CD8+ T cells to HIV proteins. We will be discussing various criteria for strong responses throughout the course of the review, but one needs to be honest that there are no precise correlates, especially in humans, for T cell-based resistance to many global infections or cancer, including the quantitative level of the protective response. As a result one tries to identify the principles that will lead to T cell responses with different features by DC-targeted protein, e.g., higher levels of immune T cells, capacity of the T cells to recognize many peptides from the protein, durability of the response, and the ability to proliferate well and make potentially protective cytokines and chemokines when an antigen challenge occurs.
Gag was the first choice for an HIV protein to be targeted to DEC, since there was evidence on many lines that T cell immunity to gag had at least some protective capacity. For example, immunity to gag has been associated with better clinical outcome [10–15] and gag as a single antigen modestly protects across MHC haplotypes following adenovirus-SIV gag immunization in monkeys [16].
It was also feasible to genetically engineer an anti-DEC-HIV gag fusion mAb that could be manufactured relatively quickly in a clinical grade. As mentioned above, it was of a high priority to us to gain objective information on the extent to which findings in mouse would pertain to humans.
Trumpfheller et al proceeded to engineer the carboxy terminus of the heavy chain of an anti-mouse DEC mAb, called NLDC-145, so that the fusion mAb would deliver gag directly to the DEC receptor on DC in mice [17]. To stimulate DC maturation, they used a combination of synthetic double stranded RNA, poly IC, and agonistic anti-CD40 mAb. While this combination of stimuli might be difficult to bring into the clinic, Trumpfheller et al wanted to find out quickly, with a single immunization of the mice, whether the DEC-targeted gag was more immunogenic than gag targeted within a control mAb. This was indeed the case, i.e., much lower doses of anti-DEC-gag mAb were needed to immunize, and the T cell responses were higher. A nice specificity control was that the anti-DEC-gag mAb failed to immunize DEC knockout mice, indicating the immunization was critically dependent on DEC. This basic finding that DEC targeting was superior to control Ig targeting, and that DEC receptor was essential, has been made in a large number of other proteins as we will illustrate in the discussion.
In addition to measuring the number of T cells making a cytokine like IFN-γ, several other potentially valuable features of the T cells were noted [17]: i) many peptides were presented in multiple MHC types of mice; ii) the immune T cells were durable, being readily recalled 3 months after immunization; and iii) in an admitted contrived assay for protection with respect to HIV, the vaccinated mice used the immune T cells to resist an airway challenge of recombinant vaccinia-gag virus. There are certainly other, even more desirable criteria of strong immunization, and these will be considered below, particularly the development of mucosal immunity and induction of CD8+ T cell response. Nevertheless, we had evidence that targeting of antigen to DC improved immunity, always sparing the required dose of antigen by factors of at least 10–100 and often inducing higher quantity and quality responses. We could turn to the issue of a suitable adjuvant particularly for human proof of concept studies.
Poly IC and poly ICLC, as effective adjuvants
As outlined above, the discovery of innate signaling receptors and chemically defined agonists has given a new face to the adjuvant field, because many of these agonists served as better adjuvants for T cell immunization relative to the time honored adjuvant, alum (which does enhance antibody immunity). Yet the field is large. There are many agonists, innate signaling receptors, possibilities for combinations, and ways to formulate the adjuvant and vaccine to be the most effective. Three events took place all at once that made us emphasize synthetic double stranded RNA, poly IC, and also a complex of poly IC with poly-L-lysine that is called poly ICLC.
First poly ICLC, made by a small biotech, Oncovir Inc., could be provided in a clinical grade by an active colleague, Dr. Andres Salazar. Poly IC was discovered to be a viral mimic in the 1960’s, able to induce large amounts of interferon, long before the discovery of innate signaling. In the early days it was not considered that this innate response was not only providing early resistance to viral challenge but also was a pivotal immunogenic cytokine affecting most types of immune cells in a positive way, including DC as below. The analogue poly ICLC was next used safely in many human studies, both as a therapy in patients with cancer and HIV infection, and also as an adjuvant for vaccination [18].
Second, our colleagues in Germany -- Christiane Stahl-Hennig, Klaus Ueberla, Paul Racz, Klara Tenner-Racz and Ralf Ignatius -- were finding that poly IC and poly ICLC could serve as adjuvants for T cell immunization in monkeys [19]. Immunization to several proteins could be achieved with poly ICLC but not other adjuvants.
Third, as will be described in detail below, M. Paula Longhi decided to compare a panel of TLR agonists in mice. All boosted antibody responses to anti-DEC-gag mAb, but it was poly IC and poly ICLC that stood out as adjuvants for T cell immunization, with TLR7/8 agonists being the second most active adjuvant [20].
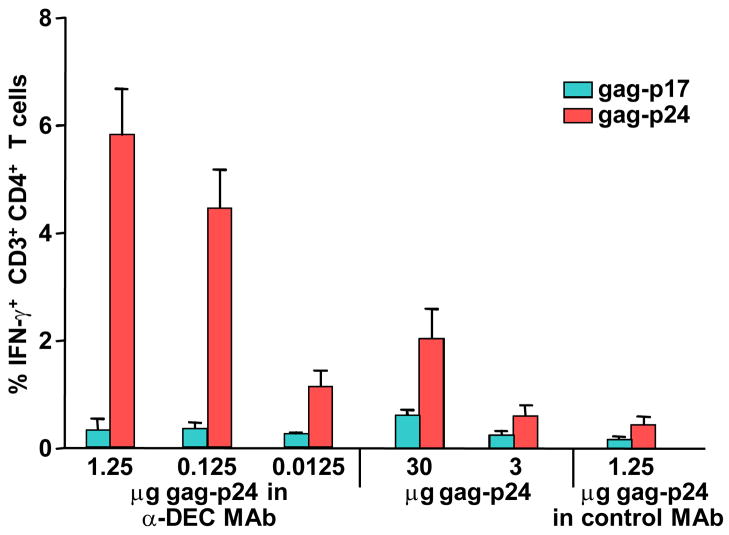
Trumpfheller et al then studied poly IC as single adjuvant [21]. The primary immune response to anti-DEC-gag mAb plus poly IC was weak, but a booster dose elicited strong CD4+ T cell immunity. The T cells could proliferate upon rechallenge with antigen and could make several cytokines like IFN-γ, IL-2, and TNF-α (often by the same T cells, where such polyfunctional T cells have been linked to protection in other infections). These T cells persisted at least 7 weeks. The T cell response was dependent on two innate receptors, not only endosomal TLR3 but also the cytosolic melanoma differentiation-associated gene-5 (MDA-5) receptor. Another targeted protein, anti-DEC-HIV nef fusion mAb, was also immunogenic when administered along with poly IC. A summary figure of immune enhancement with DEC targeting along with poly IC is provided in Figure 1.
Fig. 1.
HIV gag protein is highly immunogenic when targeted with anti-DEC mAb together with poly IC. C57BL/6 mice were injected with poly IC and graded doses of anti-DEC gag-p24 mAb or gag-p24 protein, or one dose of control-Ig gag-p24, and boosted with the same condition 6 weeks later. Here 1.25 μg of gag-p24 within anti-DEC mAb corresponds to 5 μg of anti-DEC-gag-p24 mAb. One week after boost, IFN-γ in spleen CD3+CD4+ T cells was measured in response to stimulation with HIV gag-p24 reactive peptide mix or non-reactive HIV gag-p17 peptide mix.
Poly IC as a stimulus for functional DC maturation in vivo
A stimulus for innate signaling is fundamental to the efficacy of a protein vaccine. But what are the consequences of this signaling in the in vivo setting? Curiously, the research on innate stimuli has been dominated by research on cultured cells and the production of cytokines as readouts. Longhi et al addressed what happens to DC in vivo, initially using poly IC. She focused on “functional” maturation, the ability to become immunogenic as a result of innate stimulation. One source of confusion here is that it is readily easy to measure changes in cell surface markers within hours of exposure to cytokines particularly interferons and TNF as Shin-ichiro Fujii and Kanako Shimizu had found [22]. So by definition, most innate stimuli, because they induce cytokines, are going to result in some “phenotypic” maturation, e.g., upregulation of CD86 and CD40 and downregulation of the IFN-γ receptor. Longhi et al searched for functional evidence, including cytokine production but also a direct demonstration that the DC became immunogenic.
Longhi et al designed adoptive transfer experiments with DC from elegant mixed bone marrow chimeras to prove that DC acquired the capacity to induce an immune response in a new animal [20, 23]. Mice were given anti-DEC-gag mAb with or without poly IC [20], and just 4 hrs later, the DC were isolated and transferred to naive mice that had not seen DEC-gag or poly IC. The DC from the poly IC adjuvanted mice, if they were wild type, were already educated to be immunogenic for adaptive T cell immunity. The immunization was direct. If the DC came from mice lacking MHC II molecules, they could not elicit immunity in a naive animal, i.e., the DC were not simply transferring antigen or other stimuli for the recipient mice to present. This sets the stage to figure out what happens in DC that are becoming immunogenic in that first 4 hrs.
These experiments performed by Longhi et al showed something that has since been found in other studies. The DC not only produced type I interferon when poly IC was the adjuvant, but they also required type I interferon receptors to respond [20]. Most of the interferon in the case of poly IC is made in the host, but this production not only provides innate resistance if the stimulus was a viral infection rather than poly IC, but the interferon is also key for DC to link innate and adaptive immune responses. In ongoing studies, Scott Barbuto in his PhD thesis is formally showing that, if both the antigen and an innate stimulus were targeted well to DC, the DC alone -- without needing interferon production by the host – is sufficient for initiating immunity.
Poly IC is one of many new defined agonists. In recent studies, Longhi et al pursued glucosyl pyranosyl lipid A (GLA), a synthetic agonist for TLR4. Again, a 4 hr exposure to GLA with protein vaccine, allowed DC to become immunogenic for another animal [23].
Cross-presentation of anti-DEC-gag to CD8+ T cells
While the CD4+ T cell frequencies induced by the targeted gag protein vaccine in the above studies were as high or higher than seen with other immunization methods, the absence of a measurable effector CD8+ T cell response (production of cytokines in response to antigen rechallenge, following vaccination) was striking. We tested many variables to try to improve the CD8+ T cell outcome, such as antigen and adjuvant dose, schedule and route -- but to no avail. This was a major surprise, since an early paper from the lab on targeted protein immunization had documented exceptional cross-priming of CD8+ T cells specific for ovalbumin [24]. But ovalbumin was the exception not the rule, since priming of CD4+ T cells but not CD8+ T cells was typically observed with other targeted protein antigens such as gag [21]. CD8+ T cells in C57BL/6 mice are very sensitive to one ovalbumin peptide, several hundred to a thousand fold more sensitive than most other antigens, and strong cross-priming with ovalbumin is, as a result, not representative.
In spite of the poor CD8+ T cell priming in mice, contrasting findings were made by Bozzacco et al on cross-presentation [25]. This means instead of cross priming to initiate CD8+ effector T cells, cross-presentation to already primed CD8+ T cells. Bozzacco et al studied the response of cultured T cells from well-controlled, HIV-infected individuals. The first mAb to human DEC and a control Ig were engineered to express HIV gag. These were added to mixtures of DC and T cells for a week. HIV gag targeted within an anti-human DEC mAb, but not a control Ig mAb, was cross-presented clearly to CD8+ T cells from infected individuals. The cross-presentation brought about by anti-DEC gag was seen in many MHC haplotypes, as is characteristic of unselected human populations. This was important because in the past, studies of cross-presentation had focused on single MHC haplotypes from mice and humans. Now protein vaccines seemed more feasible since to be successful, the protein must be cross-presented in the diverse population to elicit the CD8+ T cell arm of resistance.
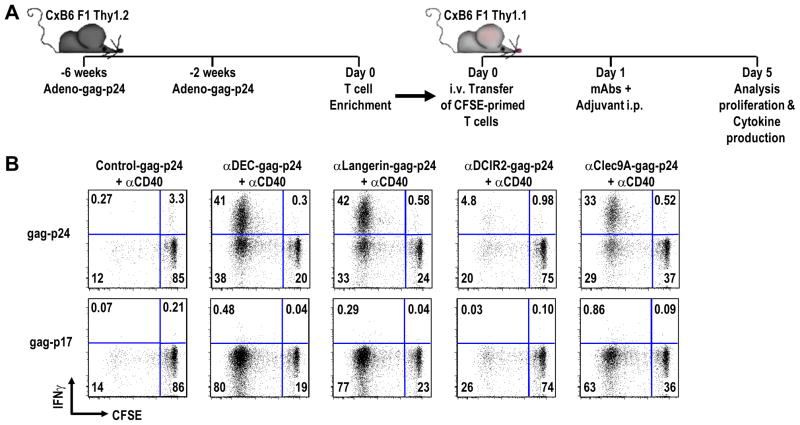
Idoyaga et al carried out a similar study in vivo in mice as republished in Figure 2 [26]. CD8+ T cells in mice were first primed to recombinant adenovirus-gag. They were then transferred to naive recipients that were boosted with anti-DEC-gag or antibodies to other DC receptors (Fig. 2). The adjuvant was anti-CD40; without adjuvant there was no boosting of the transferred gag-specific T cells. With control Ig-gag and anti-DCIR2-gag, little cross-presentation was seen. Anti-DCIR2 targets a subset of DC that lack expression of CD8αα and is poor at cross-presentation. In contrast, antibodies to the CD8αα + DC subset, including anti-DEC and anti-Langerin, were cross-presented. Specificity was shown in different ways, e.g., the responses were specific for the gag-p24 antigen used in the protein vaccine, not the gag-p17 region, and the DEC-gag mAb did not boost T cells primed with adenovirus-ovalbumin rather than adenovirus-gag. So anti-DEC mAb enhances cross-presentation dramatically, but something yet to be identified is preventing cross priming from being manifest as effector CD8+ T cell responses, in contrast to vigorous effector CD4+ T cell responses. We will return to this matter below.
Fig. 2.
Targeting of antigen to DEC receptor allows for cross-presentation on MHC class I and expansion of adoptively transferred, gag-primed, CD8+ T cells. Cross presentation refers to the capacity of nonreplicating protein to be presented on MHC class I. To show that DEC targeting of an antigen is able to greatly facilitate cross-presentation the following adoptive transfer experiment was done. (A) CxB6 F1 Thy 1.2+ mice were primed and boosted with adenovirus-gag-p24. Two weeks after the booster immunization, T cells from adenovirus-gag immunized spleens were enriched by negative selection, labeled with CFSE and one spleen equivalent was adoptively transferred into each CxB6 F1 Thy 1.1+ mouse. The following day, mice were challenged with different targeting mAbs given along with adjuvant. Four days later, the transferred T cells were evaluated for proliferation by CFSE dilution and cytokine production. (B) Adoptively transferred, gag-primed, enriched Thy 1.2+ CD3+ CD8+ T cells were challenged with 5 μg anti-DEC-gag-p24 or the other indicated fusion mAbs along the top, given along with 25 μg anti-CD40 that is essential to observe immune boosting. Four days after antibody inoculation, IFN-γ production and proliferation of the transferred T cells was assessed in a 6 hr in vitro re-stimulation assay in the presence of HIV gag-p24 reactive peptide mix, with HIV gag-p17 mix as negative control.
Other DC subsets and receptors
In mice, DEC is more highly expressed on one subset of classical DC in lymphoid tissues, the same subset that expresses high levels of CD8αα. The other CD8αα low DC subset expresses a lectin, called DCIR2, which is recognized by the 33D1 mAb. Both DCIR2 receptor and the heavy and light chain of anti-DCIR2 mAb, i.e. 33D1, were cloned by Dudziak et al [27]. Targeting to the DCIR2 receptor was excellent at presenting ovalbumin and other antigens to CD4+ transgenic T cells, whereas DEC targeting was better at presenting antigens to CD8+ ovalbumin-specific transgenic T cells.
Soares et al approached protein immunization of naive mice via DC subsets, but used the LACK antigen from Leishmania major [28]. Her interest was in vaccination against a parasite, which should be effective if CD4+ Th1 immunity could be induced and provide the large amounts of interferon gamma to activate macrophages, which in turn could kill this intracellular parasite. Both anti-DCIR2-LACK and anti-DEC-LACK were much more active than control Ig-LACK or soluble LACK in inducing CD4+ T cell immunity in Balb/c mice that are prone to make Th2 not Th1 responses. But the quality of the immunity was different when different DC subsets were targeted. Targeting the CD8αα - DC subset with anti-DCIR2-LACK led to a lower number of IFN-γ producing CD4+ T cells, while targeting the CD8αα + DC subset with anti-DEC LACK, led to a large polarized Th1 response, even in Balb/c mice that are otherwise prone to form Th2 responses. One could argue that these differences in Th outcome were due to the different receptors we targeted, not the DC subsets that expressed them. However, others had previously shown with isolated CD8αα + and CD8αα - DC that the former favored Th1 immunity with nontargeted antigen [29, 30].
The experiments were extended by Idoyaga et al with HIV gag antigen. Again anti-DCIR2-gag was weaker than anti-DEC-gag in inducing CD4+ IFN-γ producing T cells, as well as IL-2 and TNF-α producers. Moreover, in this study Idoyaga et al began to address the role of different uptake receptors expressed on the same CD8αα + DC subset. This DC subset not only expresses DEC at higher levels but selectively expresses CLEC9A and in the case of mice of the Balb/c background (or C57BL/6 x Balb/c F1 background), Langerin/CD207. The results indicated that immune priming through all 3 lectins -- DEC, CLEC9A and Langerin -- was comparable [26].
In thinking about the relevance of these data for the immunization of humans, we are currently limited by the amount of information on the distribution of DC subsets in vivo, and the expression of the different uptake receptors. However early on in the research on DEC, we made an anti-human DEC mAb and found that it stained most DC in the T cell areas of lymphoid tissues [31]. This gave us additional assurance that targeting of antigen within anti-DEC fusion mAb to human DC should be feasible in people and monkeys, which also express DEC and react with many anti-human DEC mAbs.
Getting DC-targeted protein into the clinic
Human anti-human DEC monoclonals for testing in human subjects
It is difficult to extend research into humans without a productive collaboration with industry, able to make products, involved with the science, and experienced with clinical research. Fortunately, Celldex Therapeutics and its scientific team led by Tibor Keler have been that kind of collaborator. Celldex, an antibody targeting biotech, has developed technology to make human mAbs by immunizing human immunoglobulin transgenic mice that lack mouse immunoglobulin genes but carry the human Ig locus. Chae Gyu Park prepared the large external domain of human DEC, and this was used by Celldex to prepare a panel of mAbs, many of which bound with high affinity to DEC [32]. One mAb, 3G9, was selected, and its heavy and light chains were cloned to prepare human anti-human DEC-gag fusion mAb. Celldex also made an elegant control Ig for 3G9 by mutating the CDR loops needed for DEC binding.
The 3G9-gag was nicely cross-presented to CD8+ T cells from HIV infected individuals while the control Ig-gag was not [32]. Thus human DEC was active in allowing gag protein to be processed into peptides that were presented on MHC class I.
For preclinical data in vivo, we benefited from mice in which the CD11c promoter drove expression of human DEC on DC. Cheong et al immunized these mice with 3G9-gag but not control Ig-gag. Both antibody and T cell responses were entirely dependent upon the use of a DC maturation stimulus, poly IC, but the T cell responses were again primarily CD4+, not CD8+.
3G9-gag-p24 was then produced in large amounts, and used first for successful toxicology studies in nonhuman primates, particularly since 3G9 cross-reacted well with monkey DEC receptor. The vaccine tested was the combination of 3G9-gag plus poly ICLC, and was safe. The animals developed transient injection site reactions without evidence of systemic reactogenicity. We did not and do not plan to carry out studies with 3G9-gag-p24 in the absence of adjuvant, because targeting of antigen to DC without a maturation stimulus leads to tolerance as initially discovered by Daniel Hawiger, a student in the Nussenzweig lab that has been a close collaborator in the design of DEC targeted vaccines [33]. The preparation of a clinical protocol, and the filing of the protocol with the FDA, was carried out with great efficiency by a team from our Rockefeller University hospital and Celldex Therapeutics, led by Sarah Schlesinger.
The protein vaccine, 3G9-gag-p24 together with poly ICLC, is immunogenic in rhesus macaques
Another important collaboration then was led by Robert Seder at the Vaccine Research Center of the NIH. Seder has a broad grasp of T cell-based vaccines and the many variables that go into their design and formulation. He tested our clinical product for immunogenicity in nonhuman primates [34]. Although the available number of animals was small, the 3G9-gag induced strong CD4+ T cell responses with just two doses subcutaneously, one month apart. The responses were entirely dependent upon the co-injection of poly ICLC adjuvant. Some cross-priming of CD8+ T cells was observed, and these showed some proliferative response to antigen rechallenge, but again CD8+ T cells were infrequent (0.1–0.5% of blood CD8+ T cells) relative to CD4+ T cells (>1% of CD4+ T cells). No protection experiments with SIV were carried out since the antigen was HIV gag. Many consider that protection experiments with SIV are essential for vaccines to move forward. We share the view of a large group of scientists that recently strategized on the future of AIDS vaccine development. The consensus was that monkeys are not a gatekeeper for the field, and that studies in human subjects were a priority. In part, this is because apparent protection in monkeys does not always translate to protection in humans.
3G9-gag-p24 primes CD8+ T cells for a robust response to a replication defective viral vector
An active approach in the AIDS and other vaccine fields is to combine different forms of vaccines. Notably, in the RV144 Thai trial that achieved evidence for protection against HIV infection, a combination of a recombinant POX vector (ALVAC) was used to prime several times and this was followed by boosts with envelope gp120 protein. Bob Seder, through collaboration with Gepi Pantaleo and Mariano Esteban, decided to boost the above animals that had been primed with DEC-gag protein plus poly ICLC (or either alone) with a replication defective NYVAC vector [34]. This vector had been deleted of 18 open reading frames involved in virulence and pathogenicity, so that it could only replicate in the producer cells. The NYVAC was not detectably immunogenic by itself (one or two doses) or in animals primed with just DEC-gag or poly ICLC.
But then there was a big surprise. In animals primed with both anti-DEC-gag protein and poly ICLC, there was a very large and rapid boost in gag-specific T cells upon a single injection of recombinant NYVAC, particularly CD8+ T cells, which reached levels of 0.5–20% depending on the animal. As detailed above, we had failed previously with the protein vaccine to achieve substantial CD8+ T cell immunity. However, we had not appreciated that these animals are well primed to make CD8+ T cell responses even to a replication defective vector. This finding is essentially what one wants a vaccine to do, i.e., protein vaccination enables CD8+ T cells in the vaccinated individual to respond quickly and well, even to a low level challenge.
We are now pursuing this finding, which had not previously been appreciated with protein vaccines. The vigorous boost in CD8+ T cell immunity presumably reflects the contribution of primed CD4+ and CD8+ T cells. In a prior study, Nchinda et al had found that anti-DEC-gag together with a DNA vaccine could prime mice so that they made a very large CD8+ T cell response in lungs challenged with recombinant vaccinia-gag virus. In that study, the CD4+ T cells primed by the first vaccines were essential for the CD8+ T cell response to the virus [35].
Proof of concept in human subjects
Anti-human DEC-gag-p24 plus poly ICLC appears to be immunogenic for T and B cells
A randomized dose escalation study is underway in which healthy human volunteers receive either 3G9-gag subcutaneously with poly ICLC, or poly ICLC or sterile saline only. We have fully enrolled two cohorts that received the lowest dose of vaccine, i.e. 300 μg fusion mAb (equivalent to ~100 μg of the gag protein), and the middle dose, i.e. 1 mg of fusion mAb. A fixed dose of 1.6 milligram of poly ICLC is being used. The study remains blinded, but 9/15 subjects in each of the first two cohorts had received 3G9-gag with poly ICLC and 9/15 are producing a substantial titer of IgG antibody to gag. T cell assays are underway, but in the first cohort, many individuals had T cells producing IL-2, TNF-α and IFN-γ. The immediate goal for the clinic is to boost the volunteers with a highly attenuated Poxvirus vector, as was described above in the monkey studies. Other goals are to design the vaccine to include envelope protein and to evaluate other adjuvants.
Randomized studies of the adjuvant for protein vaccines
As discussed above, the new defined agonists for innate signaling receptors very quickly instruct DC to become immunogenic. This means that events take place very early in response to the adjuvant, rather than the vaccine per se, and these dictate the adaptive immunity developing weeks to months later. Ultimately, it may be possible to compare adjuvants and their combinations by studying the early innate response of blood cells, including DC. Criteria would then be defined to predict adaptive immunity, and greatly expedite the evaluation of adjuvants.
Marina Caskey, Sarah Schlesinger and their team directed our first such study of the response of healthy subjects to poly ICLC versus placebo in a blinded fashion [36]. They made several findings together with Rafick Sekaly and his group at the Vaccine and Gene Therapy Institute in Florida, who are experts in systems approaches to the analysis of the human response to vaccines. They observed a large transcriptional response in total blood cells in all 8 individuals receiving poly ICLC subcutaneously but in none of the 4 placebos. The reliable innate response peaked in about a day and was dissipated by 3 days. It involved a dominant component of interferon-stimulated genes as well as the induction of other innate components such as inflammasomes and complement. These kinetic studies help us plan future studies of poly ICLC and other adjuvants.
Since Dr. Sekaly and his team had considerable experience with transcriptional profiling in subjects receiving the attenuated yellow fever vaccine, YF17D, we compared the changes induced by a single adjuvant, synthetic double stranded RNA, with a successful live viral vaccine. The kinetics of the transcriptional changes differed, with the response to poly ICLC being much faster. Nevertheless at the peak of the responses, the induced innate immune pathways showed considerable overlap. This was portrayed by pathway analysis of the responding genes, and about 20 such pathways were similar. This established for the first time that synthetic stimuli that are meant to mimic a microbial challenge actually do mimic the microbe in vivo and at the level of detailed and validated systems analysis.
Discussion -- expanding the scope of protein vaccines for T cell immunity
This review has focused on T cell vaccines for HIV proteins. Clearly we need to aim for an antibody component as well, to study mucosal immunization, and to generate T cells to the most conserved regions of the virus. All of these studies are underway. And we need to broaden the evaluation of adjuvants, which M. Paula Longhi began with her study of the DC maturing, TLR4 agonist GLA, as discussed above.
We feel that the themes for HIV protein vaccines have common ground with other clinical targets. For example, the idea of a targeted vaccine seems relevant for immunization against highly expressed tumor proteins like mesothelin and HER2. Bei Wang is finding that in order to immunize a mouse, it is essential to target the protein and to include a stimulus for innate immunity; otherwise the immunity is weak to imperceptible [37, and unpublished data]. Again, as with HIV, strong immunity to a tumor protein means a relatively high frequency of T cells with standard assays. While criteria for protective T cells require clinical studies, the tumor immune T cells elicited by a DEC-targeted protein vaccine are able to recognize endogenous processed antigen in tumor cells, and upon seeing specific antigen, the T cells can proliferate and make many cytokines. Of course tumors and HIV represent distinct challenges but, at the heart of new protein vaccine approaches, is the need to elicit strong, specific and durable T cell immunity. With this as a foundation, one can then explore combination approaches such as blocking immune checkpoints and tumor invasion mechanisms, and using other forms of vaccines.
But what about immune silencing? Here the principles are much less developed than immunization. The fulcrum of current attention is the suppressive T cell or foxp3+ T reg, since this cell type can develop in response to antigenic stimulation of the polyclonal repertoire and then can suppress immunity to that antigen, including in vivo as Uri Sela has now shown. A vaccine perspective has yet to be emphasized, possibly because of reports that these T reg lose suppressive function in inflammatory sites, where they would have to function in order to block for example autoimmunity, inflammatory bowel disease, graft versus host disease or allergy. In contrast, Sela et al now have evidence for long term stability of DC-induced antigen-specific foxp3+ T reg [38]. Therefore in our view, many of the variables discussed here are relevant to pursue protein vaccines that silence unwanted immunity, e.g., the selection of the appropriate antigens, the role of different DC subsets, and the need to define the environment (“adjuvants”) required by DC that will induce suppressive and stable T reg.
But returning to HIV, the data described here illustrate the value of studying patients to make findings we could not have predicted and to provide a concrete frame of reference to move forward on the design of both the adjuvant and the protein vaccine. The urgency of the AIDS epidemic and the substantial investment in research, particularly in the USA, has made it possible to develop an important parallel clinical arm to our research. Resources for clinical studies in other settings need to be found to gain antigen specific control of the immune system. This is made feasible by current knowledge on DC and the immune system more broadly. Protein vaccines that target DC are a means to expand this knowledge and to include research in human subjects to overcome the great need for T cell-based vaccines in medicine.
Acknowledgments
We thank M. Nulty and J. Chiappetta for administrative help, and J. Adams for graphics. Funding was provided by National Institutes of Health Grants AI081677 (R.M.Steinman), K23AI084855 (M. Caskey), and UL1RR024143 (Rockefeller University Hospital Center Clinical and Translational Science Award), by the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative of Bill and Melinda Gates Foundation Grant GC#334 (R.M. Steinman and S.J. Schlesinger), and Collaboration for AIDS Vaccine Discovery (CAVD) grant #38650.
Abbreviations
- DC
Dendritic cells
- mAb
monoclonal antibody
- GLA
glucopyranosyl lipid A
- poly IC
polyriboinosinic:polyribocytidylic acid
Footnotes
Conflict of Interest Statement
Tibor Keler is an employee of Celldex Therapeutics, which is developing human DEC-205-based vaccines. Ralph Steinman was on the scientific advisory board and held stock options in Celldex Therapeutics. The remaining authors declare no competing financial interests.
References
- 1.Hermansson A, Ketelhuth DF, Strodthoff D, et al. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–93. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/Hej and C57BL/10ScCr Mice: Mutations in TLr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Takeuchi O, Kawai T, et al. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 7.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (Tlr4) JExpMed. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 10.Zuniga R, Lucchetti A, Galvan P, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–5. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–22305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novitsky V, Gilbert P, Peter T, et al. Association between virus-specific T-cell responses and plasma viral load in human immunodeficiency virus type 1 subtype C infection. J Virol. 2003;77:882–90. doi: 10.1128/JVI.77.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramduth D, Chetty P, Mngquandaniso NC, et al. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J Infect Dis. 2005;192:1588–96. doi: 10.1086/496894. [DOI] [PubMed] [Google Scholar]
- 14.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 15.Geldmacher C, Currier JR, Herrmann E, et al. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J Virol. 2007;81:2440–8. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, O'Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trumpfheller C, Finke JS, Lopez CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–17. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38:1096–103. [PubMed] [Google Scholar]
- 19.Stahl-Hennig C, Eisenblatter M, Jasny E, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS pathogens. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–9. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantel A, Cheong C, Dandamudi D, et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigento elicit Th1 T-cell immunity in vivo. Eur J Immunol. 2011 doi: 10.1002/eji.201141855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozzacco L, Trumpfheller C, Siegal FP, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–94. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idoyaga J, Lubkin A, Fiorese C, et al. Comparable T helper 1 (Th1) and CD8 T-cell immnity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci USA. 2011;108:2384–9. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 28.Soares H, Waechter H, Glaichenhaus N, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-g by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszweski CE. Distinct dendritic cell subsets differentially regulate the class of immune responses in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8a+ and CD8a− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granelli-Piperno A, Pritsker A, Pack M, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–73. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheong C, Choi JH, Vitale L, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116:3828–38. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–80. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn BJ, Kastenmuller K, Wille-Reece U, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant NYVAC induces robust T cell immunity in non human primates. Proc Natl Acad Sci USA. 2011;108:7131–6. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc Natl Acad Sci USA. 2010;107:4281–6. doi: 10.1073/pnas.1000621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caskey M, Lefebvre F, Filali-Mouhim A, et al. Synthetic double stranded RNA reliably induces innate immunity similar to a live viral vaccine in humans. J Exp Med. 2011 doi: 10.1084/jem.20111171. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Kuroiwa JM, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sci. 2009;1174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific Treg that prevent graft vs. host disease and persist in mice. J Exp Med. 2011 doi: 10.1084/jem.20110466. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]