Abstract
Hematological malignancies rely heavily on support from host cells through a number of well-documented mechanisms. Host cells, specifically mesenchymal stem cells (MSCs), support tumor cell growth, metastasis, survival, bone marrow colonization, and evasion of the immune system. In multiple myeloma, similar to solid tumors, supporting cells have typically been considered healthy host cells. However, recent evidence demonstrates that many MSCs derived from myeloma patients, or MM-MSCs, demonstrate significant defects compared to MSCs from non-diseased donors (ND-MSCs). These abnormalities range from differences in gene and protein expression to allelic abnormalities and can initiate after less than 1 day of co-culture with myeloma cells or persist for months, perhaps years, after removal from myeloma influence. Alterations in MM-MSC function contribute to disease progression and provide new therapeutic targets. However, before the scientific community can capitalize on the distinctions between MM-MSCs and ND-MSCs, a number of confusions must be clarified, as we have done in this review, including: origin(s) of MM-MSCs, identification and characterization of MM-MSCs, and downstream effects and feedback circuits that support cancer progression. Further advances require more genetic analysis of MM-MSCs and disease models that accurately represent MSC-MM cell interactions.
Keywords: Multiple Myeloma, mesenchymal stem cells, stromal cells, tumor-host interactions
1 Introduction
Mesenchymal stem cells (MSCs) are a dynamic population of cells capable of self-renewal, differentiation, tumor and wound homing, and immunomodulation. Harvested from bone marrow, adipose tissue, cord blood, or a variety of other sites, MSCs play multiple roles in tumor progression, as previously reviewed(1). Complications in comparing studies and drawing conclusions arise due to different stem cell isolation, characterization, and culture protocols, and the inherent variability in stem cells within and between donors. MSCs can have both tumor supportive (pro-tumor) and inhibitory (anti-tumor) effects(2), but most myeloma-specific studies demonstrate a stimulatory, protective, and pro-tumor effect of MSCs on myeloma cells, suggesting that novel drugs could counteract these tumor-supporting effects in the bone marrow.
Local bone microenvironment activates many pathways leading to lesion growth and disease progression including the following: the PI-3K/Akt/mTOR/p70S6K cascade, the IKK-α/NF-κB pathway, Ras/Raf/MAPK, and JAK/STAT3, as reviewed extensively(3; 4). Many clinical and pre-clinical trials are aimed specifically at developing inhibitors of these pathways. Moreover, findings are emerging that alterations in local microenvironment may be not only supportive of tumor growth, but required for tumorigenesis. For example, deletion of DICER in osteoprogenitor mesenchymal cells can disrupt hematoposis and cause myelodysplasia and acute myelogenous leukemia in mice. This, among other studies, demonstrates the concept of microenvironment-induced oncogenesis (5–9). MSCs can also increase multiple myeloma (MM) cell adhesion to bone marrow, protecting the cells from chemotherapy and helping them accumulate within the bone. Adhesion is mediated by molecules including CD44, Very Late Antigen (VLA)4, VLA5, leukocyte function-associated antigen 1 (LFA1), neuronal adhesion molecule (NCAM), intercellular adhesion molecule (ICAM1), syndecan 1, and MCP1 as reviewed previously(10). Binding of CD40, located on MM cells, with its ligand (CD40L) on MSCs can further increase expression of adhesion molecules such as LFA1 and VLA4. Subsequently, MM cell adhesion is further increased, stimulating cytokine (IL6, [interleukin 6] and VEGF [vascular endothelial growth factor]) secretion by MSCs, creating a forward feedback loop for tumor growth(11; 12). In sum, stromal dysfunction is tied to neoplasia progression, implicating local stromal cells as coconspirators in tumor development.
Stromal cell-induced chemotherapy resistance in myeloma cells is well documented (13; 14), yet, many drug screens are still performed in the absence of stromal cells and therefore produce deceiving findings. Novel drug screens using stromal cell-myeloma cell co-cultures are now being developed to produce more clinically-relevant modeling tools (15; 16). Pharmaceutical developers are also now attacking tumor cells through stromal-affecting drugs, such as Bortezomib, Perifosine, and an array of bisphosphonates which target stromal cell-tumor cell interactions. Perifosine induces apoptosis even in MM cells attached to bone marrow stromal cells through the c-Jun N-terminal kinase (JNK) pathway(17) and Bortezomib, a proteasome inhibitor, was recently found to directly induce osteoblastic differentiation in MSCs to combat osteolysis through the transcription factor RUNX-2(18). As a last example, the CXCR4 inhibitor AMD3100 disrupts the interaction of MM cells and MSCs and enhances MM cell sensitivity to therapy(19). This and other work demonstrates that CXCR4 inhibitors, or other therapies that detach tumor cells from the bone matrix, can increase chemosensitivity of MM cells. The discussed pharmaceuticals were developed based on in vitro studies of healthy donor stroma cells and MM cells. We hypothesize that more effective and specific chemotherapeutic strategies will be identified using in vitro models containing MM patient MSCs. The questions are then: are there differences between non-diseased (ND-MSCs) and myelomatous MSCs, those derived from multiple myeloma patients (MM-MSCs)? How do these relate to differing interactions with MM cells? Lastly, how can we target these interactions for a therapeutic effect? These questions are herein addressed. MM-MSCs discussed were from untreated MM patients unless otherwise noted; often the status of age-matching was not reported in the studies.
2 Origin and Derivation of the MM-MSC
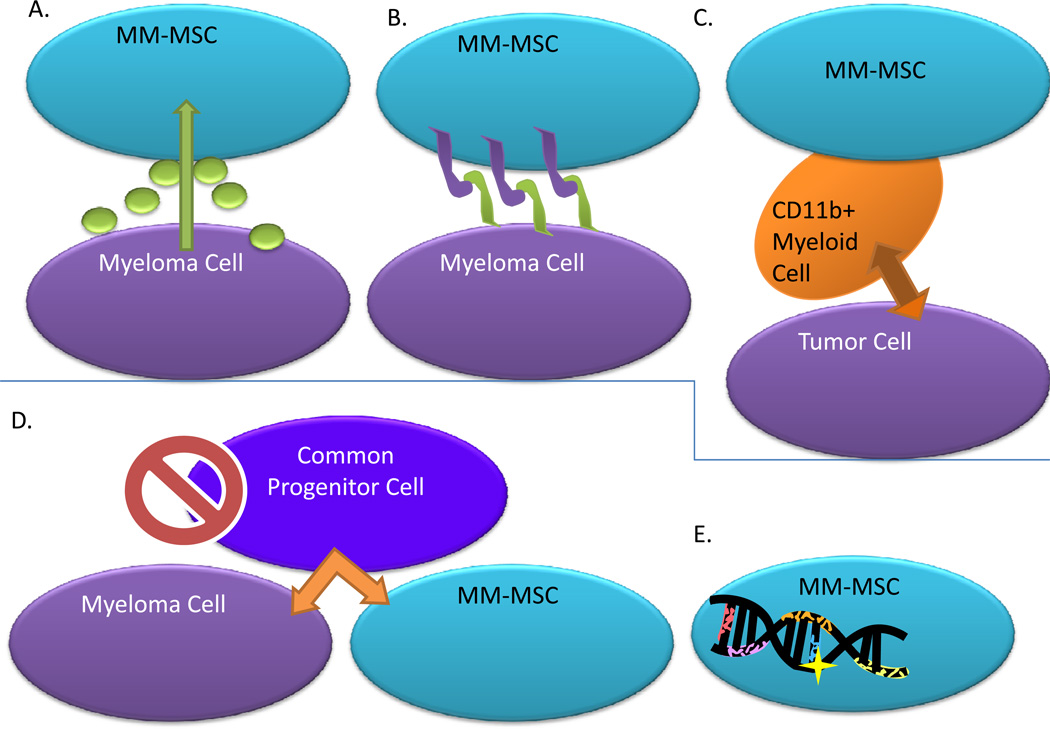
The development of MM-MSCs is poorly understood and their phenotypic and geneotypic characteristics are disputable (Figure 1). Some results suggest that MM-MSCs are inherently abnormal, and will remain abnormal despite being removed from the myeloma cell influence, while others argue that MM-MSCs are only temporarily modified in their gene expression in response to MM cells. For example, many patients survive for years with bone lesions or pathological fractures that never heal due to disrupted osteogenesis and osteoblast function, even in the absence of tumor, suggesting permanent defects within MM-MSCs(20). However, within hours of co-culture with MM cells, normal MSCs can become in vitro MM-MSCs, displaying a phenotype similar to patient-derived MM-MSCs (21). Furthermore, cell-cell contact may be necessary to create in vitro MM-MSCs, or soluble factors may be sufficient, demonstrating our lack of knowledge regarding MM-MSC evolution. Chromosomal aberrations (deletions, translocations etc.) in MM-MSCs remain once the cells are removed from MM cell co-culture(22). However, the origin of these abberations is unclear and in vitro MM cell priming of MSCs demonstrates that genetic alteration are not necessarily the source of, or required for, phenotypic variation in MM-MSCs(22). The theory that MM-MSCs and MM cells are derived from a common progenitor(23) has been disproved by chromosomal aberration comparisons (22; 24; 25). Another report suggests that a contamination of CD11b+ myeloid cells within patient derived tumor associated-stromal cells is responsible for the observed effects on tumor cells(26). Though this study utilized lung carcinoma cell lines, the same results may be true in myeloma studies. As many groups isolate “MSCs” by plastic adherence, there is a strong possibility that what are thought to MSCs are actually a diverse population containing myeloid cells. A final complication is that injection of ND-MSCs into osseous tumor lesions has returned mixed results in terms of tumor growth inhibition. While some of these MSCs retained their differentiation potential and increased osteoblastic activity and bone formation, others were functionally converted into MM-MSCs, supported tumor growth and showed decreased osteogenesis. The development of MM-MSCs is likely a consequence of multiple factors and alterations may vary between individuals, lesion locations, co-culture myeloma cell types (in vitro), and sub-populations of cells within MSC population. Identifying the governing mechanisms in the transition from normal MSC to MM-MSC and how these feedback to MM cells is vital for improved myeloma therapy(21).
Figure 1. MM-MSC Origination.
Theories regarding how MM-MSCs originate have been proposed and include the following. A) Soluble mediators released from myeloma cells can activate MSCs. B) Cell-cell contact is required between myeloma cells and MSCs to activate MSCs. C) Effects of MM-MSCs can actually be traced back to contaminating CD11b+ myeloid cells, which are the true population of cells that are able to affect tumor cells. D) MM-MSCs and myeloma cells are derived from a common progenitor cell, a theory which has been proven incorrect. E) Genetic abnormalities and chromosomal aberrations within MM-MSCs may exist and could affect their phenotype.
3 Phenotype Aberration Characteristics of MM-MSCs
Cytokine and MMP Expression
MM-MSCs differ from ND-MSCs in many aspects (Figure 2), including spontaneous or myeloma cell-induced cytokine production. Many MM stimulatory growth factors such as stem cell factor (SCF), vascular endothelial growth factor (VEGF) and interleukin(IL) 6, are secreted at higher levels by MM-MSCs than by ND-MSCs (24; 25; 27–30). Adhesion of MM cells to ND-MSCs can increase IL-6 production and downstream NF-κB pathway stimulation, suggesting direct cell-cell contact as an initiator of the MM-MSC phenotype (31). Importantly, MSC-derived IL-6 supports MM growth, demonstrating one of many forward feedback mechanisms in myeloma (32).
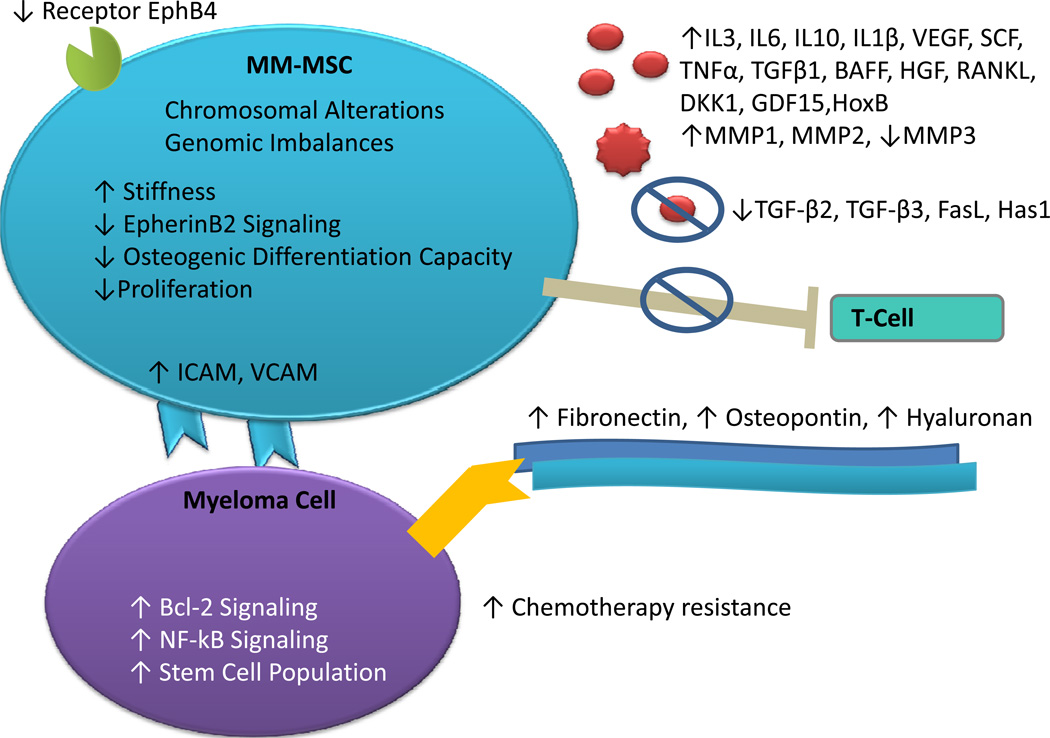
Figure 2. Phenotypic Differences: MM-MSCs vs Healthy-MSCs.
Diagram of the phenotypic differences currently described between MM-MSCs and healthy, non-diseased MSCs. As compared to healthy MSCs, MM-MSCs have the following trais. Increased expression of EphB4 receptor, ICAM, VCAM, IL3, IL6, IL10, IL1β, VEGF, SCF, TNFα, TGFβ1, BAFF, HGF, RANKL, DKK1, GDF15, HoxB, MMP1, and MMP2. Decreased expression of MMP3, TGF-β2, TGF-β3, FasL, and Has1. Increased production of fibronectin, osteopontin, and hyaluronan. Reduced immunosuppressive properties due to a loss in the ability to inhibit T-cells. Downstream effects of MM-MSCs on myeloma cells include increased chemotherapeutic resistance, Bcl-2 signaling, and NF-κB signaling and increased cancer stem cell population concentrations.
Increased expression of IL-1β, tumor necrosis factor alpha (TNF-α), and a range of other factors that can inhibit normal progenitor cell growth has also been detected in MM-MSCs compared to ND-MSCs.(29; 33–35) Recent studies document increased IL-10, B-cell-activating factor of the TNF family (BAFF), and hepatocyte growth factor (HGF) by MM-MSCs compared to ND-MSCs in response to RPMI8226 myeloma cells(29). These cytokines can induce osteoclast stimulation, tumor angiogenesis, and increased MM cell adhesion, proliferation and migration(29). Other studies have described differences in MMP (matrix metalloproteinase), transforming growth factor beta (TGF-β) family members, Receptor activator of nuclear factor kappa-B ligand (RANKL), and FasL expression and increases in cytokine production when stimulated with lipopolysaccharide (LPS) or Newcastle disease virus (NDV) (34; 36; 37). These data suggest that therapeutic interventions specifically targeting these cytokines or their downstream pathway components may be more important for MM patients than demonstrated in in vitro experiments involving no MSCs or MSCs from healthy patients. For example, anti-IL-6 therapies such as tocilizumab or other downstream JAK/STAT or NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) inhibitors may be more effective than currently realized as anti-cancer therapies when delivered specifically to areas of MSC-MM cell interactions(38).
Chemotherapy Resistance
ND-MSCs, and to a greater extent MM-MSCs, can suppress bortezomib-induced MM cell growth inhibition in a cell-cell contact dependent manner by increasing Bcl2 expression in MM cells(27). MM-MSCs, but not ND-MSCs, are also able to activate bortezomib resistance through enhanced NF-κB activity in MM cells, induced by soluble MM-MSC-derived IL-8(39). However, these MM-MSCs were from uncharacterized patients who lacked classification regarding stage or treatment and hence my not represent the typical MM-MSC phenotype. Still, the work suggests a closer examination of the potential of inhibitors of NF-κB and IL-8 within myeloma bone lesions. For example, Sunitinib, a potent inhibitor of the proto-oncogene RET was recently shown to decrease IL-8 expression but is not commonly given to MM patients. Hence, Sunitinib may be effective for MM patients and may have less off-target side effects if delivered directly to the bone marrow.
It is well known that adhesion of MM cells to bone marrow provides the cancer cells with protection against chemotherapies. MM cells have been found to become chemosensitized when their adhesion to marrow stromal cells via the CXCR4 receptor is inhibited(19). Moreover, expression of CXCR4 was increased in MM side-populations (the more stem-like tumor cells) when cultured with MM-MSCs compared to ND-MSCs(40). In sum, MM-MSCs may be extremely important for 1) attracting and increasing the adherence of MM cells to bone marrow, 2) protecting MM cells from chemotherapy, and 3) specifically increasing the tumor-initiating cancer cell populations within bone marrow. Targeting the CXCR4-SDF1 signaling between MSC and MM cells is one of the most promising therapeutic avenues because it can be targeted, and we know this pathway is very relevant to the accumulation, adhesion, and survival of MM cells, specifically of the more stem-like tumor cell population. We propose that more studies into methods of de-adhesion of tumor cells via CXCR4 inhibitors, or other inhibitors, will have a significant impact on the effectiveness of chemotherapy drugs as chemosensitizing agents.
ND-MSCs can also upregulate survivin in MM cells through direct cell-cell contact, though the ability for MM-MSCs to do this has not been investigated(41). Lastly, MSCs from acute myeloid leukemia, Hodgkin disease, and MM demonstrated similar capacities to protect MM cells from IL-6R antagonist therapies, although the ability in ND-MSCs was not studied(32).
ECM Expression
Fifteen years ago, MM stromal cells were found to deposit fewer extracellular matrix proteins (ECM) with simpler organization than ND-stromal cells, specifically regarding fibronectin, laminin and collagen IV(42). More recently, MM-MSCs were instead found to have increased fibronectin and collagen IV expression compared to ND-MSCs(33; 43). These proteins provide feedback to MM cells, as they express moieties for adhesion and survival through cell adhesion mediated-drug resistance [CAM-DR]. Research demonstrates fibronectin adhesion is mediated through integrins such as VLA-4 (α4β1) and VLA-5 (α5β1), among many others(44; 45). Fibronectin binding upregulates p27, induces NF-κB activation, and has been shown to alter expression of 469 gene products in MM cells(46).
Reports also show increased osteopontin (OPN) production from MM-MSCs compared to ND-MSCs in response to myeloma cells(29). Interestingly, MM-MSCs also displayed increased hyaluronan synthase 1 (Has1) expression, decreased Has2 expression, and increased total hyaluronan production(47). MM adhesion to hyaluronan also confers CAM-DR to MM cells(48). OPN has been shown to mediate multidrug resistance in other cancers by enhancing hyaluronate binding and may act similarly in MM(49). Moreover, MM-MSCs have been found to express higher levels of intracellular receptor for hyaluronan-mediated motility (iRHAMM) compared to ND-MSCs, suggesting an increased dependence on hyaluronan for MM-MSC migration(33).
Adhesion, Phenotype and Biomechanics
Differences in MM- versus ND-MSC adhesion molecule expression may facilitate MM cell entrapment in the bone marrow. MM-MSCs express adhesion molecules that bind MM cells (Inter-Cellular Adhesion Molecule 1 (ICAM-1) and Vascular Cell Adhesion Molecule 1 (VCAM-1)) at higher levels than ND-MSCs(27; 33; 39). Beta-1 and beta-2 integrin-mediated MM cell adhesion may also be stronger to MM-MSCs than to ND-MSCs, though these MM-MSCs were from uncharacterized patients(50).
Most reports describe a similar phenotypic appearance and MSC marker expression profile in MM-MSCs and ND-MSCs(25). However, MM-MSCs may be significantly stiffer than ND-MSCs(51). Forward feedback mechanisms through FAK (Focal Adhesion Kinase) activation between MM-MSCs and MM cells, and specifically the MM cancer stem cell population, may govern stiffening of both cell types(51).
Proliferation and Differentiation Capacity
MM-MSC proliferation and rate of osteogenic differentiation is much slower, in part due to their reduced expression of growth factor receptors and increased expression of inflammatory cytokines such as TNF-α, relative to their normal counterparts(24; 52; 53). Dysregulated ephrinB2/EphB4 signaling in MM-MSCs may also decrease their osteogenic potential and increase their MM cell support(54). MM-MSCs have also demonstrated a fivefold higher expression of the osteoblast inhibitor DKK1 at transcript and protein levels, suggesting a direct role in osteolytic lesion propagation through autocrine and paracrine signaling(52). In contrast, others have found similar proliferative and differentiation potentials in MM- and ND-MSCs, although this not commonly found and is often not quantified(25; 28).
Immunomodulation and Downstream Effects on Tumor Stem Cells
MM-MSCs demonstrate an impaired ability to inhibit T-cell proliferation, compared to ND-MSCs, but no difference in their ability to support hematopoiesis(24; 25). The ability to stimulate osteoblastic differentiation is also decreased in T-cells isolated from myeloma patients and in ND-MSCs co-cultured with MM cells(34).
Corre et al., report enhanced support of tumor cell proliferation by MM-MSCs compared to ND-MSCs, suggesting that the abnormal niche created by MM-MSCs may be more efficient at supporting myeloma progression(24). MM cells also demonstrate low baseline miRNA-15a, increased miRNA-15a expression after bortezomib treatment, and a subsequent decline in expression after co-culture with MM-MSCs, suggesting another potential protective mechanism of MM-MSCs(27). In ovarian cancer, carcinoma-associated MSCs (CA-MSCs) derived from cancer patients significantly promoted tumor cell growth compared to MSCs from healthy individuals(55). Overexpression of BMP-2, -4 and -6 in CA-MSCs compared to ND-MSCs and subsequent promotion of the cancer stem cell population was deemed the underlying mechanism of increased tumor growth.(55) Of course, in MM other factors are likely involved since BMP signaling would likely promote osteogenesis, not tumor growth. MM-MSCs may also enhance cancer stem cell colony-forming ability (in vitro) and proliferation (in vitro and in vivo) as compared to ND-MSCs(40; 51). MM-MSCs also appear to support proliferation of the stem-like population of MM cells to a greater extent than ND-MSCs do, suggesting that MM-MSCs are more specifically selective for the growth of tumor-initiating cells than ND-MSCs(40). Indeed, the theory that the less mature, more resistant CD138-negative myeloma cell fraction develops in response to interactions with local mesenchymal cells is becoming increasingly credible and suggests an urgent need to unlock the underpinnings of their association(56).
Gene Signature / Chromosomal Aberrations
Microarray gene expression data has identified 104 transcripts upregulated in rat MSCs exposed to conditioned media from human colorectal cancer cells for 24 hours versus control medium, demonstrating tumor-cell induced MSC gene expression modifications(57). Two gene expression profiling studies have extracted many genes differentially expressed by human MM- and ND-MSCs. One study used human U133 plus 2.0 GeneChip microarrays and identified 145 genes differentially expressed including IL-6, DKK1, “growth and differentiation factor-15” (GDF15)(24). The other study examined expression profiles of MM-associated bone cells in relation to osteolytic disease. Using microarray analysis, the report examined MM-MSCs and MM-osteoblasts (OB) in osteolytic and nonosteolytic MM patient samples and healthy donors. ND-MSCs and MM-MSCs displayed distinct transcriptional patterns in 78 genes. ND-OBs vs MM-OB samples had 29 specifically modulated genes. Many of the HOXB genes were highly expressed in both MSCs and OBs of myeloma patients, although the significance of this finding remains elusive(37). MSCs from osteolytic vs non-osteolytic patients also displayed different expression of 45 genes, but no difference in OB gene expression was found between these groups(37). However, whole-genome array-comparative genomic hybridization analysis found no chromosomal abnormalities in MM-MSCs or MM-OBs(37).
In contrast, others have reported genomic imbalances in MM-MSCs absent in ND-MSCs(22). They note, however, that these were more evident in passage 3 (P3) MSCs compared to P0 MSCs, suggesting this may be due to MSC adaptation to culture conditions and/or clonal selection for abnormal MM-MSCs(22). Similarly, studies examining chromosomal differences in MDS (myodysplastic syndromes)-MSCs and normal MSCs report severe chromosomal alterations in MDS-MSCs(58). MDS-MSCs also secrete more IL-1β and after treatment with TNFα, secrete more SCF compared to their normal counterparts(58). Of note, ND-MSCs and MDS-MSCs had no difference in adhesion molecule or ECM protein expression or in differentiation ability, suggesting that dramatic chromosomal abnormalities may not cause drastic changes in function. Hence, although disputed, the results suggest potential MM-MSC genomic alterations.
Lastly, in all studies that positively identify chromosomal differences in MM- vs ND-MSCs, the specific abnormalities of MM-MSCs were unique from the mutations/abnormalities observed in patient-matched myeloma cells, implying the absence of a common progenitor cell for MM-MSCs and MM cells(24; 25). Rather, the data suggests a coevolution of genomic alterations in juxtatumoral MSCs during tumorigenesis in response to the same carcinogens or mutagens responsible for plasma cell transformation(22). In summary, though chromosomal aberrations may be present between normal and MM-MSCs, they do not explain most of the functional and gene expression differences observed.
4 Disease models for reproduction of the MM-MSC phenotype
Understanding how MM-MSCs evolve from healthy ND-MSCs requires in vitro experiments and models of ND-MSCs co-cultured with MM cells. With those tools, we can determine parameters that initiate this phenotypic switch that may include: A) time periods of co-culture, B) soluble or direct cell-cell contact requirements, C) other cell types, and D) mutagens, among other parameters. Whether the transition involves intermediate phenotypes or one dramatic switch and whether the phenotype change is an initiator of, or a downstream reaction to, genetic alterations or myeloma disease progression can be elucidated.
In one model, primary ND-MSCs were differentiated down the osteoblastic lineage and then cultured with MM cells for 7–10 days(21). In this model system, each cell types was grown on opposite sides of a 1 µm-pore membrane. While some osteoblast samples supported tumor growth, others inhibited it and it was determined that MM proliferative response to MSCs was based on traits within the MM cells themselves, not the MSCs(21).
Co-culture of ND-MSCs with MM cells can also induce changes in MSCs. MM cells can induce VEGF and IL-6 upregulation and bFGF (basic fibroblast growth factor) downregulation in MSCs, giving these a similar phenotype to patient derived-MM-MSCs(59). Affymetrix microarray analysis of MM and MSC mRNA after 18 hours of co-culture revealed rapid induction of gene expression changes in both cell types, but results have not been peer-reviewed(60). Still, the work suggests that 2-D culture of MSCs with MM cells may be able to produce MM-MSC like cells very rapidly. Advantages of using induced MM-MSCs rather than patient-derived MM-MSCs include reproducibility, controllability, greater cell numbers and proliferation rates, the ability to analyze development of the MM-MSC outside the body, and better controls (ie. the same MSC population cultured without MM cells).
Although long recognized in the field of tissue engineering, the importance of using 3-dimensional (3-D) rather than 2-dimensional (2-D) models to elucidate biologically relevant interactions has only recently been recognized in MSC-myeloma cell interaction modeling. Cytokine production was compared in 2-D and 3-D cultures of MM-MSCs in response to RPMI8226 myeloma cells using a gelatin sponge and demonstrated that MSCs in 3-D culture produce more IL-11 and HGF and less IL-10 than in the 2D cultures(61). Furthermore, MM cells responded with increased production of sIL-6R (soluble IL-6 receptor) after contact with MM-MSCs in 3-D compared to 2-D. Other researchers have also described models for 3-D cell culture, but the models lack mineralization and poorly mimic the strength, rigidity, or complexity of bone(62).
Several in vivo MM models have been described to study stromal cell-myeloma cell interactions. One model, the SCID-hu model, improves upon previous mouse models by humanizing the bone compartment by using xenograft human fetal bone chips implanted into CB-17 SCID mice. The model reproduces homing of myeloma cells to these and the subsequent bone-tumor cell interactions observed in myeloma(14). A drawback to this is that MSCs and osteoblasts in this bone are healthy and not necessarily representative of MM-MSCs or MM-OBs found in patients. Subsequent models have utilized 3-D poly-ε-caprolactone polymeric scaffolds seeded with MM-MSCs and MM cells, providing a more realistic microenvironment model(63).
5 Conclusion
MM-MSCs demonstrate a number of functional differences, many of which allow them to specifically support MM cells. Transient protein/mRNA-based differences and long-term chromosomal differences were identified between ND-MSCs and MM-MSCs, but the cause of these alterations remains largely unknown. More research is necessary understand the evolution of allelic imbalances and non-chromosomal-based differences between healthy and tumor-associated cells, which have been identified not only in myeloma, but in many other tumors as well(23; 64–66). Specifically, it would be beneficial to characterize and study MSCs from a variety of patients with well-defined clinical data. There is little to no data regarding MM-MSCs from high-risk, MGUS and smoldering myeloma patients. There is rarely any clinical follow-up or characterization of the MSCs from myeloma patients, and age- or sex-matched comparisons are rare. Hence, more data needs to be collected on the properties of MSCs from broader, well-documented populations of healthy and myeloma patient donors to more fully and accurately understand the evolution and interactions of myeloma and myeloma MSCs.
Statement of Translational Relevance.
Interactions between cancerous and non-cancerous cells are driving factors in tumor development and progression. This knowledge has led to the development of drugs specifically targeted against cancer-stroma interactions or stromal cells explicitly. For example, bisphosphonates target osteoclasts within bone, which are overly active in osteoclastic lesions due to the tumor-induced process termed the “vicious cycle”. However, most research in this field assumes cancer patient stromal cells are equivalent to those found in healthy individuals, and, though often this simplification allows for development of effective drugs, it is becoming clear that cancer-associated stromal cells are distinctly abnormal. This review details phenotypic abnormalities within bone marrow-derived mesenchymal stem cells (MSCs) associated with multiple myeloma cells and describes mechanisms for their evolution. The review details their abilities to support tumorigenesis, distinct from their normal counterpart MSCs, which suggests novel pathways to target for inhibition and provides a greater understanding of the disease.
Acknowledgements
Supported in part by NIH 1R01CA133799 and 1R01CA152607.
Footnotes
Contribution: M.R.R. researched and wrote the paper and created the figures; I.M.G. reviewed and edited. Both identified the theme of the review.
Conflict-of-interest disclosure: M.R.R. declares no competing financial interests. IMG received research funding from Millennium, BMS, Noxxon and is on the advisory board or consultant for Millennium, Celgene, Novartis, BMS, Noxxon, and Polyphor.
References
- 1.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer metastasis reviews. 2010 Jun;29(2):249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Research. 2010 Dec;70(24):10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KC, Ghobrial IM. Multiple Myeloma: Translational and Emerging Therapies. Informa Healthcare. 2007 [Google Scholar]
- 4.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. European journal of cancer (Oxford England: 1990) 2006 Jul;42(11):1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009 Oct 22;461(7267):1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008 Oct 18;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 7.Yang F-C, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell. 2008 Oct 31;135(3):437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Raaijmakers MHGP, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010 Apr 8;464(7290):852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nature reviews. Cancer. 2007 Aug;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 11.Urashima M, Chauhan D, Uchiyama H, Freeman GJ, Anderson KC. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood. 1995 Apr 1;85(7):1903–1912. [PubMed] [Google Scholar]
- 12.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem cells (Dayton Ohio) 2006 Apr;24(4):986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001 Sep 20;20(42):5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 14.Tassone P, Neri P, Carrasco DR, Burger R, Goldmacher VS, Fram R, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005 Jul 15;106(2):713–716. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nature Medicine. 2010 Apr;16(4):483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMillin DW, Delmore J, Negri J, Ooi M, Klippel S, Miduturu CV, et al. Microenvironmental influence on pre-clinical activity of polo-like kinase inhibition in multiple myeloma: implications for clinical translation. PloS one. 2011 Jan;6(7):e20226. doi: 10.1371/journal.pone.0020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006 May 15;107(10):4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. The Journal of Clinical Investigation. 2008 Feb;118(2):491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azab AK, Runnels JM, Pitsillides C, Moreau A-S, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009 Apr 30;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clinical orthopaedics and related research. 1983 Sep;(178):297–302. [PubMed] [Google Scholar]
- 21.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006 Feb 1;91(2):192–199. [PMC free article] [PubMed] [Google Scholar]
- 22.Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009 Aug 9;23(8):1515–1527. doi: 10.1038/leu.2009.65. [DOI] [PubMed] [Google Scholar]
- 23.Tuhkanen H, Anttila M, Kosma V-M, Heinonen S, Juhola M, Helisalmi S, et al. Frequent gene dosage alterations in stromal cells of epithelial ovarian carcinomas. International journal of cancer. Journal international du cancer. 2006 Sep 15;119(6):1345–1353. doi: 10.1002/ijc.21785. [DOI] [PubMed] [Google Scholar]
- 24.Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007 May;21(5):1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnulf B, Lecourt S, Soulier J, Ternaux B, Lacassagne M-N, Crinquette A, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia. 2007 Jan;21(1):158–163. doi: 10.1038/sj.leu.2404466. [DOI] [PubMed] [Google Scholar]
- 26.Dawson MR, Chae S-S, Jain RK, Duda DG. Direct evidence for lineage-dependent effects of bone marrow stromal cells on tumor progression. American journal of cancer research. 2011 Jan;1(2):144–154. [PMC free article] [PubMed] [Google Scholar]
- 27.Hao M, Zhang L, An G, Meng H, Han Y, Xie Z, et al. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leukemia & lymphoma. 2011 May 3; doi: 10.3109/10428194.2011.576791. [DOI] [PubMed] [Google Scholar]
- 28.Zhu B-D, Ren J, Wang X-Y, Li X, Nie J. [Biological properties of mesenchymal stem cells derived from bone marrow of patients with multiple myeloma] Zhongguo shi yan xue ye xue za zhi / Zhongguo bing li sheng li xue hui = Journal of experimental hematology / Chinese Association of Pathophysiology. 2006 Dec;14(6):1138–1142. [PubMed] [Google Scholar]
- 29.Zdzisińska B, Bojarska-Junak A, Dmoszyńska A, Kandefer-Szerszeń M. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Archivum immunologiae et therapiae experimentalis. 2008;56(3):207–221. doi: 10.1007/s00005-008-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lokhorst HM, Lamme T, de Smet M, Klein S, de Weger RA, van Oers R, et al. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures. Blood. 1994 Oct 1;84(7):2269–2277. [PubMed] [Google Scholar]
- 31.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996 Feb 1;87(3):1104–1112. [PubMed] [Google Scholar]
- 32.Chatterjee M, Hönemann D, Lentzsch S, Bommert K, Sers C, Herrmann P, et al. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood. 2002 Nov 1;100(9):3311–3318. doi: 10.1182/blood-2002-01-0102. [DOI] [PubMed] [Google Scholar]
- 33.Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001 Apr 1;91(7):1219–1230. doi: 10.1002/1097-0142(20010401)91:7<1219::aid-cncr1122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Fu J, Chen P, Zhuang W. Impairment in immunomodulatory function of mesenchymal stem cells from multiple myeloma patients. Archives of medical research. 2010 Nov;41(8):623–633. doi: 10.1016/j.arcmed.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Jaramillo G, Vela-Ojeda J, Flores-Guzmán P, Mayani H. In vitro growth of hematopoietic progenitors and stromal bone marrow cells from patients with multiple myeloma. Leukemia research. 2011 Feb;35(2):250–255. doi: 10.1016/j.leukres.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Zdzisińska B, Walter-Croneck A, Dmoszyńska A, Kandefer-Szerszeń M. Matrix metalloproteinase and cytokine production by bone marrow adherent cells from multiple myeloma patients. Archivum immunologiae et therapiae experimentalis. 2006;54(4):289–296. doi: 10.1007/s00005-006-0033-z. [DOI] [PubMed] [Google Scholar]
- 37.Todoerti K, Lisignoli G, Storti P, Agnelli L, Novara F, Manferdini C, et al. Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Experimental hematology. 2010 Feb;38(2):141–153. doi: 10.1016/j.exphem.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Betts BC, St Angelo ET, Kennedy M, Young JW. Anti-IL-6 receptor alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive T-cell responses. Blood. 2011 Sep 22; doi: 10.1182/blood-2011-06-363390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markovina S, Callander NS, O’Connor SL, Xu G, Shi Y, Leith CP, et al. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Molecular cancer. 2010 Jan;9:176. doi: 10.1186/1476-4598-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y, Wen J, Mike P, Choi DS, Eshoa C, Shi Z-Z, et al. Bone marrow stromal cells from myeloma patients support the growth of myeloma stem cells. Stem cells and development. 2010 Sep;19(9):1289–1296. doi: 10.1089/scd.2010.0010. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zhang Z, Yao C. Survivin is upregulated in myeloma cell lines cocultured with mesenchymal stem cells. Leukemia research. 2010 Oct;34(10):1325–1329. doi: 10.1016/j.leukres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Gregoretti MG, Gottardi D, Ghia P, Bergui L, Merico F, Marchisio PC, et al. Characterization of bone marrow stromal cells from multiple myeloma. Leukemia research. 1994 Sep;18(9):675–682. doi: 10.1016/0145-2126(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 43.Tancred TM, Belch AR, Reiman T, Pilarski LM, Kirshner J. Altered expression of fibronectin and collagens I and IV in multiple myeloma and monoclonal gammopathy of undetermined significance. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2009 Mar 1;57(3):239–247. doi: 10.1369/jhc.2008.952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999 Mar 1;93(5):1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 45.Noborio-Hatano K, Kikuchi J, Takatoku M, Shimizu R, Wada T, Ueda M, et al. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene. 2009 Jan 15;28(2):231–242. doi: 10.1038/onc.2008.385. [DOI] [PubMed] [Google Scholar]
- 46.Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene. 2003 Apr 24;22(16):2417–2421. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]
- 47.Calabro A, Oken MM, Hascall VC, Masellis AM. Characterization of hyaluronan synthase expression and hyaluronan synthesis in bone marrow mesenchymal progenitor cells: predominant expression of HAS1 mRNA and up-regulated hyaluronan synthesis in bone marrow cells derived from multiple myeloma patients. Blood. 2002 Oct 1;100(7):2578–2585. doi: 10.1182/blood-2002-01-0030. [DOI] [PubMed] [Google Scholar]
- 48.Vincent T, Molina L, Espert L, Mechti N. Hyaluronan, a major non-protein glycosaminoglycan component of the extracellular matrix in human bone marrow, mediates dexamethasone resistance in multiple myeloma. British journal of haematology. 2003 Apr;121(2):259–269. doi: 10.1046/j.1365-2141.2003.04282.x. [DOI] [PubMed] [Google Scholar]
- 49.Tajima K, Ohashi R, Sekido Y, Hida T, Nara T, Hashimoto M, et al. Osteopontin-mediated enhanced hyaluronan binding induces multidrug resistance in mesothelioma cells. Oncogene. 2010 Apr 1;29(13):1941–1951. doi: 10.1038/onc.2009.478. [DOI] [PubMed] [Google Scholar]
- 50.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993 Dec 15;82(12):3712–3720. [PubMed] [Google Scholar]
- 51.Feng Y, Ofek G, Choi DS, Wen J, Hu J, Zhao H, et al. Unique biomechanical interactions between myeloma cells and bone marrow stroma cells. Progress in biophysics and molecular biology. 2010 Sep;103(1):148–156. doi: 10.1016/j.pbiomolbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Garderet L, Mazurier C, Chapel A, Ernou I, Boutin L, Holy X, et al. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leukemia & lymphoma. 2007 Oct;48(10):2032–2041. doi: 10.1080/10428190701593644. [DOI] [PubMed] [Google Scholar]
- 53.Li B, Shi M, Li J, Zhang H, Chen B, Chen L, et al. Elevated tumor necrosis factor-alpha suppresses TAZ expression and impairs osteogenic potential of Flk-1+ mesenchymal stem cells in patients with multiple myeloma. Stem cells and development. 2007 Dec 25;16(6):921–930. doi: 10.1089/scd.2007.0074. [DOI] [PubMed] [Google Scholar]
- 54.Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Barlogie B, et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009 Aug 27;114(9):1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. The Journal of clinical investigation. 2011 Jul 1; doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuhler GM, Baanstra M, Chesik D, Somasundaram R, Seckinger A, Hose D, et al. Bone marrow stromal cell interaction reduces syndecan-1 expression and induces kinomic changes in myeloma cells. Experimental cell research. 2010 Jul 1;316(11):1816–1828. doi: 10.1016/j.yexcr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem cells(Dayton Ohio) 2007 Feb;25(2):520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 58.Flores-Figueroa E, Montesinos JJ, Flores-Guzmán P, Gutiérrez-Espíndola G, Arana-Trejo RM, Castillo-Medina S, et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leukemia research. 2008 Sep;32(9):1407–1416. doi: 10.1016/j.leukres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001 Dec;15(12):1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 60.Herman D, Williams DR, Freeman J, Shaughnessy J, Barlogie B, Epstein J. 2738 Myeloma Cell Interaction with Mesenchymal Stem Cells: Heterogeneity of Myeloma Cell Survival and Gene Expression Studies; Conference Poster Session: Myeloma - Biology and Pathophysiology, Excluding Therapy Poster II, 6:00 PM–8:00 PM Hall A (Moscone Center) Poster Board II-832; San Francisco, CA. USA: 2008. [Google Scholar]
- 61.Zdzisińska B, Roliński J, Piersiak T, Kandefer-Szerszeń M. A comparison of cytokine production in 2-dimensional and 3-dimensional cultures of bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2009 Jan;47(1):69–74. doi: 10.2478/v10042-009-0015-1. [DOI] [PubMed] [Google Scholar]
- 62.Kirshner J, Thulien KJ, Martin LD, Debes Marun C, Reiman T, Belch AR, et al. A unique three-dimensional model for evaluating the impact of therapy on multiple myeloma. Blood. 2008 Oct 1;112(7):2935–2945. doi: 10.1182/blood-2008-02-142430. [DOI] [PubMed] [Google Scholar]
- 63.Calimeri T, Battista E, Conforti F, Neri P, Di Martino MT, Rossi M, et al. A unique three-dimensional SCID-polymeric scaffold (SCID-synth-hu) model for in vivo expansion of human primary multiple myeloma cells. Leukemia. 2011 Apr;25(4):707–711. doi: 10.1038/leu.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005 Dec 16;123(6):1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Weber F, Xu Y, Zhang L, Patocs A, Shen L, Platzer P, et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA: the journal of the American Medical Association. 2007 Jan 10;297(2):187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- 66.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer research. 2000 May 1;60(9):2562–2566. [PubMed] [Google Scholar]