1. Structure
The human and mouse genes for lens fiber major intrinsic protein (MIP) reside on chromosome 12q13 (MIP; Gene ID: 4284) and chromosome 10 (Mip; Gene ID: 17339), respectively. Each gene comprises four exons encoding a hydrophobic, transmembrane protein of 263-amino-acids (Mr ~28-kDa). MIP is a member of the aquaporin (AQP) family of water channels (pfam00230) and also referred to as aquaporin-0 (AQP0) (reviewed by Chepelinsky 2008).
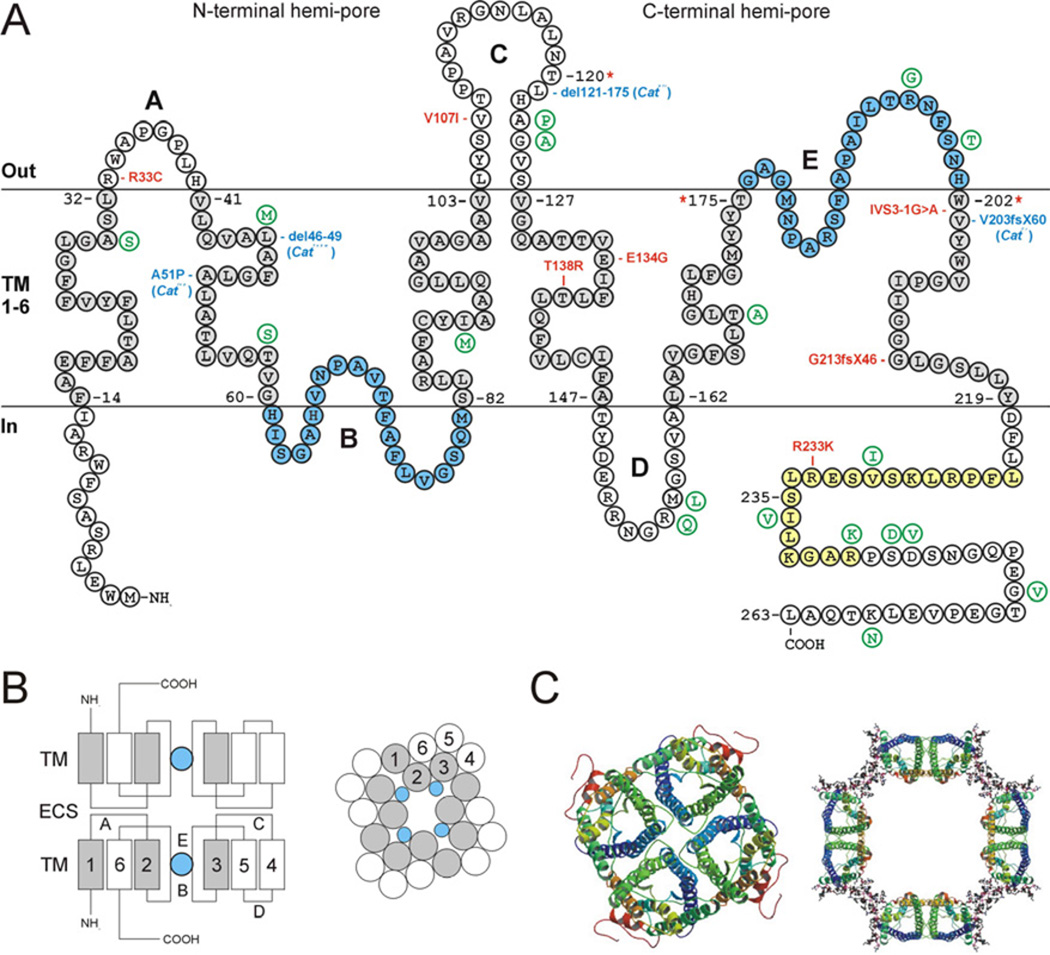
The first cloning and sequence analysis of a bovine MIP cDNA in 1984 predicted a monomeric protein topology with six plasma membrane-spanning α-helices (Fig. 1). The subsequent discovery that MIP shared homology with aquaporins led to the “hourglass” structural model which reflected a key gene-duplication event in the aquaporin gene family. MIP monomers were found to contain an internal structural symmetry of two tandem repeats, each containing an amino- and carboxy-terminal “hemi-pore” of three transmembrane-helices and a hydrophobic-loop (loops B and E) with a highly conserved Asn-Pro-Ala (NPA) motif that fold into the membrane to form a functional water pore. X-ray and electron crystallography techniques have largely substantiated the hourglass structure of MIP monomers, and further confirmed that MIP forms homo-tetramers, consistent with the square-arrays observed by freeze-fracture electron microscopy of fiber cell membranes in the lens core or nucleus. In addition to single-layered 2D crystals of tetramers, MIP purified from the lens core can form double-layered 2D crystals of MIP octamers (i.e. in register MIP tetramer-MIP tetramer junctions) with similar dimensions to the prominent “thin-junctions” (11–14 nm) observed by thin-section transmission electron microscopy of lens fiber cell membranes in situ. Conversion of “non-junctional” MIP tetramers to “junctional” MIP octamers has been associated with proteolytic truncation of the cytoplasmic amino- and carboxy- termini, and subsequent stabilization by proline-mediated hydrophobic interactions between the extracellular loops (A and C) of apposed tetramers (Fig. 1). However, immuno-electron microscopy of thin-junctions in situ reveals mostly out-of-register domains of MIP in apposing fiber cell membranes (i.e. MIP tetramer-membrane lipid junctions). Recently, atomic force microscopy (AFM) of native lens membranes has further revealed that MIP is assembled into junctional microdomains consisting of full-length and truncated MIP tetramers edged by gap-junction connexons (Mangenot et al. 2009).
Fig. 1.
Structure of Mip/Aqp0. A. Schematic of mouse Mip secondary structure (single letter amino acid code), showing six transmembrane domains (TM 1–6, shaded grey), three extracellular loops (A, C, and E), two intracellular loops (B and D), and cytoplasmic amino- and carboxy- termini. The aquaporin NPA-motifs (loops B and E) that line the water channel are shaded blue. The CaM-binding domain is shaded yellow and includes a conserved phosphorylation site at serine-235. Amino acid residues at the boundaries of exons 1–3 are numbered with an asterisk. Variations in the human MIP sequence are shown in green. The relative locations of four mouse mutations (blue) and seven human mutations (red) are indicated. The CatTohm mutation causes an in-frame 12-bp deletion in exon-1, predicted to result in the loss of four amino-acids (del46LAFG49) in the second transmembrane domain. The Cathfi mutation deletes 76-bp of exon-2 including the donor (5´) splice-site causing skipping of exon-2 and resulting in a truncated protein lacking amino-acids 121–175, which constitute the fourth and fifth transmembrane domains and cytoplasmic loop D. The CatFr mutation substitutes an ETn LTR sequence specifying 59 novel amino-acids for the C-terminal 61 amino acids normally encoded by exon-4. The resulting Mip-LTR fusion protein lacks most of the sixth transmembrane domain and the entire C-terminal domain of native Mip. B. Schematic of junctional Mip showing side-view (left) of apposed monomers docking through loops A and C with water pores (blue) in-register, and surface-view (right) of an Mip tetramer. ECS – extracellular space. TM – transmembrane domains (1–6). The N-terminal hemi-pore (TM 1–3) is shaded grey. For a model of Mip junctions in lens fiber cells see Grey et al. 2009. Differentiation 77, 70–83. C. Crystal structure of Mip in the open-pore tetramer state (left), and closed-pore octamer state (right). Transmembrane domains are shown as colored helices. The cytoplasmic-tail is colored red. Membrane lipid molecules are represented by ball-and-stick regions (right). Reproduced with permission from Gonen et al., 2005. Nature 438, 633–638.
2. Function
Proteomic analysis has indicated that MIP constitutes about 40% of the plasma membrane proteins in lens fiber cells. Based largely on electron microscopy observations, MIP was originally thought to function as a gap-junction protein. However in 1995, physiological studies using the classical Xenopus oocyte expression system revealed, for the first time, that MIP functioned as a modest water channel (>10-fold less active than AQP1). Accordingly, MIP was designated AQP0. Despite its relatively low conductivity, subsequent water permeability studies comparing lens membrane vesicles derived from wild type and Mip-deficient mice, confirmed that Mip accounts for about 80% of water transport across lens cortical fiber cell membranes. MIP water channel activity in lens vesicles is insensitive to Hg2+-inhibition; however, it is increased at low pH (6.5), or elevated Ca2+ ion concentration, in a calmodulin-dependent manner. NMR-spectroscopy has revealed that two calmodulin (CaM) molecules bind a single MIP tetramer in a non-canonical manner to block channel permeability, and that phosphorylation at a conserved site (Ser-235) within the CaM-binding domain (Fig 1.) inhibits this interaction. These observations suggest that co-operative asssembly of MIP monomers is important for regulation of MIP function (Reichow et al. 2008).
The number of water molecules filling the channel in the MIP crystals, reportedly ranges from 0–3 molecules in “closed” (junctional) octamers to 7–8 molecules in “open” (non-junctional) monomers and tetramers. However, recent molecular dynamic simulations indicate that MIP monomers, tetramers, and octamers transport water at similar rates, with an average channel occupancy of about five non-continuous water molecules (Jensen et al. 2008). These calculations suggest that MIP has evolved a dual function, simultaneously facilitating water transport and thin-junction formation. Water transport may contribute to fluid micro-circulation throughout the avascular lens, whereas, thin-junctions are believed to collapse the extracellular space between fiber cells thereby minimizing light-scattering by the lens.
Beyond self-interactions, MIP is believed to interact through its C-terminus with other lens proteins including, connexin 45.6, the beaded-filament structural proteins filensin and phakinin, and several crystallins, raising the possibility of additional lens-specific functions. Moreover, MIP expression has been detected at trace levels in other tissues, including retinal bipolar cells where it has been implicated in the maintenance of synaptic junctions.
3. Disease Involvement
In 1996, the first genetic link between MIP and disease was reported in two historically important mouse mutants that inherit spontaneous forms of cataract. The shriveled (Svl) or Fraser (CatFr) mutant was found to harbor an embryo transposon (ETn) insertion mutation in intron-3 of the Mip gene that results in mis-splicing of exon-3 to a long terminal repeat (LTR) sequence (Fig. 1). By contrast the lens opacity (Catlop) mutant inherits a missense mutation in exon-1 that results in the non-conservative substitution of proline for a conserved alanine at codon 51 (p.A51P) predicted to lie in the second transmembrane domain (Fig. 1). Subsequently, deletion mutations in the mouse MIP gene were found to underlie radiation-induced cataract in the hydropic fibers (Cathfi) mutant, and spontaneous cataract in the Tohoku (CatTohm) mutant (Fig 1). The resulting cataract phenotypes are described as semi-dominant primarily affecting the lens nucleus and inner cortex with increased severity in homozygotes associated with small eyes (microphthalmia) and lenses (microphakia). Similarly, mice totally lacking Mip develop small eyes and lenses with severe congenital, central opacities. Hemizygous loss of Mip function results in optically disturbed lenses with reduced focusing power, and is sufficient to trigger adult-onset (~6 months) central polymorphic opacities consistent with a dosage-dependent “refractive” function in the lens. The fact that both deleterious gain- and loss- of function mutations have been reported for MIP may in part reflect its proposed dual function as both a water-channel and a cell-junction structural component in the lens.
Genetic studies in humans have so far identified at least seven mutations in the MIP gene associated with autosomal dominant forms of cataract (Fig. 1). Five of these are predicted to result in missense amino acid substitutions, one in a reading frame-shift in exon-4 causing premature termination of translation, and one splice-site mutation resulting in mis-splicing of exon-4 (Fig. 1). The resulting lens opacities are clinically heterogeneous, and variously described as nuclear, lamellar, sutural, punctate, polymorphic and total. Functional expression and sub-cellular immuno-localization studies of mutant forms of MIP indicate that loss of plasma-membrane water channel activity is associated with intracellular accumulation of the mutant protein in endoplasmic reticulum (ER)-like membranes. These observations suggest that membrane targeting defects are a common trigger for MIP-related cataract and lens fiber cell pathology in humans and mice.
4. Future studies
Despite recent progress in elucidating the structure, function and pathology of MIP several questions require further investigation. First, the functional relationship between MIP-mediated water transport and junction formation in lens homeostasis remains unclear. There is general agreement that full-length MIP can form monomers and tetramers of open water channels, particularly in the lens cortex. However within the lens core, there is conflicting evidence for junctional-octamers composed of closed (truncated) water channels versus junctional-tetramers composed of open water channels. Second, the pathogenetic mechanisms underlying MIP-related cataract are poorly understood. Both gain-of-function and loss-of-function mutations in the human and mouse genes for MIP result in lens opacities. However, the causative relationships between impaired water-channel activity, impaired junction-formation, impaired membrane-targeting and cataract formation have yet to be determined. Third, MIP is believed to interact with several other lens-abundant proteins, however, the importance of these protein-protein interactions in defining the highly specialized architecture of the lens fiber cell membrane is largely unknown. Finally, the detection of MIP expression in other cell-types raises the likelihood of additional functions and phenotypes associated with this iconic lens membrane protein.
Acknowledgements
Supported by NIH/NEI grant EY012284.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chepelinsky AB. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb. Exp. Pharmacol. 2009;190:265–297. doi: 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- Jensen MØ, Dror RO, Xu H, Borhani DW, Arkin IT, Eastwood MP, Shaw DE. Dynamic control of slow water transport by aquaporin 0: implications for hydration and junction stability in the eye lens. Proc Natl Acad Sci USA. 2008;105:14430–14435. doi: 10.1073/pnas.0802401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangenot S, Buzhynskyy N, Girmens JF, Scheuring S. Malformation of junctional microdomains in cataract lens membranes from a type II diabetes patient. Pflugers Arch. 2009;457:1265–1274. doi: 10.1007/s00424-008-0604-4. [DOI] [PubMed] [Google Scholar]
- Reichow SL, Gonen T. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure. 2008;16:1389–1398. doi: 10.1016/j.str.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]