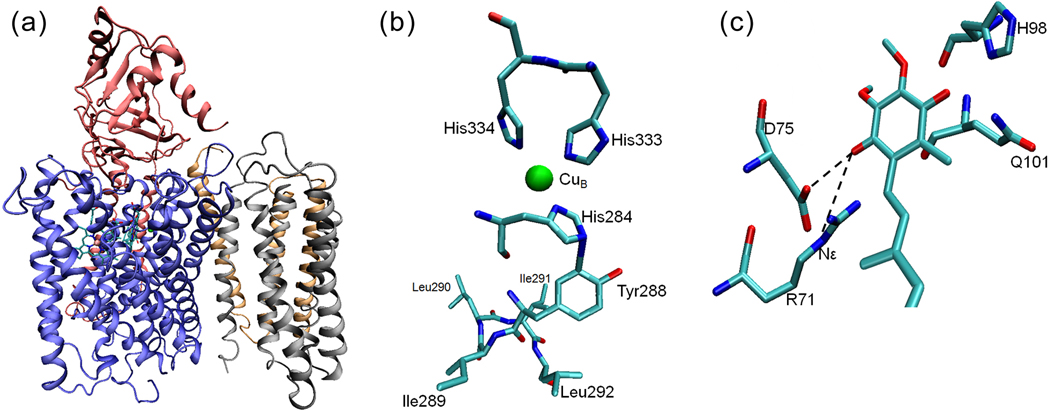
Figure 5.
The X-ray crystal structure of cytochrome bo3 from E. coli[18]. (a) A cartoon representation of the four subunits of cyt bo3 with heme b, heme o3 and CuB. (b) The residues near the CuB site. His333, His334 and His284 are ligands to CuB. The proposed cross-link between His284 and Tyr288 is immediately followed by a unique quartet of ILIL. All residues shown are from subunit I. (c) A cartoon model of the high-affinity ubiquinone binding site (QH) of the wide-type cyt bo3 showing the strong H-bonds between the ubisemiquinone and the Nε of R71 as well as the side-chain carboxylic group of D75.