Abstract
Studies in methamphetamine (METH) abusers showed that the decreases in brain dopamine (DA) function might recover with protracted detoxification. However, the extent to which striatal DA function in METH predicts recovery has not been evaluated. Here we assessed whether striatal DA activity in METH abusers is associated with clinical outcomes. Brain DA D2 receptor (D2R) availability was measured with PET and [11C]raclopride in sixteen METH abusers both after placebo and after challenge with 60 mg oral methylphenidate (to measure DA release) to assess if it predicted clinical outcomes. For this purpose METH abusers were tested within 6 months of last METH use and then followed up for 9 months of abstinence. In parallel, 15 healthy controls were tested. METH abusers had lower D2R availability in caudate than controls. Both METH abusers and controls showed decreased striatal D2R availability after MPH and these decreases were smaller in METH than in controls in left putamen. The 6 METH abusers who relapsed during the follow up period had lower D2R availability in dorsal striatum than controls and had no D2R changes after MPH challenge. The 10 METH abusers who completed detoxification did not differ from controls neither in striatal D2R availability nor in MPH-induced striatal dopamine changes. These results provide preliminary evidence that low striatal DA function in METH abusers is associated with a greater likelihood of relapse during treatment. Detection of the extent of DA dysfunction may be helpful in predicting therapeutic outcomes.
Keywords: methamphetamine, dopamine release, positron emission tomography, relapse, early withdrawal
Introduction
Several preclinical studies have been reported on the deleterious effects of methamphetamine (METH) to dopamine (DA) cells. These studies have shown that METH can produce long-lasting changes on DA cell markers1,2 including decreases in brain DA content, tyrosine hydroxylase activity, DA uptake and DA transporter (DAT) levels.3,4
METH-induced changes in these DA markers have been associated with motor and learning deficits in rodents indicating that they are functionally significant.5,6 These DA effects have been interpreted as evidence of neurotoxicity, which is corroborated by studies that show METH-induced DA nerve terminal degeneration and reactive gliosis in striatum.7,8 More recent human imaging studies revealed correlation of memory deficits and decrement of DAT binding potential9 as well as reduced striatal DA D2 receptor (D2R) and impulsivity in METH abusers.10 Though it is generally believed that the effects of METH are limited to the terminals while sparing the soma, DA cell death has also been reported.11 Non-human primate studies report that the DA changes induced by METH are long lasting and present at least 6 months after drug administration.12 However, more recent work has documented significant recovery from METH-induced DA terminal changes. Studies investigating the recovery of DA brain neurotransmission after METH have shown results that vary from complete recovery to persistent long lasting changes. In rats, a marked reduction in baseline DA levels and amphetamine induced increases in DA release were observed one week after METH administration with recovery to normal values 12 months post treatment.13 Significant, though not complete, recovery in DA function after METH administration was reported in a rodent study that showed marked depletions in DA (30% of controls) one week after METH that recovered 7 months post treatment (80% of controls).14 Similarly, imaging studies in non-human primates have shown significant recovery of DAT in animals exposed to METH 12–15 month post treatment.15 Our human positron emission tomography (PET) study showed increased DAT after protracted (12–17 months) abstinence.16 However, one non-human primate study documented striatal DA reductions still evident after 4 years post-METH treatment.2
Both preclinical and clinical data provide evidence that METH is toxic to DA cells but that some of the damage may recover with detoxification. However, all of the clinical studies in METH users are based on findings showing decreases structural markers of DA terminals (transporters). In this study we used PET and [11C]raclopride to evaluate changes in extracellular DA in the striatum, which allowed direct assessment of the function of DA cells. PET with [11C]raclopridehas been used to study the involvement of DA in drug reward and addiction in the human brain.17 This includes studies that investigate the relationships between the ability of various drugs to modulate DA and their reinforcing effects, both in non-dependent and drug dependent individuals.18–20 Using [11C]raclopride, these studies have shown that stimulant drugs (i.e. methylphenidate, amphetamine) increase DA in striatum in the human brain and that these responses are attenuated in drug-addicted subjects (review21). In this study PET imaging was performed after placebo and after oral methylphenidate (MPH, 60 mg). The METH abusers were initially tested within 6 months of last METH use and then followed for 9 months to assess if striatal DA function in METH predicted abstinence at the end of this time period.
Methods
Subjects
The Institutional Review Boards at the Stony Brook University/Brookhaven National Laboratory, at Harbor-University of California – Los Angles (HUCLA) Medical Center and at the Portland Veterans Administration Medical Center (PVAMC) approved the protocol. Written informed consent was obtained after the experimental procedure was explained to the subjects. METH abusers (3 female and 13 male, 39.2±4.9 year old) were recruited from HUCLA and PVAMC. METH was the subjects’ primary drug of abuse via the smoked or intravenous routes with a use frequency of at least 5 times/week and 0.5 g/day. Subjects that met a minimum 12-month history of METH abuse, DSM IV diagnosis for METH dependence, and a minimum 2-week period of detoxification and desired to stop using METH were recruited for the study. The control group (2 female and 13 male, 37.2±4.3 year old) was chosen to match the age range of the METH group. Exclusion criteria for both groups were: Present or past history of neurological or DSM IV Axis I psychiatric disease (apart from METH dependence), past or present history of affective disorder or ADD/ADHD, abuse or dependence of drugs other than METH (excluding nicotine), medical illness that can affect brain function, past or present history of cardiovascular disease or high blood pressure, scores on the Hamilton Depression Scale > 15, which may indicate clinical depression. Urine screening tests for psychoactive drugs were performed to corroborate lack of drug use.
Study design
Each subject was tested with two [11C]raclopride scans [once after placebo and once after 60 mg of oral methylphenidate (MPH), which was be given an hour prior to [11C]raclopride] to measure D2R availability and DA cell function. To avoid the carry over effects of MPH on subsequent scans, the [11C]raclopride PET scan was done first after placebo administration, and two hours later after MPH administration. Blood samples were obtained to measure plasma MPH concentration prior to and at 30 and 60 min after MPH. The plasma concentration of MPH was analyzed at Dr. Thomas Cooper's laboratory (Nathan Kline Institute, New York). In addition, cardiovascular response (continuously recording vital signs and EKG) and self-report rating of drug effects (high, anxiety, restlessness, alertness, drug effect) to pharmacological doses of MPH were obtained prior to and at every 5 minutes after placebo and MPH administration.
After the scan visits, the METH abusers were followed up in outpatient treatment facilities with random urine screening tests for psychoactive drugs for the 9 months that followed their PET scan study. All enrollees attended the same substance abuse day treatment program weekly. The program consists of an intensive outpatient drug rehabilitation program that includes group and individual therapy and educational group sessions. The program includes early recovery skills and relapse prevention techniques. Family counseling is also available. Anger management and domestic violence awareness and prevention programs are also available at the center for up to 52 weekly sessions. The subjects received behavioral and psychological treatments. The subjects did not receive any medical treatments during the rehabilitation process.
PET Scanning
The PET scans were carried out on an HR+ Siemens tomograph using the 3-D acquisition mode. An external chinstrap device was used in addition to the individual headholder to minimize head motion during the scan. After a transmission image obtained for attenuation correction, scans were started immediately after injection of 5–10 mCi of [11C]raclopride and were carried out for a total of 60 minutes. The following scanning sequences were used: 15 × 1 min.; 3 × 5 min.; 2 × 15 min. Each study included a measurement of the plasma clearance of total radioactivity and the measurement of the fraction of labeled tracer in plasma at selected times.
Image Analysis
Both manual drawn preselected regions of interest (ROIs) and Statistical Parametric Mapping (SPM) analyses were used. The ROI in dorsal striatum (caudate, putamen), ventral striatum and cerebellum were outlined by superimposing boundaries from a neuroanatomical atlas using a template22 Briefly, the regions of interest were initially outlined on the individual’s summed baseline [11C]raclopride image (images obtained between 15–60 min) and were then projected into the dynamic [11C]raclopride images to generate time–activity curves for the striatal regions (caudate, putamen and ventral striatum) and cerebellum. These time–activity curves for tissue concentration, along with the time–activity curves for unchanged tracer in plasma were used to calculate [11C]raclopride’s transfer constant from plasma to brain (K1) and the total tissue distribution volume (VT). VT corresponds to the equilibrium measurement of the ratio of tissue concentration to plasma concentration in striatum and cerebellum using a graphical analysis technique for reversible systems23 The ratio of VT in striatum to that of VT in cerebellum corresponds to BPND +1 where BPND is the in vivo binding potential (nondisplaceable). BPND is proportional to the number available binding sites Bavail/Kd. The response to drug administration (with placebo or with MPH) was quantified as the difference in Bmax/Kd with respect to the baseline, placebo condition.
Data Analysis
Differences in BPND between conditions were tested using a repeated measures ANOVA model with two conditions (placebo and MPH) and two groups (METH abuser and control) and post-hoc t-tests were then used to determine for which conditions the effects differed from the baseline condition. Plasma MPH concentration after oral administration were compared between the groups using Students’ t test. The effects of the MPH on the cardiovascular responses and behavioral self-reports were tested by comparing the scores obtained prior to the drug administration (placebo and MPH) and the averaged scores obtained between 70–100 min after oral administration using a repeated measures ANOVA. Pearson product moment correlations were used to assess the relationship between the MPH-induced changes in BPND and the behavioral effects of the MPH. Using these statistics, we also tested whether clinical indices as cigarette smoking, years of METH use, amount of METH use and days of last METH use affected placebo or MPH-induced DA release in the abusers and as a function of abstinence vs. relapse.
D2R image template
A spatial normalization template matching the average D2R image contrast in the brain was developed using VT images from 34 healthy control subjects. These parametric images were obtained from [11C]raclopride PET scans collected with the same scanning sequence used for the present study. All images were carefully inspected to ensure whole brain coverage and exclude images with potential artifacts. A 12-parameter affine transformation with 16 nonlinear iterations was used to register the images to the PD.mnc Montreal Neurological Institute (MNI) template provided with the SPM2 package. The dimensions of bounding box of the output volumes were 90:90, 126:90, and 72:108 along x, y, and z, respectively, and the voxel size was 2×2×2 mm3. Bilinear interpolation was used for reslicing. The average normalized and smoothed image (full with half maximum-FWHM = 8 mm) was computed to obtain the D2R image template in the MNI stereotactic space.
SPM analysis
Differences in D2R availability between MPH and placebo in the METH abusers and in the controls as well as between first visit and second visit were also tested on the voxel-level using the software package for SPM. Prior to the analysis, each subject’s VT image was converted to the Analyze format, mapped onto the MNI stereotactic space using the custom D2R image template and the 12-parameter affine transformation in SPM2 with a 2×2×2 mm3 voxel size, smoothed via a Gaussian kernel with FWHM of 8 mm, and rescaled to the VT in cerebellum (VT ratio images). The VT ratio images were included into a repeated measures ANOVA model with two conditions (placebo and MPH) and two groups (METH abuser and control) for the group analyses in SPM2. Corrections for multiple comparisons were performed via the random field theory, an approach that is less conservative than the traditional Bonferroni method. Imaging voxels that were significantly different (p ≤ 0.005, minimum cluster size = 100 voxels) between conditions were identified. The statistical significance of condition × group interactions effects in the striatum were corrected for multiple comparisons using small volume corrections with a 10-mm diameter spherical volume in SPM. Only regions with corrected p-values ≤ 0.05 at the cluster-level were considered significantly. The MNI coordinates of the significant clusters were transformed to the Talairach space using a best-fit transform that minimizes bias associated with reference frame and scaling.24 The brain regions were labeled according to the Talairach daemon25 and a query range of 5-mm. We further checked the labels of the hubs using the Automated Anatomical Labeling atlas26 and the Brodmann atlas, which is included in the MRIcro software.
Results
The METH abusers differed from the controls in that they had lesser years of education (12.7±1.6 vs. 14.3±8.1 years, p < 0.001) and there was a greater proportion of cigarette smokers (16 METH abusers vs. 4 controls, p < 0.001) and heavier cigarette smoking. Four METH abusers had moderate alcohol use and none of the controls use alcohol more than once per week. The METH abusers had a history of 13.1±7.2 years METH use and 1.2±1.0 grams per day.
After placebo administration, the 16 METH abusers had lower D2R availability in caudate (2.5±0.2) than controls (2.8±0.3, p < 0.04), Table 1. Both METH abusers and controls reported greater ratings of drug effect (+1.5±1.7, p < 0.003; +2.3±2.8, p < 0.007) and alertness (+2.0±2.0, p < 0.001; +1.2±1.7, p < 0.02) after MPH than after placebo administration but the magnitude of these effects did not differ between groups. The controls but not the METH abusers reported greater ratings of “high” (+1.6±2.5, p < 0.03) after MPH. Neither group reported increased anxiety or restlessness after MPH. MPH administration increased pulse rate (METH abusers: +28.5±28.1%, p < 0.001; control: 19.8±16.8%, p < 0.0004) and diastolic pressure (METH abusers: +6.3±10.7%, p < 0.03; subjects: +7.8±12.1%, p < 0.03) in both groups and increased systolic pressure in the METH abusers (+8.5±6.5%, p < 0.0001) but not in the controls (+3.8±9.2%, NS). However, the self-report ratings and cardiovascular changes after MPH were not significantly different between the groups. The plasma MPH concentrations at 30 and at 60 minutes were similar for the METH abusers (5.9±6.1, 19.1±6.2 ng/ml) and the controls (8±11.5, 21.8±8.9 ng/ml).
Table 1.
Comparison of DA D2 receptor availability of METH subjects during early withdrawal and control subjects during their first visit after oral placebo and after oral methylphenidate (60 mg) administration.
| METH users (n = 16) | Control subjects (n = 15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | MPH | Placebo | MPH | |||||||
| Regions | Mean | SD | Mean | SD | P value | Mean | SD | Mean | SD | P value |
| Caudate | 2.5 | 0.2 | 2.4 | 0.1 | 0.04 | 2.8* | 0.3 | 2.7 | 0.3 | 0.008 |
| Putamen | 3.1 | 0.2 | 3.0 | 0.3 | 0.01 | 3.4 | 0.5 | 3.1 | 0.4 | 0.001 |
| Ventral striatum | 2.5 | 0.5 | 2.4 | 0.4 | NS | 2.7 | 0.4 | 2.5 | 0.5 | 0.007 |
Control subjects had higher DA D2 receptor availability for caudate than METH users after oral placebo (p < 0.04). ANOVA analyses of the interaction among groups (METH users vs. control subjects), condition (placebo or MPH) and striatal regions were significant (p < 0.05) in the left putamen.
After MPH administration, both METH abusers (caudate: −4±7.3%, p < 0.04; putamen: −5.5±8.3%, p < 0.01; ventral striatum: −4.1±14%, p < 0.06) and controls had decreased D2R availability (caudate: −6.4±9.2%, p < 0.002; putamen: −10.5±10%, p < 0.001; ventral striatum: −9.5±13.5%, p < 0.007; Table 1) presumably reflecting DA increases. Comparison for groups’ interaction effects was only significant for MPH-induced DA in left putamen (p < 0.05). The MP-induced decreases in left putamen in METH abusers were significantly smaller than in controls. There was no association between placebo or MPH-induced DA release with clinical variables as cigarette smoking, years of METH use, amount of METH use and days of last METH use in the METH abusers. The plasma MPH levels were not associated with MPH-induced DA increases neither in METH abusers nor controls.
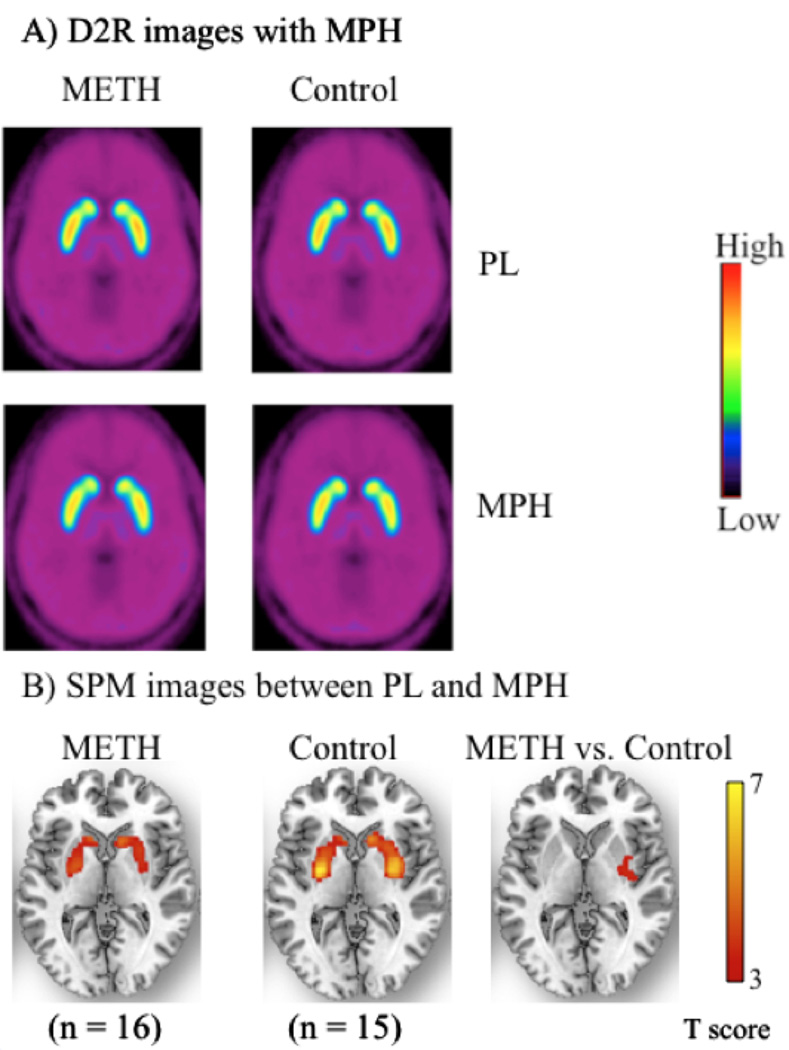
SPM analysis, using a correction for small volume and multiple comparisons (p < 0.005), showed significantly decreased D2R availability in striatum after MPH in both controls and METH abusers. The METH abusers had less decrement in putamen than controls [Coordinates in the MNI frame of reference: x = 38, y = −12, z = 6 mm; cluster volume = 482 voxels (2-mm isotropic); statistical significance: Z-score = 2.5; p < 0.036, FDR-corrected for multiple comparisons using a small volume correction (10-mm spherical volume), Figure 1]. There were no significant correlations between MPH-induced changes in BPND and the behavioral effects of MPH.
Figure 1.
A) Averaged DA D2 receptor availability (D2R) images with oral methylphenidate (MPH 60 mg) and placebo between MPH and placebo (PL) of 16 METH abusers and 15 control subjects. The D2R images are scaled with respect to the maximum Vd ratio value of the control subjects with oral PL. The images are presented by using the rainbow scale, where red represents highest value and violet represents lowest value. Binding of [11C]raclopride is lowest in the METH abusers with oral MPH. B) SPM images (p < 0.005, small volume correction, multiple comparison) are superimposed on a structural MR image. The images of differences between MPH and PL for the METH abusers and control subjects as well as the Δ (MPH – placebo) image between METH abusers and control subjects are presented by using yellow-red color, where yellow represents highest value and red represents lowest value.
Six METH abusers (2 female and 4 male) relapsed after the PET scan visit. Four of these subjects had positive drug screens during the course of the study and two of the subjects were lost to follow up but the center was given informal feedback that the subjects had relapsed. There were no demographic (age, education) or clinical differences (years of METH, alcohol, tobacco use, amount of METH, alcohol, tobacco use and days of last METH use) between the six METH abusers who relapsed and those who did not. There were also no differences in self-report ratings and cardiovascular response to MPH administration between those who relapsed and those who did not.
The six METH abusers who relapsed showed no D2R availability changes after MPH (caudate: −0.7±11%, NS; putamen: −0.5±10%, NS) whereas in 10 METH abusers who did not relapse MPH induced significant reduction in D2R availability (caudate: −6.6±5%, p < 0.002; putamen: −8.5±5.6%, p < 0.001, Table 2). Post hoc analysis showed that METH abusers who relapsed had significant lower D2R availability in caudate (p < 0.014) and putamen (p < 0.015) at baseline and less DA release after MPH in putamen (treatment × diagnosis, p < 0.037). The amount of METH used by the 6 relapsed subjects did not correlate with baseline D2R availability and not with MPH-induced DA changes. The 10 METH abusers who remained abstinent showed a trend for lower baseline D2R availability (p < 0.07) and less MPH-induced DA release (treatment × diagnosis, p < 0.07) in caudate than the controls.
Table 2.
Comparison of DA D2 receptor availability of METH subjects (n = 10) with protracted abstinence and METH users (n = 6) relapsed after scan visit after oral placebo and after oral methylphenidate (60 mg).
| METH subjects with protracted abstinence |
METH users relapsed after scan visit |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | MPH | Placebo | MPH | |||||||
| Regions | Mean | SD | Mean | SD | p value | Mean | SD | Mean | SD | P value |
| Caudate | 2.6 | 0.2 | 2.4 | 0.1 | 0.002 | 2.5 | 0.2 | 2.5 | 0.2 | NS |
| Putamen | 3.2 | 0.2 | 3.0 | 0.3 | 0.001 | 3.0 | 0.1 | 3.0 | 0.3 | NS |
| Ventral Striatum | 2.5 | 0.4 | 2.4 | 0.4 | NS | 2.5 | 0.4 | 2.3 | 0.4 | NS |
Discussion
The results show that METH abusers had lower baseline D2R availability (placebo condition) in the caudate during early withdrawal, which replicates our prior findings in a different population of METH abusers.27 Animal studies have implicated DA D2 receptors located on striatal cells post-synaptic to the DA terminals in METH-induced toxicity.28,29 Imaging studies have also shown that long-term METH abuse leads to extensive neuronal damage in the human brain, which is associated with persistent cognitive impairment.4
The subjects in this study received 60 mg MPH orally (0.75–1 mg/kg) and showed the expected increases in extracellular DA concentration secondary to MPH-induced DAT blockade. The DA increases were not correlated with self-ratings of high, which is consistent with our prior studies showing that MPH-induced DA increases are only associated with the “high” after intravenous but not after oral administration.22 The difference between the intravenous and oral administration of MPH with respect to their reinforcing effects most likely reflects the fact that the intravenous route leads to DA changes that are much faster than those observed after oral administration. Thus, the failure of oral MPH to induce a “high” is likely due to slower pharmacokinetics (slower brain uptake). This is because the speed with which drugs of abuse enter the brain is a key parameter that affects their reinforcing effects; the faster their brain uptake the more intense their reinforcing effects.30
The METH users showed lesser DA increases with MPH administration at early withdrawal than the controls. Since DA increases induced by MPH are dependent on DA release (a function of DA cell firing), it is likely that the difference may reflect decreased dopaminergic cell activity in the METH abusers. While evaluating the results of PET studies based on the competition of [11C]raclopride by endogenous DA, it is critical to remember that the results merely reflect the fraction of D2R that is vacant and thus capable of binding the tracer. As a consequence, any reduction in D2R measured with this technique could reflect either decreases in levels of D2R and/or increases in DA release (competing for binding with [11C]raclopride for the D2R availability) in striatum (including nucleus accumbens). The increases in DA release could include pre-existing DA that was present prior to MPH administration or a combination of pre-existing DA plus DA that is released following MPH administration. If the presynaptic neuron in a METH abuser releases smaller amounts of DA (but fires at the same rate) the same observation would be seen. Other potential reasons for MPH-induced reductions in [11C]raclopride binding were decreased DA affinity for the D2R or decreased efficacy of MPH to prevent DA reuptake. However, the fact that the METH abusers and other drug-addicted subjects (i.e. cocaine and alcohol) show blunted reductions in specific binding (indicative of decrease DA release) when administered MPH indicates that these individuals had both a reduction in the levels of D2R as well as a decrease in DA release in striatum.20,31 Each deficiency would contribute to the overall decreased sensitivity in drug-addicted subjects to natural reinforcers.
In this study the METH abusers who showed greater DA increases after MPH administration during early withdrawal stayed sober and returned for the follow up visit. In contrast the METH users who showed no DA increases after MPH during early withdrawal relapsed. A similar association was recently reported by a study in cocaine abusers that showed lower baseline striatal D2R availability and MPH-induced DA increases in cocaine abusers who relapsed versus those who did not.32 Moreover in a prior study these same investigators had shown a negative correlation between the DA increases induced by amphetamine administration and their choice of cocaine over money; the lower the DA increases the more likely they were to select cocaine over a monetary reinforcer33. Since the study also reported reduced DA increases in cocaine abusers when compared with controls, this could indicate that drug-addicted subjects with the most severe decreases in brain dopaminergic activity are the ones more likely to choose drugs over other reinforcers (such as money). Therefore, the failure of DA depleted METH users to stay sober could occur because these individuals have a greater deficit in their ability to shift their reward directed behavior to natural reinforcers. In this study we found the METH users who relapsed had a blunted DA response to oral MPH in the dorsal striatum (caudate and putamen). During the development of drug addiction, drug-seeking behavior progresses from seeking the rewarding effects of drugs to being triggered and maintained by drug-associated cues and stressful environments.34 Prolonged drug seeking in vulnerable individuals results in habitual compulsive drug using behavior35,36 and in increased DA release in dorsal striatum triggered by conditioned drug-cues.36 In human cocaine abusers increases in DA elicited by cocaine-cues in the dorsal striatum are associated with the degree of drug craving and addiction severity.37,38 The dorsal striatum is also a major site that appears to be involved in relapse after prolonged abstinence from cocaine self-administration.39 Greater deficits in dorsal striatal DA transmission in METH abusers might promote habitual drug use and a loss in the ability to stay sober.
Limitations for this study are the relatively small sample sizes and even though we found group differences between relapse and control groups, we cannot ascribe a direct relationship between D2R availability and the prediction of relapse. Prospective studies in a larger number of METH abusers are needed to determine the sensitivity of striatal DA measures in predicting relapse vs. abstinence. In this study we excluded METH abusers with any history of an axis I disorder other than METH dependence, which limits the generalization of our findings since co-morbidity in METH abusers is high. Also, most of the METH abusers were cigarette smokers and continued to smoke throughout the study, which might have confounded the comparisons with controls most of whom were non-smokers.
Conclusion
The results revealed lower baseline striatal D2R availability and DA release in METH abusers than controls, which replicates our prior studies. We also show that METH abusers with better DA function during the early withdrawal period were more likely to stay sober at follow up than those with low striatal DA function. This coupled with similar findings in cocaine abusers32 suggest that poor DA function may be a biomarker that predicts a greater likelihood for relapse and a greater addiction severity. Thus early detection of the extent of DA dysfunction may be helpful in predicting clinical outcomes.
Figure 2.
Averaged D2R images with MPH and placebo of 10 METH abusers with protracted abstinence and images of 6 METH abusers who relapse after the scan visit.
Acknowledgments
The PET study was carried out at Brookhaven National Laboratory. The recruitment and psychological screening were at University of California –Los Angels and Veteran hospital at Oregon. Hospital. We thank David Schlyer and Michael Schueller for cyclotron operations; Donald Warner, David Alexoff and Paul Vaska for PET operations; Richard Ferrieri, Colleen Shea, Youwen Xu, Lisa Muench and Payton King for radiotracer preparation and analysis, Karen Apelskog-Torres for study protocol preparation, and Barbara Hubbard and Pauline Carter for patient care.
Financial support and other disclosures: The PET study was carried out at Brookhaven National Laboratory with infrastructure support from the U. S. Department of Energy OBER (DE-ACO2-76CH00016) and under support in part by NIH: R01DA06891 (Dr. Wang), MO1RR10710 (the General Clinical Research Center of Stony Brook University) and Z01AA000550 (Dr. Volkow).
Footnotes
Disclosures
All the authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr. 1996;163:251–276. [PubMed] [Google Scholar]
- 2.Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- 3.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- 6.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol. 1991;194:71–76. doi: 10.1016/0014-2999(91)90125-a. [DOI] [PubMed] [Google Scholar]
- 7.Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- 9.McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- 12.Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- 13.Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 15.Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Quantification of Behavior Sackler Colloquium: Addiction: Beyond dopamine reward circuitry. Proc Nat Acad Sci USA. 2011 Mar 14; doi: 10.1073/pnas.1010654108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Zhu JP, Angulo JA. Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse. 2005;58:110–121. doi: 10.1002/syn.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong J, Ross BM, Schmunk GA, Peretti FJ, Kalasinsky KS, Furukawa Y, et al. Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase activity in human methamphetamine users. Am J Psychiatry. 2003;160:896–903. doi: 10.1176/appi.ajp.160.5.896. [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 32.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging Dopamine Transmission in Cocaine Dependence: Link Between Neurochemistry and Response to Treatment. Am J Psychiatry. 2011 Mar 15; doi: 10.1176/appi.ajp.2010.10050748. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 34.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 35.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 36.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]