Abstract
IL-10 is a broadly acting immune inhibitory cytokine that is generally thought to support tumor growth. Here we challenge this view with evidence that genetic ablation of IL-10 in the mouse significantly heightens sensitivity to chemical carcinogenesis, growth of transplanted tumors and formation of metastases. Tumor growth in IL-10-deficient (IL-10−/−) mice was associated with an increased level of myeloid-derived suppressor cells (MDSCs) and CD4+Foxp3+ regulatory T(Treg) cells in both the tumor microenvironment and the tumor-draining lymph nodes (TDLN). IL-10−/− MDSCs express high levels of MHC and IL-1 and they efficiently induced formation of Treg cells. IL-1 signaling blockade reduced tumor growth mediated by IL-10 deficiency, associated with a partial rescue of tumor infiltration and function of effector T cells and a decrease in tumor angiogenesis and tumor infiltration by Treg cells. Taken together, our findings establish that endogenous IL-10 inhibits inflammatory cytokine production and hampers the development of Treg cells and MDSCs, two key components of the immunosuppressive tumor microenvironment, thereby inhibiting tumor development, growth and metastasis.
Keywords: IL-10, IFNγ, tumor immunity, regulatory T cell, myeloid derived suppressor cell, IL-1
Introduction
Interleukin-10 (IL-10) was first described as a product of T helper 2 (Th2) cells that inhibited cytokine production by T helper 1 cells (Th1) (1–2). In addition to Th2 cells, IL-10 is produced by different T cell subsets including Th1 cells (3), B cells, myeloid APCs (1–2), keratinocytes (4) and epithelial cells (5). IL-10 has been thought to be largely immunosuppressive, enacting multiple inhibitory effects on APCs. IL-10 restrains antigen presentation via its inhibition of major histocompatibility complex (MHC) and co-stimulatory B7 family member molecules (1–2), stimulates inhibitory B7 family members (6–8), downregulates IL-12 production, and inhibits dendritic cell differentiation and maturation (9). Therefore, most attention has been devoted to the immunosuppressive roles of IL-10 in a variety of experimental settings, particularly in infectious disease models (9–11). In addition to immunosuppressive effects, IL-10 also exerts some immunostimulatory effects, such as promoting the generation of cytotoxic T lymphocytes, activating B cells (1, 3, 12), and up-regulating a small number of genes in TLR-activated macrophages and dendritic cells (DCs) (13).
Although the underlying mechanisms are poorly understood, the stimulatory effects of IL-10 have also been observed in the context of tumor. Transfection of tumor cells with IL-10 or systemic IL-10 administration significantly suppressed tumor growth and led to tumor rejection (14–17). These data suggest that the biological activities of IL-10 in tumor pathology may be highly context-dependent.
Over the past few years, we and others have achieved important insights into tumor immunopathogenesis in patients with cancer. We and others have demonstrated that the tumor microenvironment is comprised of Treg cells (18–19), MDSCs (20–28) and dysfunctional APCs (6–7, 29–30) that form a suppressive network to defeat tumor-specific immunity and promote tumor growth. Because IL-10 is functionally linked to Treg cells and MDSCs (15–17), we revisited the role of IL-10 in tumor pathology in this study. We have demonstrated that IL-10 inhibits inflammatory cytokine production, hampers the development of MDSCs and Treg cells in the tumor microenvironment, and retards tumor development, growth and metastasis.
Methods
Tumor models
6–8 week old C57BL/6 mice (strain #000664) were purchased from the Jackson Laboratory. IL-10−/− mice (strain #2251) and IL-1R−/− mice (strain #3245) were purchased from the Jackson Laboratory (Jackson Laboratory, Maine) and bred in-house. The mice were maintained in specific pathogen-free conditions. This research was approved by the committee on Use and Care of Animals at the University of Michigan. 1×106 MC38 mouse colon carcinoma cells were inoculated subcutaneously into the left flank of IL-10−/−, IL-10+/+, or IL-1R−/− C57/BL6 mice. Tumor size was measured each three days using calipers fitted with a Vernier scale. Tumor volume was calculated based on three perpendicular measurements. For other experiments, 2×105 MCA310 fibrosarcoma cells were inoculated intravenously into the tail vein of IL-10+/+ and IL-10−/− mice. At 2 weeks post-inoculation, mice were sacrificed and their lungs were harvested, perfused with India Ink, and fixed in paraformaldehyde. The numbers of lung foci were quantified with the aid of a magnifier. MC38 was from Walter Storkus and MCA310 was from Drs. Alfred Chang and Bernard Fox. MC38 was tested in 2009 (31) and MCA310 was tested in 2009 (32) and 2010 (this work) for in vivo tumor formation in mice.
AOM/DSS treatment
Mice were given 10mg/kg azoxymethane (Sigma) via intraperitoneal injection. Five days later they were allowed free access to water containing 2% dextran sodium sulfate (DSS, MP Biomedicals/Fisher) for five days, followed by 16 days of regular water. This cycle was repeated twice and mice were sacrificed 2 weeks after the end of the last DSS cycle or at the end of 9 weeks. Colons were harvested, flushed of feces and slit open longitudinally to count tumors with the aid of a magnifier.
IL-1Ra treatment
The human recombinant IL-1 receptor antagonist Anakinra (Kineret®) was administered at 150mg/kg to mice intraperitoneally for 5 days before tumor inoculation and each day thereafter for three weeks. Mice not receiving Anakinra were injected with PBS vehicle.
Flow cytometry analysis
Single-cell suspensions were made from spleen, tumor-draining lymph nodes and tumor. Cells were labeled with fluorescence-conjugated antibodies to CD45 (Invitrogen), CD4, CD8, Gr-1, CD11b, CD90, CD115 (all eBioscience), IFNγ, MHC I, CD19 (all BD Pharmingen), and/or Foxp3 (eBioscience). For cytokine profiles, the cells were stimulated with 50ng/mL PMA (Sigma) and 1 μM Ionomycin (Sigma-Aldrich, St. Louis, MO) for 4 hours in the presence of GolgiPlug and GolgiStop (BD Biosciences, San Jose, CA). Cells were first stained extracellularly with specific antibodies, then fixed and permeabilized with Fix/Perm solution (eBioscience), and finally stained intracellularly with specific antibodies. Samples were acquired on a special order LSR II flow cytometer (BD Biosciences), and data were analyzed with DIVA software (BD Biosciences) (7, 18).
Immunofluorescence analysis
Immunofluorescence analysis was performed as previously described (7, 18). Briefly, harvested tissues were frozen in OCT and then fixed with paraformaldehyde. Permeabilized tissues were stained with ratanti-mouse CD8 (1:50, BD Pharmingen), rat anti-mouse CD31 (1:00, BD Pharmingen), and/or rabbit anti-mouse Foxp3 (1:500, Abcam) followed by goat anti-rat and goat anti-rabbit secondaries conjugated to Alexa Fluor 488 and AlexaFluor 568, respectively (both 1:2000; Molecular Probes/Invitrogen). Nuclei were stained with DAPI (Molecular Probes/Invitrogen). Fluorescent images were acquired on a fluorescence microscope (Leica) and analyzed by ImagePro Plus software.
MDSC Suppression and Treg Induction
For the immunosuppressive assay, MDSCs were isolated and sorted with a high speed sorter (FACSAria, BD) from tumor in tumor-bearing mice, and responder T cells were isolated from spleen in normal wild-type (IL-10+/+) mice. Normal spleen T cells were stimulated for 3 days with anti-CD3 (2.5 μg/ml) and anti-CD28 (1.25 μg/ml) in the presence of different concentrations of MDSCs. Thymidine was added in the last 16 hours. T cell proliferation was determined by thymidine incorporation. In Treg induction experiments, tumor-associated MDSCs were cultured with normal naïve CD4+ T cells isolated from lymph nodes in the presence of anti-CD3 (2.5 μg/ml) for 6 days. On day 6 the cells were removed from culture, permeabilized, and stained with antibodies to Foxp3, CD4, CD90, and CD11b. FoxP3 expression in total CD4+ T cells was analyzed via FACS gated on CD4+CD90+CD11b− cells.
Real-time reverse-transcriptase polymerase chain reaction (RT-PCR)
CD11b+ cells were isolated from IL-10+/+ or IL-10−/− splenic single-cell suspensions with CD11b positive selection microbeads (Miltenyi), and CD11c−CD11b+ cells were further sorted with FACSAria. mRNA was isolated with Trizol (Gibco BRL). Cytokine transcripts were detected by real-time RT-PCR as previously described (33). Gene-specific primer pairs and Fast SYBR Green Master Mix (Applied Biosystems) were used in a Multiplex instrument (Eppendorf). Data analysis is based on the Ct method with normalization of raw data to a housekeeping gene (HPRT).
Statistics
Most experiments were evaluated using the Mann-Whitney test, with P < 0.05 considered significant. Some cases were evaluated with Student’s T test, and quadratic regression model, also with P < 0.05 considered significant. Statistics were performed in the GraphPad Prism program suite (GraphPad Software, Inc., La Jolla, CA) and Statistica program suite (StatSoft, Tulsa, OK).
Results
IL-10 deficiency increases tumor incidence, growth and foci formation
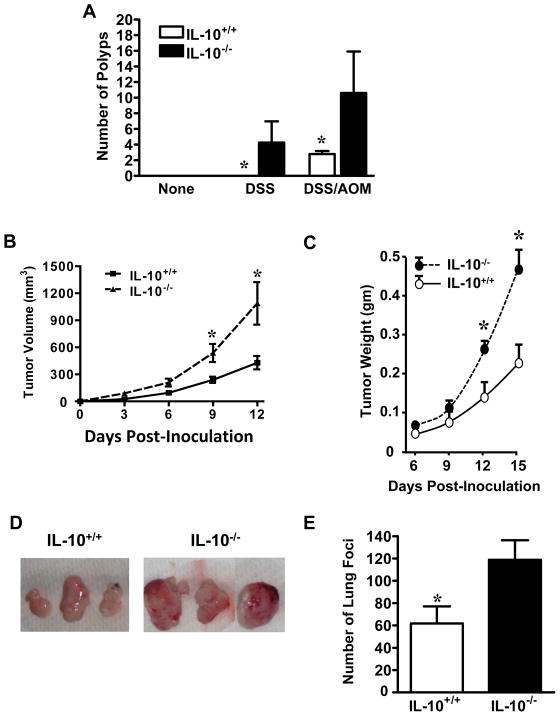
The immune-inhibitory role of IL-10 has been well-defined in numerous experimental settings. However, the in vivo effects of endogenous IL-10 on tumor immune responses and tumorigenesis are not well understood. We compared tumor incidence, growth and foci formation in IL-10-deficient (IL-10−/−) and wild-type (IL-10+/+) mice. The mice were subjected to administration of dextran sodium sulfate (DSS) and/or azoxymethane (AOM), as previously described (34). IL-10−/− mice, but not IL-10+/+ mice treated with DSS developed numerous colon polyps. In the presence of DSS and AOM, colon polyps developed in both IL-10−/− and IL-10+/+ mice. However, there were more polyps in IL-10−/− mice than IL-10+/+ mice (Figure 1A). We next subcutaneously injected a colon cancer cell line, MC38, into mice and observed tumor growth over a period of two weeks. MC38 had accelerated growth in IL-10−/− mice as compared to IL-10+/+ mice as shown by tumor volume measurement (Figure1B). This was confirmed by measuring tumor weight at multiple time points (Figure1C). Examples of final tumors excised from IL-10+/+ and IL-10−/− mice are shown in Figure 1D. We further examined the effect of endogenous IL-10 on the development of mouse lung metastatic foci. To this end, MCA310, a methylcholanthrene-induced sarcoma, was intravenously injected into the two groups of mice. IL-10−/− mice had more tumor foci in the lungs than IL-10+/+ mice (Figure 1E). Thus, IL-10 deficiency increases tumor incidence, growth and lung foci formation.
Figure 1. IL-10 deficiency increased tumor incidence, growth, and foci formation.
(A). Chemically-induced tumor incidence in IL-10+/+ and IL-10−/− mice. IL-10+/+ and IL-10−/− mice were given no treatment, DSS, or DSS and AOM as described in Material and Methods. Numbers of colon polyps were recorded. Results are expressed as the mean of colon polyps +/− SEM. 6 mice per genotype. *, P < 0.05. (B–D). Tumor growth in IL-10+/+ and IL-10−/− mice. MC38 cells were inoculated subcutaneously into the left flank of IL-10+/+ and IL-10−/− mice. B. Tumor volume was monitored and recorded. 10 mice per genotype. *, P < 0.05. C. Tumors excised at each time point were weighed. 5 mice per time point per genotype. *, P < 0.05. D. Six actual tumors are shown. (E). Tumor lung foci in IL-10+/+ and IL-10−/− mice. MCA310 cells were injected intravenously into IL-10+/+ and IL-10−/− mice. The numbers of lung tumor foci were counted two weeks after tumor inoculation. 6 mice per genotype. *, P < 0.05.
IL-10 deficiency increases Treg cells in the tumor
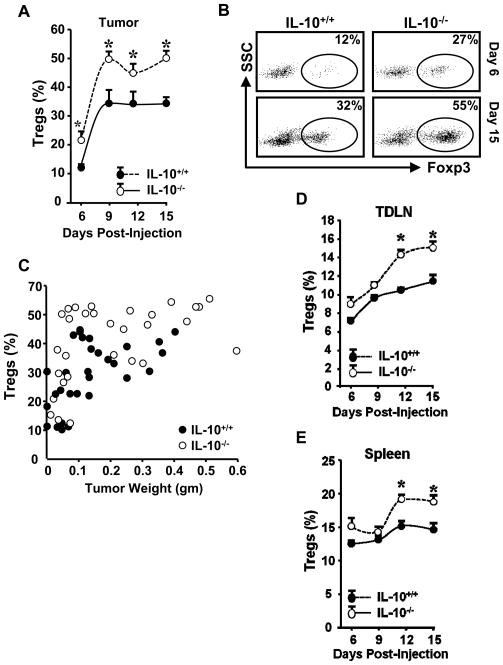
We next investigated the well-defined immunosuppressive immune cell subsets in the tumor microenvironment, including Treg cells. The percentages of intratumoral Treg cells during the course of MC38 tumor development were higher in IL-10−/− mice as compared to their wild-type counterparts beginning on day 6 (Figure 2A, B). There was a significant correlation between tumor weight and percentage of intratumoral Treg cells in both IL-10+/+ and IL-10−/− mice. Furthermore, a quadratic regression analysis revealed that Treg cell levels were higher in IL-10−/− mice than IL-10+/+ mice across different tumor volumes (Figure 2C). Interestingly, although Treg populations do not differ in the spleens and lymph nodes of unchallenged, tumor-free mice (Supplementary Figure 1A, B), they were considerably increased in the tumor-draining lymph nodes and spleen of tumor-bearing mice on day 12 and day 15 (Figure 2D, E). Larger Treg populations were also observed in TDLNs of IL-10−/− mice bearing MCA310 (not shown). The data indicate that IL-10 deficiency results in increased Treg cells in tumor-bearing mice.
Figure 2. IL-10 deficiency increased Treg cells in tumor bearing mice.
(A–E). Single-cell suspensions were made from tumor tissues, spleen, and tumor-draining lymph nodes (TDLN). These cells were stained with anti-CD3, CD45, CD4, and FoxP3. Antigen and cytokine expression was analyzed by FACS. A. Treg cells were quantified as the percentage of Foxp3+ cells in CD4+CD3+ cells isolated from tumor tissue. 5 mice per time point. *, P < 0.05. B. Representative FACS dot plots from data in A. C. Correlation between tumor weight and Treg percentage. Cells were analyzed as in A and the percentage of Treg cells in CD45+ cells was plotted against corresponding tumor weight. Treg cells versus tumor weight in IL-10−/−(n=30) and IL-10+/+ (n=30) mice. Quadratic regression analysis between IL-10−/− and IL-10+/+ mice across tumor volumes (P <0.05). D. Treg cells were quantified as the percentage of FoxP3+ cells in CD4+CD3+ cells isolated from TDLN. 5 mice per time point. *, P < 0.05. E. Tregs were quantified as the percentage of FoxP3+ cells in CD4+CD3+ cells isolated from spleens in tumor-bearing mice. 5 mice per time point. *, P < 0.05.
IL-10 deficiency increases MDSC in the tumor
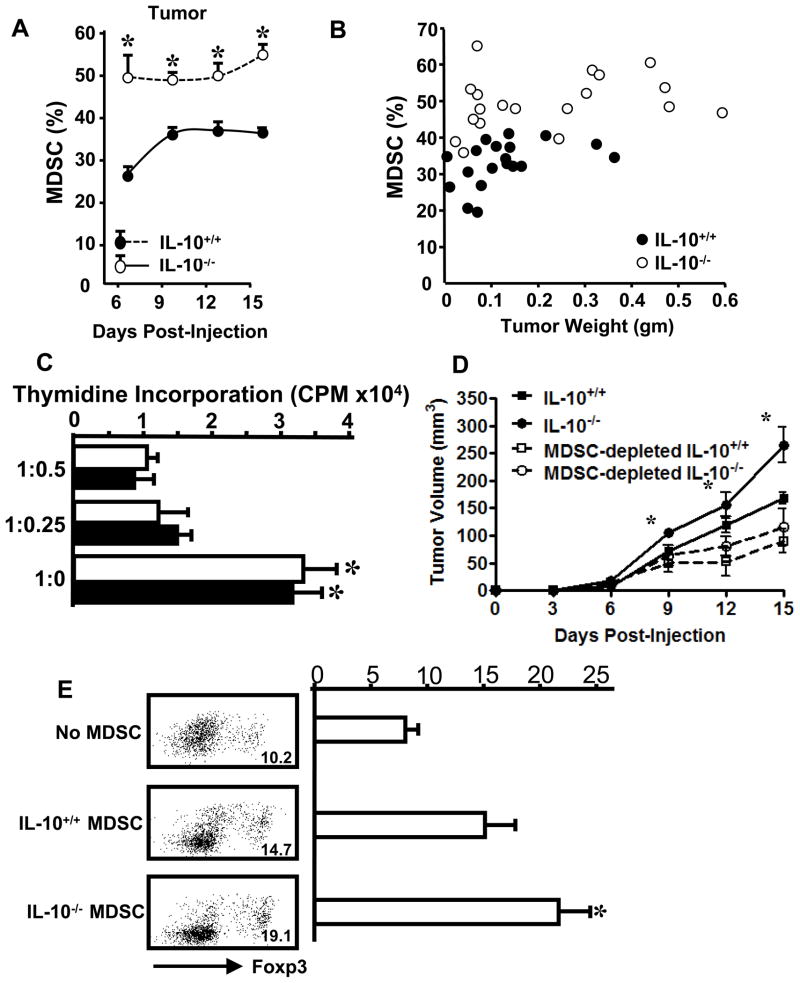
In addition to Treg cells, MDSCs are also an important immunosuppressive component in the tumor bearing hosts. We observed that intratumoral Gr-1+CD11b+ MDSCs were consistently increased in IL-10−/− mice in comparison to IL-10+/+ mice throughout the duration of tumor growth (Figure 3A, Supplementary Figure 2A). Similar to intratumoral Treg cells, a quadratic regression analysis revealed that intratumoral MDSC levels were higher in IL-10−/− mice than IL-10+/+ mice across different tumor volumes (Figure 3B). Not surprisingly, MHC I and II expression was increased on TDLN MDSCs (Supplementary Figure 2B, and not shown) in IL-10−/− mice. Once again, although MDSC populations do not differ in unchallenged mice (Supplementary Figure 3, day 0), they were consistently increased in the spleens of IL-10−/− tumor-bearing mice in comparison to IL-10+/+ counterparts (Supplementary Figure 3). This trend was not dependent upon tumor weight (Supplementary Figure 4).
Figure 3. IL-10 deficiency increased MDSCs in tumor bearing mice.
(A, B). Single-cell suspensions were made from tumor tissues and stained with anti-CD3, CD45, CD4, CD8, Gr-1, and CD11b for membrane antigen expression. The expression of antigens was analyzed by FACS. A. Increased MDSCs in tumor-bearing IL-10−/− mice. Gr-1+CD11b+ MDSCs were analyzed in MC38 tumors of IL-10−/− and IL-10+/+ mice. 5 mice per genotype per time point. *P < 0.05, IL-10−/− versus IL-10+/+ mice. B. Tumor MDSC levels were higher in IL-10−/− mice than IL-10+/+ mice. Cells were analyzed as in A and the percentage of MDSCs in CD45+ cells was plotted against corresponding tumor weight. Quadratic regression analysis between IL-10−/− (n=20) and IL-10+/+ (n=20)mice across tumor volumes (P <0.05). C. Tumor-associated IL-10−/− MDSCs mediate immune suppression in vitro. MDSCs were sorted from MC38 tumors. MDSCs were cultured with naïve T cells in different ratios as detailed in Materials and Methods. T cell proliferation was determined by thymidine incorporation. Results are expressed as the mean of CPM +/− SD in triplicates. Empty bars: IL-10+/+. Filled bars: IL-10−/−. One of three experiments is shown. *P < 0.01 compared to groups with MDSCs. D. MDSCs suppressed antitumor immunity in vivo. IL-10+/+ mice were lethally irradiated (2 × 600 rad, three hours apart, for a total of 1200 rad) and given an infusion of IL-10+/+ or IL-10−/− whole spleen cells or spleen cells depleted of MDSCs from tumor bearing mice. On the same day, mice were injected subcutaneously with MC38. Tumor growth was measured over a period of two weeks. 3–4 mice per group. *P < 0.05, Day 9–15: IL-10−/− vs. IL-10+/+, IL-10−/− vs. MDSC-depleted IL-10−/−; and Day 12–15: IL-10+/+ vs. MDSC-depleted IL-10+/+. E. Tumor-derived IL-10−/− MDSCs were superior to IL-10+/+ MDSCs in Treg cell induction. MDSCs were isolated from MC38 tumors and co-cultured with IL-10+/+ CD4+ T cells for 6 days. CD4+ FoxP3+ cells were analyzed via FACS in cultured T cells. One of three experiments with triplicates per experiment is shown. IL-10+/+ vs. IL-10−/− MDSC in 3 independent experiments, *P<0.05.
We further examined the roles of MDSCs on T cell activation and tumor growth. In an in vitro immune suppression assay, similar to IL-10+/+ MDSCs, tumor-derived IL-10−/− MDSC suppressed T cell proliferation in a dose-dependent manner (Figure 3C). To directly test the effects of MDSCs in vivo, we completed immune cell adoptive transfusion experiments. We initially irradiated IL-10+/+ mice and infused them with total mononuclear or MDSC-depleted spleen T cells from IL-10+/+ or IL-10−/− mice. Subsequently, we subcutaneously injected MC38 tumor cells into these mice, and monitored tumor growth. We observed that MDSC depletion resulted in significantly reduced tumor growth in mice receiving either IL-10−/− or IL-10+/+ immune cells. This indicates that IL-10−/− and IL-10+/+ MDSCs mediate immune suppression in vivo. However, although tumor volumes were bigger in irradiated mice receiving IL-10−/− than in those receiving IL-10+/+ immune cells (Figure 3D), which is in line with our observations of non-irradiated mice (Figure 1B–D), we showed that after MDSC depletion, tumor volumes were similar in irradiated mice receiving either IL-10−/− or IL-10+/+ immune cells (Figure 3D). The data indicate that MDSCs serve as important suppressive components of the IL-10−/− immune system in vivo.
We then hypothesized that MDSCs might induce Treg cells in tumor-bearing mice as previously reported (22). In support of this, we observed that both tumor-derived IL-10−/− and IL-10+/+ MDSCs from MC38-bearing mice induced Treg cells. However, Treg cells were more efficiently induced by IL-10−/− MDSCs, as compared to IL-10+/+ MDSCs (Figure 3E). Altogether, our results indicate that IL-10 deficiency increases Treg cells and MDSCs, and suggest that increased MDSCs may induce Treg cells in tumor-bearing mice.
IL-1 contributes to increased tumor growth in IL-10−/− mice
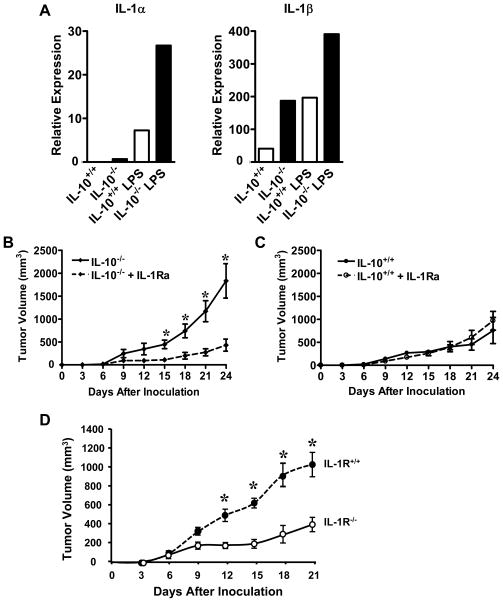
After demonstrating the cellular mechanisms which may contribute to increased tumor incidence, growth and foci formation in IL-10−/− mice, we further examined the relevant molecular patterns. We showed that myeloid cells from tumor-free mice expressed higher levels of IL-1α and IL-1β in IL-10−/− than IL-10+/+ mice (Figure 4A). We then investigated the role of IL-1 in tumor growth in IL-10−/− mice. Anakinra, the recombinant human IL-1 receptor antagonist (IL-1Ra), has demonstrated biological efficacy in mice (35). We treated tumor-bearing IL-10−/− and IL-10+/+ mice with Anakinra or vehicle over a period of three weeks. We observed that IL-10−/− mice treated with Anakinra had reduced tumor burden (Figure 4B). Interestingly, tumor growth was comparable in IL-10+/+ mice treated with Anakinra and those treated with PBS vehicle (Figure 4C). In further support of the relevance of IL-1, we showed that tumor volume was decreased in IL-1 receptor-deficient (IL-1R−/−) mice as compared to IL-1R+/+ mice (Figure 4D). It is likely that IL-1 contributes to increased tumor growth in IL-10−/− mice.
Figure 4. IL-1 blockade reduced tumor growth in IL-10−/− mice.
(A). IL-10−/− myeloid derived cells expressed high levels of IL-1. Myeloid derived cells were isolated from spleens in tumor-free IL-10−/− and IL-10+/+ mice and not stimulated or stimulated with LPS for 8 hours. Real-time PCR was performed to determine the expression of IL-1α and IL-1β. One of 3 experiments is shown. (B, C). Anakinra treatment reduced tumor burden in IL-10−/− mice but had no significant effect on tumor burden in IL-10+/+ mice. MC38 was injected subcutaneously into IL-10−/− and IL-10+/+ mice. The mice were treated with Anakinra or vehicle as described in Materials and Methods. Tumor volume was measured every 3 days. Results are shown as the mean values of tumor volume +/− SEM. N = 4–5 mice per group. *, P < 0.05, Anakinra versus control in IL-10−/− mice. (D). Reduced tumor growth in IL-1R−/− mice. MC38 was injected subcutaneously into IL-1R−/− and IL-1R+/+ mice. Tumor volumes were measured every 3 days. Results are shown as the mean values of tumor volume +/− SEM in IL-10−/− mice. N = 3–4 mice per group. *, P < 0.05, IL-1R−/− versus IL-1R+/+ mice.
IL-1 blockade enhances effector T cell tumor infiltration and reduces tumor angiogenesis in IL-10−/− mice
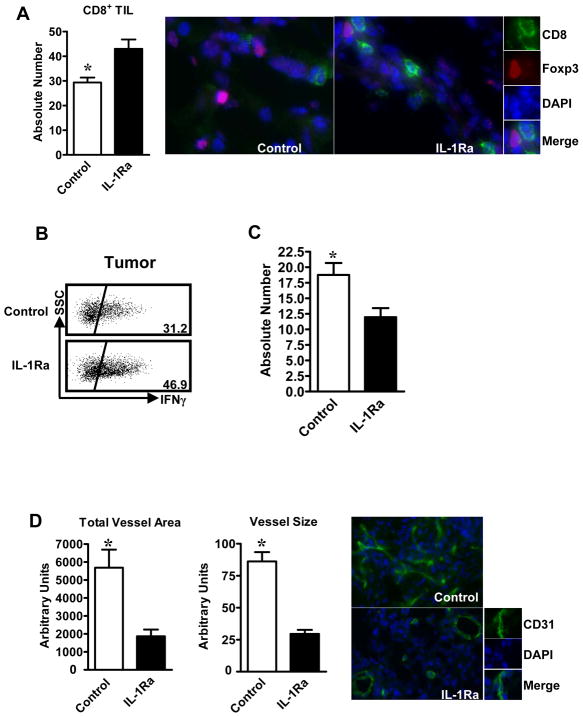
We next examined tumor-infiltrating T cells in IL-10−/− mice treated with Anakinra. Immunofluorescent staining revealed that there were more tumor-infiltrating CD8+ T cells in IL-10−/− mice treated with Anakinra as compared to control (Figure 5A). The levels of intratumoral IFNγ+CD8+ T cells were also increased in mice treated with Anakinra (Figure 5B). Although the levels of MDSCs were comparable in the two groups (not shown), the numbers of tumor infiltrating Treg cells were reduced in mice treated with Anakinra (Figure 5C). Furthermore, tumor microvessel intensity and size (Figure 5D) were reduced by Anakinra treatment. Altogether, the data suggest that IL-1 blockade promotes tumor immune surveillance and reduces tumor angiogenesis.
Figure 5. IL-1 blockade altered immune phenotype and tumor vascularization in IL-10−/− mice.
(A). Increased tumor-infiltrating CD8+ T cells in Anakinra-treated IL-10−/− mice. Immunofluorescent staining was performed on tumor tissue sections. The absolute numbers of CD8+ T cells were counted. Results are expressed as the mean values +/− SEM per 15 high-powered fields (HPF). N = 5 mice per group. *P < 0.05, Anakinra versus control. (B). Increased tumor-infiltrating IFNγ+CD8+ T cells in Anakinra-treated IL-10−/− mice. Single-cell suspensions were made from tumor tissues. The cells were stained for CD8 and intracellular IFNγ. Results are expressed as the mean percent of IFNγ+CD8+ T cells in CD8+ cells +/− SEM. N = 4–5 mice per group. *, P < 0.05, Anakinra versus control. (C). Reduced tumor-infiltrating Treg cells in Anakinra-treated IL-10−/− mice. Immunofluorescent staining was performed on tumor tissue sections. The absolute numbers of Foxp3+ T cells were counted. Results are expressed as the mean values +/− SEM per 15 high-powered fields (HPF). N = 5 mice per group. *, P < 0.05, Anakinra versus control. (D). Reduced tumor vascularization in Anakinra-treated IL-10−/− mice. Immunofluorescent staining was performed to analyze the expression of CD31 in tumor tissue sections. The areas of CD31+ vessels and the size of CD31+ vessels were analyzed as detailed in Materials and Methods. Results are expressed as the mean area or size of vessels +/− SEM in 10 HPF. N = 4–5 mice per group. *, P < 0.05, Anakinra versus control.
Discussion
IL-10 has been thought to be largely immune suppressive. However, to our surprise, chemically-induced tumor incidence, transplanted tumor growth and lung foci formation are increased in IL-10−/− mice. This is associated with a number of immune phenotypic, inflammatory and functional signatures.
The prevalence of MDSCs and Treg cells is similar in tumor-free IL-10-deficient versus wild-type mice. However, there are significantly more MDSCs and Treg cells in IL-10-deficient tumor-bearing mice than in wild-type tumor bearing mice. MDSCs and Treg cells are the most important immunosuppressive components in the tumor microenvironment. Our observations suggest that IL-10 may impact the development of MDSCs and Treg cells in the context of tumor, and in turn regulate tumor immune responses. Consistent with our observations, earlier studies in mice have shown that IL-10 promoted tumor immunity and led to reduced tumor growth or tumor rejection (15–17). However, the underlying mechanisms by which IL-10 mediates suppression of tumor growth despite its defined immune-inhibitory functions remained elusive. In addition to regulating the immunosuppressive components, we do not rule out the possibility that IL-10 directly affects T cell activation. In line with this, it has been reported that IL-10 may promote CD8 differentiation and expansion (36–38). In fact, humans treated with IL-10 showed a reduction of IL-1 and increased IFNγ (39–40). Our studies suggest that the biological activities of IL-10 may be highly context-dependent, and vary in the presence of different cellular targets, phases of immune responses and disease model systems. A similar example of this phenomenon is MyD88 signaling, which has been implicated in the promotion of cancer in several mouse carcinogenesis models. However, under chronic colitis conditions induced by DSS-induced mucosal damage, MyD88 has a protective role in colon cancer (12).
Given that IL-10 can be immune stimulatory and inhibitory, the balance of these effects may determine whether IL-10 is beneficial or detrimental in a given model system and/or disease stage. The immune regulatory roles of IL-10 are prominently defined in infectious disease models. In these scenarios, APCs are the immediate and predominant targets of IL-10 (38). Infectious pathogens rapidly activate Toll-like receptors on APCs, and induce antigen-specific T cell priming. IL-10 inhibits the maturation and function of APCs via reduced MHC expression and IL-12 production, and subsequently suppresses T cell priming and Th1-type responses (1–2). In a tumor-bearing host, however, when there is no obvious acute phase of immune response (30), tumor-associated antigen (TAA)-specific priming may slowly occur, IL-10 may preferentially target MDSCs and Treg cells before potent TAA-specific effector T cell immunity is established in tumor-bearing hosts (38). This may partially explain the discrepancy of IL-10 biology in the literature.
It has been reported that IL-1 supports the development of MDSCs in tumor-bearing mice (25–26, 41). In line with this possibility, IL-10−/− myeloid cells express high levels of IL-1, and there are more MDSCs in IL-10−/− tumor-bearing mice. MDSCs promote Treg cell development (22). Consistent with this, we have observed that IL-10−/− MDSCs efficiently suppress T cell expansion and are stronger inducers of Treg cells than IL-10+/+ MDSCs. It has been suggested that loss of MHC or mutations in MHC expression machinery may result in limited tumor antigen recognition and presentation (42). Interestingly, IL-10−/− MDSCs express high levels of MHC molecules, and may efficiently present self-antigens to Treg cells, thus activating and expanding Treg cells as we have observed. This may explain why IL-10−/− MDSCs are superior to IL-10+/+ MDSCs in Treg cell induction. Perhaps most tellingly, depletion of MDSCs in our immune cell-transfer model profoundly reduces tumor volume, demonstrating that the presence of MDSCs has a negative impact on host immunity in vivo. Additionally, tumor burden in mice receiving IL-10−/− cells depleted of MDSCs was reduced by a larger volume than in those mice receiving IL-10+/+ cells depleted of MDSCs. This suggests that MDSCs that develop in the absence of IL-10 may mediate more powerful suppressive activities in tumor-bearing mice. However, although IL-1 promotes the development of MDSCs in tumor-bearing mice (25–26, 41), intratumoral MDSCs were not changed after IL-1Ra administration in IL-10−/− mice, while effector T cells were increased and Treg cells were reduced. Further studies will determine why IL-1 blockade did not affect tumor associated MDSCs.
IL-10 suppresses the expression of inflammatory cytokines in chronic inflammation models (1–2). IL-10−/− mice develop chronic colitis in conventional conditions (43) and are prone to develop colon tumors under the stimulation of only DSS, or of both DSS and AOM. Although there is no obvious inflammation (including colitis) in IL-10−/− mice when housed in specific pathogen-free facilities, IL-10−/− myeloid cells express high levels of IL-1, which may suggest the presence of microscopic inflammation in these mice. This inflammation may be increased after tumor inoculation or induction. Inflammation is often linked to increased tumor vascularization, and IL-1 has long been known as a pro-angiogenic cytokine (44) and promoter of tumorigenesis (45). In line with this, IL-1 blockade reduces tumor microvessel density and size in IL-10−/− mice. The data suggests that IL-10 negatively regulates inflammatory cytokine production, and directly inhibits inflammation-associated cancer development and growth. However, it is also possible that the reduced density of tumor microvessles may be due to the resulting enhanced T cell immunity that includes the production of IFNγ, a potent anti-angiogeneic factor (46). Finally, we have demonstrated that IL-1 blockade reduces tumor growth in IL-10−/− mice. Accordingly, the levels of intratumoral Treg cells are reduced, and the numbers of tumor-infiltrating effector T cells and effector cytokines are enhanced. Altogether, the increased tumor development, growth and metastasis in IL-10−/− mice result from multiple intertwined mechanisms: increased immunosuppression, enhanced inflammation and possible reduced effector T cell function and tumor trafficking in tumor-bearing hosts.
In summary, contrasting the established views, we demonstrate that IL-10 is negatively linked to the development of immunosuppressive MDSCs and Treg cells, subverts tumor immune suppression, and in turn contributes to reduced tumor development, growth and metastasis. Our data indicate that the biological activities of IL-10 can be highly context-dependent.
Supplementary Material
Acknowledgments
The work is partially supported by the NIH/NCI grants (W. Zou). The authors would like to acknowledge the members of the Zou lab for constructive discussion and technical assistance, especially Linda Vatan and Wojciech Szeliga.
Footnotes
Contributions: TT, CMW, and IK designed the research, performed experiments, analyzed data, and made the figures; JK, GYC and GN helped with experimental design and research protocol; TT, CMW, and WZ wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Vicari AP, Trinchieri G. Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev. 2004;202:223–36. doi: 10.1111/j.0105-2896.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Garra A, Murphy KM. From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce T(H)1 development. Nat Immunol. 2009;10:929–32. doi: 10.1038/ni0909-929. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–43. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92–5. [PubMed] [Google Scholar]
- 5.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 7.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Hagenbaugh A, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–10. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006;213:159–79. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 12.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–36. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Tahara H, Narula S, Moore KW, Robbins PD, Lotze MT. Viral interleukin 10 (IL-10), the human herpes virus 4 cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J Exp Med. 1995;182:477–86. doi: 10.1084/jem.182.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–84. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT. Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice. J Immunol. 1996;157:231–8. [PubMed] [Google Scholar]
- 17.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98:2143–51. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fostersimmune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 21.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 23.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 24.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 25.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 26.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 30.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 31.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–9. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei S, Kryczek I, Namm J, Szeliga W, Vatan L, Chang AE, et al. Response: Endogenous IL-17, tumor growth, and metastasis. Blood. 2010;115:2256–7. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–7. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surguladze D, Deevi D, Claros N, Corcoran E, Wang S, Plym MJ, et al. Tumor necrosis factor-alpha and interleukin-1 antagonists alleviate inflammatory skin changes associated with epidermal growth factor receptor antibody therapy in mice. Cancer Res. 2009;69:5643–7. doi: 10.1158/0008-5472.CAN-09-0487. [DOI] [PubMed] [Google Scholar]
- 36.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–73. [PubMed] [Google Scholar]
- 37.Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147:528–34. [PubMed] [Google Scholar]
- 38.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]
- 39.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–9. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 40.Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJ, Schreiber S, et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191–5. doi: 10.1136/gut.50.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–94. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 44.Voronov E, Carmi Y, Apte RN. Role of IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol. 2007;601:265–70. doi: 10.1007/978-0-387-72005-0_28. [DOI] [PubMed] [Google Scholar]
- 45.Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–71. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 46.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.