Abstract
Background
The pathology of essential tremor is increasingly being studied; however, there are limited studies of biochemical changes in this condition.
Methods
We studied several candidate biochemical/anatomical systems in the brainstem, striatum and cerebellum of 23 essential tremor subjects who came to autopsy, comparing them to a control population.
Results
Striatal tyrosine hydroxylase, a marker of dopaminergic neurons, was 91.7 ±113.2 ng/mg versus 96.4±102.7 ng/mg (not significant) in cases and controls. Locus ceruleus dopamine beta-hydroxylase, a marker of noradrenergic neurons, was not significantly different between essential tremor and control groups. Parvalbumin, a marker of GABAergic neurons, was 199.3±42.0 versus 251.4±74.8 ng/mg (p=0.025) in the pons in the region of the locus ceruleus of essential tremor versus controls, while there was no difference in cerebellar parvalbumin.
Conclusion
These results are supportive of a possible role for reduced GABAergic function within the locus ceruleus in essential tremor. The hypothesis that essential tremor represents early Parkinson’s disease was not supported as striatal dopaminergic markers were not reduced compared to control subjects.
Search terms: tremor, pathology, GABA, norepinephrine
Introduction
Essential tremor is increasingly being studied to determine the pathophysiological underpinnings. Pathological studies have supported the role of cerebellar Purkinje cell loss1, 2, and cerebellar pathology in general3. However, a recent study has called into question whether cerebellar cell loss is a primary finding in ET.4 Further investigations into alternate hypotheses are warranted.
One group has suggested that some cases of ET are due to Lewy Body pathology.2 However, we have found that the occurrence of Lewy bodies in ET cases is not any more common than in similarly evaluated control subjects.3 It is possible that ET-like tremor may be a manifestation of the underlying biochemical changes of PD. The presence of Lewy bodies without classic motor features of Parkinson’s disease or dementia is termed incidental Lewy body disease (ILBD). We and others have reported ILBD is associated with striatal dopaminergic deficiency,3, 5, 6 supporting ILBD being pre-motor PD, but similar studies in ET are lacking. If some cases of ET are simply early PD then we would hypothesize that ET cases would have a dopaminergic deficiency much like is found in ILBD.
One study of three subjects with ET found higher levels of noradrenaline in the locus ceruleus (LC), dentate and cerebellar cortex.7 It is well known that beta-blockade is an effective treatment for ET8, perhaps acting through this cerebellar noradrenergic pathway. A second recent post-mortem study found an increase in cerebellar GABAA receptor protein α1 in small group of ET subjects compared with controls.9
In this study, we have tested the hypothesis that ET is a pre-Parkinson’s state by quantifying striatal tyrosine hydroxlase (TH) concentration. By analogy with our previous work on ILBD, if ET is an early stage of PD, then it might be expected that subjects with ET have lower striatal TH concentrations than age-similar normal control subjects. We also tested the hypothesis that ET is associated with noradrenergic neuron upregulation in the LC, assessing LC concentrations of dopamine beta hydroxylase (DBH). Additionally, we examined parvalbumin, a marker of GABAergic interneurons, in both LC region and cerebellar cortex.
Methods
Subjects in the Banner Sun Health Brain and Body Donation Program (BBDP)3 were assessed annually with a general neurological examination, a movement disorder evaluation and neuropsychological testing. IRB approval was obtained and written consent provided by the subjects. The movement disorder evaluation consisted of the Unified Parkinson’s Disease Rating Scale (UPDRS), tremor rating scale,10 and assessment of other involuntary movements (dystonia, myoclonus). Subjects were diagnosed with ET if they had the presence of a grade 2 postural and/or kinetic tremor of the hands or forearms without identifiable secondary cause or other exclusion criteria (e.g. prominent unilateral tremor, rigidity or bradykinesia). 11, 12 Subjects with a tremor score between 0.5 and 2 were also considered ET if the research evaluations found the tremor was present for at least 3 years with similar exclusion criteria or medical records supported longstanding ET. Subjects with Parkinson’s disease or other movement disorders were excluded, as were those with dementia. Subjects meeting Petersen’s criteria for mild cognitive impairment (MCI) on neuropsychological testing were included in the study.13 Controls had the same inclusion and exclusion criteria except for absence of tremor. Alzheimer’s (plaque density and Braak tangle stage) and Lewy Body pathology was specifically examined in both groups, as per our previously published pathology report, with no baseline differences in this expanded cohort.
Brain pathology was evaluated according to standard protocols with descriptive assessment of cortex, basal ganglia, brainstem and cerebellum as described in our previous papers.3, 14 Measurements of TH levels were carried out using an ELISA procedure as previously described.15 The concentration of TH in each sample was calculated from a standard curves constructed using known amounts of recombinant TH (kindly provided by Dr. Paul Fitzpatrick, Texas A & M University, TX).
Due to low concentrations of protein, DBH levels in cerebellar and LC brain extracts were measured using a western blot method. In brief, samples of total brain extract (20 μg/lane) were separated through 4–12 % Bis-Tris NOVEX gradient gels and transferred to nitrocellulose membranes. DBH immunoreactive bands were detected using two sheep polyclonal antibodies (R&D Systems) to N and C terminal peptide sequences of human DBH protein. Detection and quantification of bands were carried out as previously described.16
ELISA for measuring concentrations of parvalbumin in brain extracts were developed in our laboratory. The design of the ELISA follows the same principals as for the TH assay, with capture antibody being sheep anti human parvalbumin,(R&D Systems), and both detection antibody rabbit anti human parvalbumin and purified recombinant parvalbumin protein from ABCAM, Cambridge MA. Due to small size of the LC, anatomical localization of parvalbumin was performed using immunohistochemistry. Paraffin embedded tissue sections (5 μm) of midbrain (containing pons and LC) were used to identified parvalbumin immunoreactive structures in the LC. Sections were deparaffinized according to standard procedures. Antigen retrieval was carried out by heating sections in 50 mM citric acid solution (pH 8.0) at 95°C for 30 minutes. For detection of parvalbumin, sections were incubated in a rabbit anti parvalbumin (1:1000 dilution, AbCAM, Cambridge, MA) antibody for 18 hours, followed by reaction with biotinylated anti rabbit immunoglobulin (Vector Labs, 1:1000 dilution for 2 hours) and then in Avidin-Biotin-Peroxidase complex (Vector Labs, 1:1000 dilution for 1 hour). Sections were developed in nickel enhanced diaminobenzidine solution.16 Stained sections were counterstained with neutral red and coverslipped for microscopy.
Adequate frozen tissue for biochemical assessment varied depending on the region studied. Dissection of the striatum has been previously published. The LC dissection was performed to include only the very small region defined by black pigment, about 0.3 cc. The cerebellar tissue was removed by taking a block from the far lateral aspect of the hemisphere. Median/mean post mortem interval was 2.5/3.0 (SD 3.1) hours. For clarity, the demographics of the ET population are reported for the TH sample, given that it was the largest.
All results were compared between groups using t-test unless otherwise indicated. Summary results are expressed as mean± SD Correlation analysis was done using Pearson’s correlation coefficient. Significance was set at p<0.05.
Results
There were 23 ET and 37 control subjects that met study criteria. Sixteen of the 23 subjects were previously reported in our pathology study3, while the remaining subjects were prospectively added since that report. Mean age was 86.2 yrs for the ET group and 83.8 yrs for controls. Mild cognitive impairment was seen in 3/23 ET subjects and 11/37 controls. ET was previously diagnosed by the subjects’ private physicians in 9/23 (39%) subjects, while 14/23 (61%) were diagnosed through their research evaluations. Sixty-five percent noted that they were bothered by tremor (UPDRS part II tremor score ≥ 1). The mean duration of tremor was 14.7 years. There were 3 ILBD cases in each group (NS by chi square analysis).
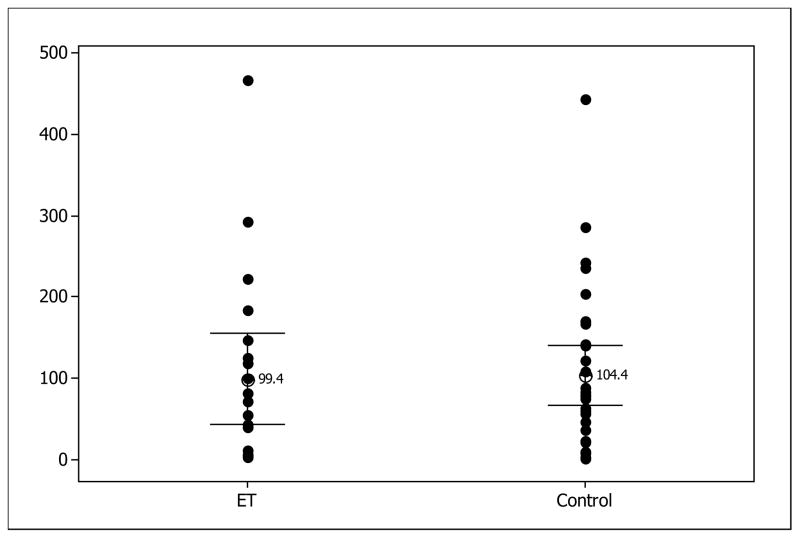
For striatal TH analysis the mean ± SD TH value was 91.7 ± 113.2 (ET) versus 96.4± 102.7 (controls) ng/mg (Table 1). Removing the 3 cases of ILBD from each group resulted in TH values of 99.4±119.3ng/mg (ET) and 104.4±103.7 ng/mg (controls). All differences between groups were not significant by t-test. Figure 1 depicts the range of values for TH, including ILBD.
Table 1.
| Location | N (ET/Control) | Marker | ET | Controls |
|---|---|---|---|---|
| Striatum | 23/37 | TH | 91.7±113.2 ng/mg | 96.4±102.7 ng/mg |
| LC | 15/16 | PVB | 199.3±42.0 ng/mg | 251.4±74.8 ng/mg* |
| DBH | 0.69±0.31 RU | 0.62±0.21 RU | ||
| CBM | 20/20 | PVB | 1152.6±240.6 ng/mg | 1182±241.2 ng/mg |
Legend: TH=tyrosine hydroxylase; LC=locus ceruleus; PVB=parvalbumin; DBH=dopamine beta hydroxylase; CBM= cerebellum;
p < 0.05 between ET and controls
Figure 1.
TH in ET vs. Controls, minus ILBD, results expressed in ng/mg
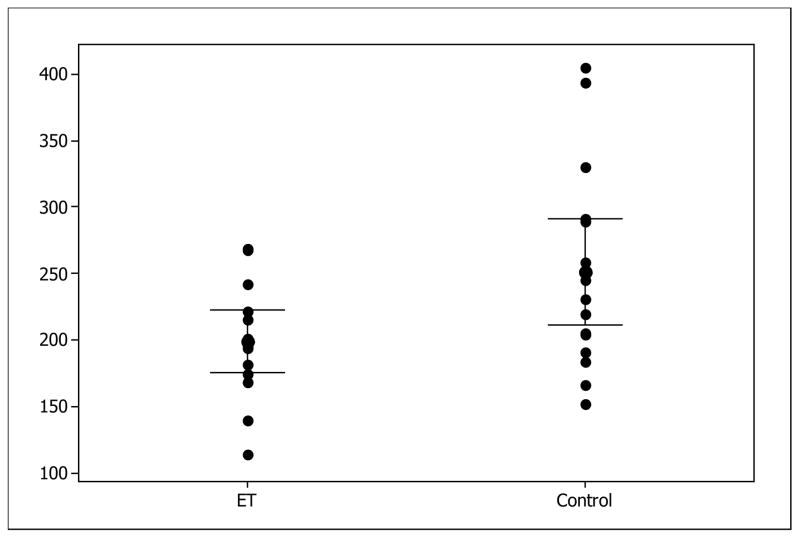
For the LC analyses there were 15 ET cases and 16 controls with adequate locus ceruleus tissue to analyze. Mean age at death was 86.7 and 85.9 respectively. Parvalbumin levels (see Figure 2) were significantly lower in ET, (199.3±42.0 ng/mg) versus controls (251.4±74.8 ng/mg) (p=0.025). Although not significant, there was modest correlation of LC-PVB with tremor duration with longer duration tremor having higher levels (R=0.46, p=0.09). For immunohistochemistry, we examined 3 control cases with the highest ELISA values of parvalbumin and compared them to 3 essential tremor cases with lowest ELISA values. Figure 3(A) shows that in a representative control case, in the LC, punctuate staining in bundles was observed running through the LC. These are presumptive neuronal fibers. These were adjacent to the large neuromelanin neurons (noradrenergic) found in the LC. Figure 3(B) show that in a representative ET case, a similar pattern was observed but the amount of punctuate staining was noticeable less. Figure 3(C) shows that staining with this antibody was selective; this panel shows distinct immunoreactive cells in the pons with strong parvalbumin staining compared to other cells with no staining. Similarly, immunoreactive fibers can also be seen in this region.
Figure 2.
Parvalbumin-LC ET versus controls expressed as ng/mg
Figure 3.
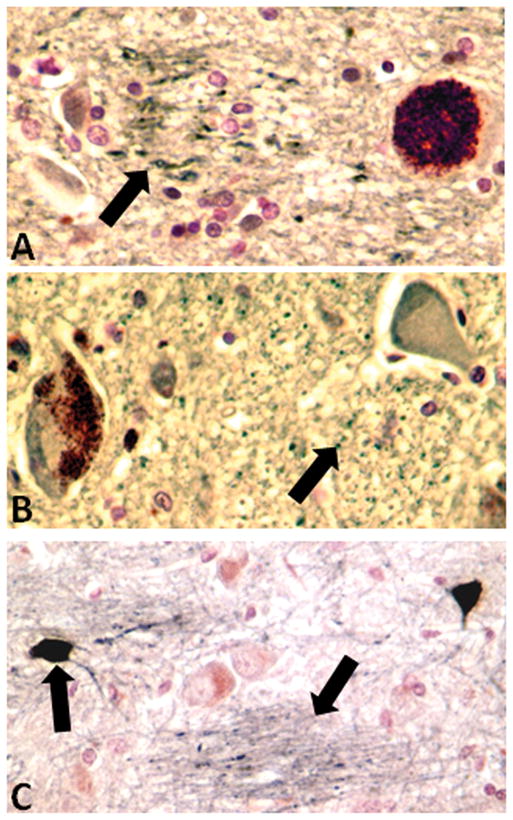
Parvalbumin immunoreactivity in locus coeruleus of control case. Arrow indicates regions with punctuate parvalbumin immunoreactivity.
B) Parvalbumin immunoreactivity in locus coeruleus of an essential tremor case. Arrow indicates region with punctuate parvalbumin immunoreactivity. It is noticeable that this was more restricted compared with panel A.
C) Parvalbumin immunoreactivity in pons of control case. This panel demonstrates the specificity of staining by the selective immunoreactivity of individual neurons (Arrow) and fibers (Arrow).
Western blot analysis for DBH in LC was 0.69±0.31 (relative units) in ET versus 0.62±0.21 (relative units) in controls (p=0.44). Due to the absence of standard protein, results are expressed as relative intensities of western blot bands (75 kD) protein normalized for intensities of β actin bands.
For cerebellar tissue analyses there were 20 ET cases and 20 controls. Parvalbumin was 1152.6±240.6 ng/mg versus 1182.52±241.2 ng/mg, also not significant (p=0.70). DBH was not reliably measured in the cerebellum despite multiple attempts; these results are not presented.
Discussion
The main findings of this study were the significantly reduced levels of parvalbumin in the region of the LC in ET versus controls and the lack of any difference in striatal TH in ET compared to controls. The parvalbumin changes were only found in the pons and locus ceruleus with no difference in the cerebellum suggesting a more focal abnormality in this particular brain region.
Parvalbumin is a marker for GABAergic interneurons within the central nervous system. It is mostly studied in cortical structures, particularly with respect to epilepsy. Previous study of GABA in ET demonstrated reduced GABA levels in the CSF of ET17 patients and increased GABA-A receptor in the cerebellar cortex.9 Medications that enhance GABAergic tone, such as primidone, gabapentin and benzodiazepines, are effective in treating ET.8 Taken together with our previous pathology studies showing more generalized pathology in the LC, these data support GABA neuronal loss and/or dysfunction in the brainstem in ET. We did not find changes in parvalbumin levels within the cerebellum, however, suggesting that this might be a more focal and specific finding. A recent study using flumazenil Positron Emission Tomography (PET) in ET suggested that the GABAergic findings are more diffuse, including the cerebello-thalamocortical loop.18 Confirmation of this would require a more detailed assessment of parvalbumin at varying levels of the neuroaxis in ET and controls. There was a trend toward higher parvalbumin levels correlating with longer tremor duration. This is somewhat unexpected but would support that there may be other factors involved in tremor expression besides simply an alteration in GABA. Alternately, it might suggest that shorter duration, elderly onset tremor may not be the same as classical familial ET with onset prior to age 65. How a modification of GABAergic tone in the LC might contribute to tremor is not fully clear. However, if may be that reduced inhibition to the adrenergic LC neurons results in a generalized increase in adrenergic tone in ET. This is supported by previous work showing an increase in norepinephrine in the LC, cerebellum and dentate in 3 ET patients compared with controls.7
DBH is responsible for the conversion of dopamine to norepinephrine and was not significantly changed in the locus ceruleus in ET compared with controls. This finding seems in contrast to the study that found elevated norepinephrine in ET.7 However, it may be that our subjects likely had more mild ET (since many were not bothered by tremor or formally diagnosed) and given that there is significant loss of LC neurons with normal aging, differences in enzyme levels might be difficult to see in a small study. The study population in our brain and body donation does have mild, elderly cases and this is a limitation. However, it seems likely that if norepinephine changes were a primary pathological finding in ET, it should be reflected in changes in DBH even in this small number of patients and therefore, the adrenergic changes may be secondary, perhaps related to changes in GABAergic function as discussed.
Regarding the relationship between ET and PD, we found no evidence that ET may be the first neurological sign of this neurodegenerative condition. Previous pathological studies have suggested that ET patients may harbor Lewy bodies in greater frequency than controls.2 Clinical studies suggest that the prevalence of PD in the ET population is 24 times greater than expected.19 Given that age is the most prominent risk factor for PD, studying the ET/PD interaction in a longitudinal, prospectively examined elderly cohort, such our BBDP, is helpful. We have previously shown no increase in the prevalence of Lewy bodies in ET compared with controls.3 Conversely, our subjects with ILBD were no more likely to exhibit tremor than those without ILBD.20 Now, with this study, we demonstrate that the striatal biochemical levels of a dopaminergic marker, TH, are similar between patients and controls. Whether there may be a subset of ET cases that may be genetically predisposed to both disorders or that a small portion of ET with onset of tremor before age 65 may be predestined to develop PD in later life cannot be excluded.21
Future studies should continue to search for the anatomical locus of ET. The present work and studies to date indicate the need for further exploration of the status of the GABAergic neuronal systems and locus ceruleus function. Further investigation of tremor dominant PD where there is often a clinical overlap between ET and parkinsonism may help clarify action tremor as it related to PD.
Acknowledgments
Support: This research is supported by the International Essential Tremor Foundation, the Michael J. Fox Foundation for Parkinson’s Research (The Prescott Family Initiative), the Arizona Biomedical Research Commission (contracts 4001, 0011 05-901, 1001), the Arizona Department of Health Services (contract 211002) and the National Institute on Aging (P30 AG19610).
Footnotes
Author roles:
Research project: A Conception B: Organization C: Execution
Statistical Analysis A: Design B: Execution C: Review and Critique
Manuscript: Writing of the first draft B: Review and Critique
Shill: 1A, 1B, 1C, 2A, 2B, 3A
Adler 1A, 2C, 3B
Beach 1C, 2C, 3B
Lue 1C, 2C, 3B
Caviness 1A, 2C, 3B
Sabbagh 1C, 2C, 3B
Sue1B, 2C, 3B
Walker 1A, 1C, 2A, 2B, 3B
Disclosures:
Dr. Shill is a consultant for Ipsen and Merz pharmaceuticals as well as Medtronic. She receives research support from Chelsea Therapeutics, Teva Neuroscience, Schering-Plough, Kalaco Scientific, Avid Radiopharmaceuticals, International Essential Tremor Foundation, Michael J Fox Foundation for Parkinson Research, Arizona Biomedical Research Commission, and NIH.
Dr. Adler has served as a consultant to Allergan, Biogen Idec, Eli Lilly, GlaxoSmithKline, Ipsen, Medtronic, Merck Serono, and Merz.
Dr. Beach has received research support from the Arizona Alzheimer’s Consortium, Glaxo-Smith-Klein, Inc., National Parkinson’s Foundation, National Institutes of Health, University of Arizona Department of Ophthalmology, Science Foundation Arizona, Arizona Biomedical Research Commission, Avid Radiopharmaceuticals, Inc. Schering-Bayer Pharmaceuticals, Inc. and the Michael J. Fox Foundation for Parkinson’s Research.
Dr. Lue has no disclosures.
Dr. Caviness has received an honorarium from Teva Pharmaceuticals. He reports no other disclosures.
Dr. Sabbagh serves as an advisory board member for Janssen/Pfizer, Amerisciences, Eisai, and GlaxoSmithKline. He receives royalties from Amerisciences and Wiley. He receives research support (clinical trials) from BMS, Avid, GE, Bayer, Baxter, Wyeth, Janssen, Lilly, and Medivation.
Ms. Sue and Dr. Walker have no disclosures.
Contributor Information
Holly A. Shill, Email: Holly.shill@bannerhealth.com.
Charles H. Adler, Email: cadler@mayo.edu.
Thomas G Beach, Email: Thomas.beach@bannerhealth.com.
Lih-Fen Lue, Email: Lihfen.lue@bannerhealth.com.
John N. Caviness, Email: jcaviness@mayo.edu.
Marwan N. Sabbagh, Email: Marwan.sabbagh@bannerhealth.com.
Lucia I Sue, Email: Lucia.sue@bannerhealth.com.
Douglas G. Walker, Email: Douglas.walker@bannerhealth.com.
References
- 1.Axelrad JE, Louis ED, Honig LS, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 3.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 4.Rajput AH, Robinson CA, Rajput ML, Rajput A. Cerebellar Purkinje cell loss is not pathognomonic of essential tremor. Parkinsonism Relat Disord. doi: 10.1016/j.parkreldis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 5.DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 6.Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 7.Rajput A, Hornykiewicz O, Deng Y, et al. Increased noradrenaline levels in essential tremor brain. Neurology. 2001;56:A302. [Google Scholar]
- 8.Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 9.Rajput A, Luo C. GABAa receptor alpha protein is increased in the cerebellar cortex of essential tremor patients. Neurology. 2009;72(Suppl3):A100. [Google Scholar]
- 10.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. 2. Baltimore: Williams and Wilkens; 1993. pp. 271–280. [Google Scholar]
- 11.Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology. 2000;54:S7. [PubMed] [Google Scholar]
- 12.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13 (Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 13.Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 14.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2007 doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2007 doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mally J, Baranyi M, Vizi ES. Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. J Neural Transm. 1996;103:555–560. doi: 10.1007/BF01273153. [DOI] [PubMed] [Google Scholar]
- 18.Boecker H, Weindl A, Brooks DJ, et al. GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study. J Nucl Med. 51:1030–1035. doi: 10.2967/jnumed.109.074120. [DOI] [PubMed] [Google Scholar]
- 19.Geraghty JJ, Jankovic J, Zetusky WJ. Association between essential tremor and Parkinson’s disease. Ann Neurol. 1985;17:329–333. doi: 10.1002/ana.410170404. [DOI] [PubMed] [Google Scholar]
- 20.Adler CH, Connor DJ, Hentz JG, et al. Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord. 25:642–646. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]