Abstract
Outer hair cells (OHCs) in the cochlea are crucial for the remarkable hearing sensitivity and frequency tuning. To understand OHC physiology and pathology, it is imperative to use mouse genetic tools to manipulate gene expression specifically in OHCs. Here, we generated 2 prestin knockin mouse lines: 1) the prestin-CreERT2 line, with an internal ribosome entry site (IRES)-CreERT2-FRT-Neo-FRT cassette inserted into the prestin locus after the stop codon, and 2) the prestin-CreERT2-NN line, with the FRT-Neo-FRT removed subsequently. We characterized the inducible Cre activity of both lines by crossing them with the reporter lines CAG-eGFP and Ai6. Cre activity was induced with tamoxifen at various postnatal ages and only detected in OHCs, resembling the endogenous prestin expression pattern. Moreover, prestin-CreERT2 +/− (heterozygotes) and +/+ (homozygotes) as well as prestin-CreERT2-NN +/− mice displayed normal hearing. These 2 prestin-CreERT2 mouse lines are therefore useful tools to analyze gene function in OHCs in vivo.
Keywords: Prestin, Cre recombinase, inner ear, outer hair cells
Introduction
Postnatal cochlear outer hair cells express high level of prestin, a membrane motor protein that undergoes voltage-dependent conformational changes and consequently cell length changes or electromotility (Zheng et al., 2000). Prestin-mediated OHC electromotility is necessary for cochlear amplification that is critical for remarkable hearing sensitivity and frequency tuning (Dallos et al., 2008; Liberman et al., 2002). In addition, prestin mRNA, not protein, is also detected at low levels in brain, testis and vestibular hair cell (Adler et al., 2003; Zheng et al., 2003). However, the function of many other proteins in OHC cytoskeleton regulation, synaptic formation and stereociliary mechanics, is still poorly understood, because of the early embryonic lethality or the complex pleiotropic effects associated with loss-of-function mutations in the corresponding genes. Moreover, OHCs are the most vulnerable cellular target in deafness caused by aging, acoustic injury and ototoxic drugs (Henderson et al., 2008; Ohlemiller and Frisina, 2008; Rybak et al., 2008). Therefore, studies of OHC physiology and pathology in vivo can be greatly benefited if inactivation of genes is inducible at specific times only in postnatal OHCs.
The Cre/loxP system has been widely applied to generate tissue-specific knockout mice. Many Cre mouse lines have been established for inner ear hair cells (Chow et al., 2006; Gao et al., 2004; Tian et al., 2006; Yang et al., 2010a; Yang et al., 2010b) (NIH Neuroscience Blueprint Cre Driver Network-Database http://www.credrivermice.org/database). These mouse lines expressing Cre at different developmental ages (embryonic to neonatal) are powerful tools to study gene function in inner ear hair cells. Although these Cre lines are specific to hair cells in the cochlea, they can also express Cre in other tissues. Furthermore, the recently reported hair cell Cre lines Atoh1-Cre and Gfi1-Cre cannot be induced in a temporally controlled manner (Yang et al., 2010a; Yang et al., 2010b). Atoh1-CreER™ (Cre recombinase fused with estrogen receptor or ER) transgenic mice can be induced postnatally to control the Cre activity in cochlear hair cells. However, Atoh1-CreER™ is downregulated after P7 in cochlear hair cells (Chow et al., 2006). Moreover, no OHC-specific Cre or CreER lines have been reported.
In the present study, we generated prestin-CreERT2 knockin mice that specifically express inducible Cre recombinase in OHCs at postnatal and adult ages. Prestin is first turned on at postnatal day 0–2 (P0–2) and maintained at high levels in adult OHCs. We generated 2 prestin knockin mouse lines: 1) prestin-CreERT2, in which an internal ribosome entry site (IRES)-CreERT2–FRT-Neo-FRT cassette is inserted into the prestin locus after the stop codon; and 2) prestin-CreERT2-NN, in which the FRT-Neo-FRT cassette is subsequently removed from prestin-CreERT2. The spatiotemporal inducible activity of Cre recombinase in both lines was examined by crossing prestin-CreERT2 mice with the CAG-eGFP or Ai6 reporter mice (Madisen et al., 2010), and the hearing thresholds of mice were also tested. We found inducible prestin-CreERT2 activity specifically in postnatal developing and mature OHCs. These mouse lines are therefore valuable tools for examining gene functions in OHCs.
RESULTS AND DISCUSSION
Generation of the prestin-CreERT2 knockin mouse line
The prestin-CreERT2 knockin mouse line was created by using ES cell gene targeting and FLP-FRT technology (Fig. 1a). CreERT2 is a modified version of CreER™ (Feil et al., 1997; Indra et al., 1999); FRT is the recognition site for Flp recombinase (Dymecki, 1996); and Neo is the selection marker for the ES cell screen. A cassette with an internal ribosome entry site (IRES)-CreERT2 and the Neo marker flanked by 2 FRT sites was inserted into the prestin locus after the stop codon (Fig. 1a). After homologous recombination, correctly targeted ES cells with IRES-CreERT2 and the Neo marker were confirmed by Southern blotting. Forty-five (~14%) of 325 ES cell clones showed correct homologous recombination. Five colonies were chosen from these 45 colonies for karyotyping and 3 colonies with normal diploid were chosen for injection into blastocysts. High-level chimeras were chosen to obtain germline transmission. By using the 3′ external probe, it was confirmed that the germline-transmitted prestin-CreERT2 knockin mice were correctly targeted by Southern blotting analysis of expected DNA fragments 23kb (wild type) and 10kb (targeted) from SpeI-digested tail genomic DNA (Fig. 1b). Also, PCR genotyping showed a wild-type allele (300 bp) and the prestin-CreERT2 allele (228-bp) (Fig. 1c). Both the prestin-CreERT2 +/− (heterozygous) and prestin-CreERT2 +/+ (homozygous) mice were viable and had no detectable developmental defects.
FIG. 1. Generation of prestin-CreERT2 knock-in mice.
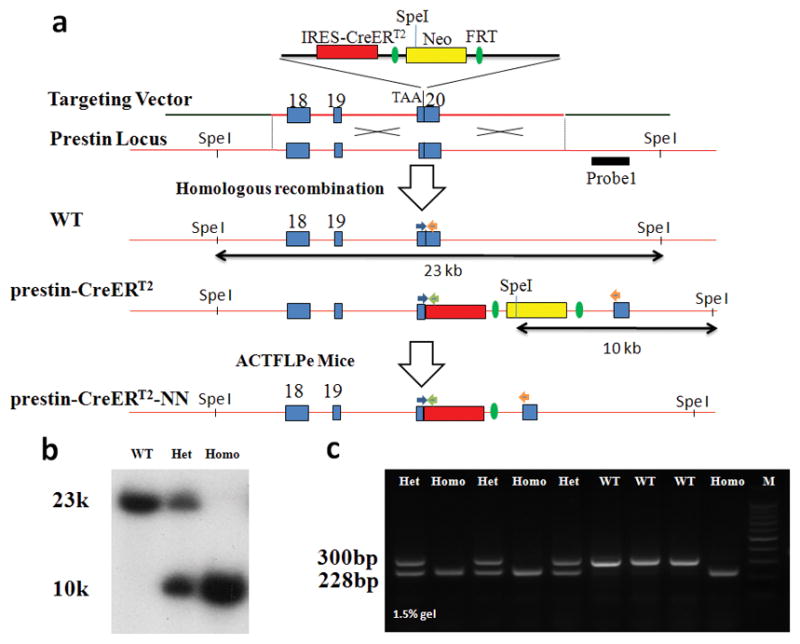
(a) Schematic diagram of generation of prestin-CreERT2 +/− and prestin-CreERT2–NN (No Neo cassette) knock-in mice. Solid blue rectangles represent exon18–20 of the prestin gene. The external 3′-Southern probe is shown as the black bar. Arrows indicate the approximate positions of the PCR genotyping primers. Abbreviations: IRES, internal ribosome entry site; CreERT2, Cre recombinase fused to a mutant estrogen ligand-binding domain; Neo, PGK-neo cassette; FRT, FLP recombinase target.
(b) Southern blot genotyping analysis of prestin-CreERT2 +/− and prestin-CreERT2 +/+ mice. Genomic tail DNA was digested with SpeI and a 3′-external probe indicated in (a) was used to identify the 23-kb wild-type and the 10-kb targeted DNA fragments.
(c) PCR genotyping shows 300-bp product (wild type) and 228-bp product (prestin-CreERT2 +/− mice).
OHC-specific, inducible Cre activity of prestin-CreERT2 knockin mice
To investigate the Cre recombinase inducible activity and tissue specificity of prestin-CreERT2 mice, prestin-CreERT2 +/− mice were bred with mice from reporter lines CAG-eGFP (Okabe et al., 1997) and Ai6 (Madisen et al., 2010). The eGFP or ZsGreen reporter gene is expressed only after Cre-mediated excision of a loxP-flanked stop cassette and the level of eGFP or ZsGreen expression is dictated by the CAG or ROSA26/CAG promoter, respectively. To determine CreERT2 activities are inducible, prestin-CreERT2;Ai6 mice were first intraperitoneally (IP) injected with tamoxifen at a dose of 3mg/40g body weight from P2 to P4. P7 cochleae from tamoxifen-injected mice displayed a strong green fluorescence in almost all OHCs (Fig. 2d–f), but those from mice not injected with tamoxifen did not (Fig. 2a–c).
FIG. 2.
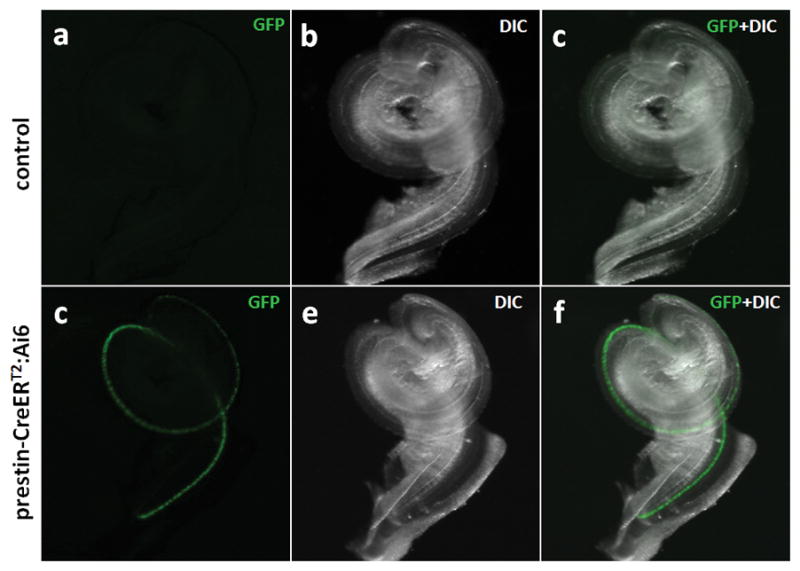
Inducible Cre recombination in prestin-CreERT2 +/− mice. Images of a whole cochlea (P7) from a prestin-CreERT2 +/−; Ai6 mouse without (a–c) and with (d–f) tamoxifen injection at P2–4.
Although in the developing cochlea, prestin can be detected as early as P0 (Belyantseva et al., 2000), we found no detectable reporter expression in the prestin-CreERT2 +/− cochleae at P6 when tamoxifen (at 3mg/40g body weight) was injected at P0. However, when tamoxifen (at 3mg/40g body weight) was injected once daily at P0–P2, prestin-CreERT2 +/− cochleae showed an increasing gradient in reporter expression from apex (16.9±5.1%), middle (35.7±3.2%), to base (58.4±6.7%) when analyzed at P6 (Fig. 3a–c‴). This gradual Cre expression pattern is consistent with endogenous prestin expression in different turns at P0–P2 (Legendre et al., 2008). After P2, almost all OHCs expressed the reporter with 2–5 continuous once-daily injections of tamoxifen (3mg/40g body weight) at different ages (Table 1). Only P10 apical-turn images from prestin-CreERT2 +/−;Ai6 mice induced tamoxifen at P5–7 are shown here because all image were similar at all turns and ages after P2 (Fig. 3d-d‴).
FIG. 3.
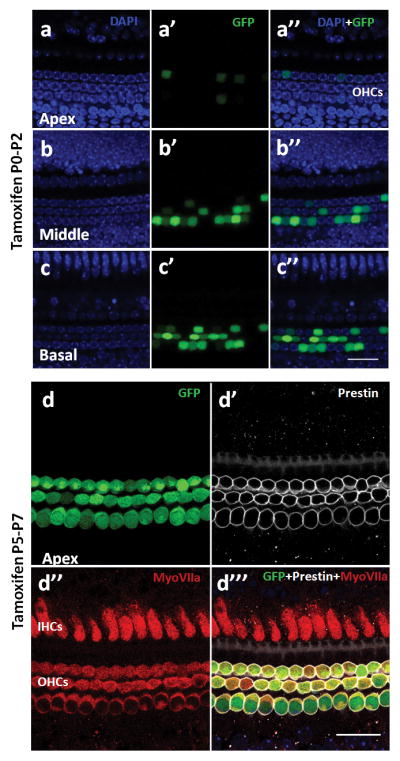
Cre recombination in all OHCs at various postnatal ages. (a-c″) prestin-CreERT2 +/− shows a gradual decrease pattern in reporter expression. prestin-CreERT2 +/−; CAG-eGFP mice were injected with tamoxifen (3mg/40g body weight) at P0–2. (d-d‴) prestin-CreERT2 +/−; Ai6 mice were injected with tamoxifen at P5–7. Images of GFP (d), anti-prestin (d′), anti-myo VIIa (d″), and merger (d‴). IHCs: Inner hair cells, OHCs: outer hair cells. Scale bar: 20 μm.
Table 1.
Injection schedule for inducible Cre activity in all OHCs
| Tamoxifen (once per day) | Injection day | Analysis day |
|---|---|---|
| 3mg/40g body weight | P2–P4 | P7–8 |
| 3mg/40g body weight | P5–P7 | P10–12 |
| 3mg/40g body weight | P9–P10 | P14–16 |
| 3mg/40g body weight | P14–P16 | P20–P21 |
| 3mg/40g body weight | P21–P25 | P28–P30 |
| 9mg/40g body weight | P21–P22 | P25–28 |
To reduce tamoxifen injection frequencies while achieving maximal (100%) Cre activity in OHCs, we increased tamoxifen doses to 9mg/40g body weight for adult mice. Cre activity was detected in all OHCs after a once-daily injection for 2 consecutive days.
Although prestin is highly specific for OHCs in the cochlea, it is expressed at low levels in the brain and testis (Zheng et al., 2003) and vestibular hair cells (Adler et al., 2003). Therefore, we checked for the presence of Cre activity in vestibular organs, testis, and brain in Prestin-CreERT2; CAG-eGFP mice. There is no tamoxifen-induced Cre activity in these tissues (data not shown). Either prestin level is too low, or the IRES cassette before the CreERT2 may reduce Cre expression in these tissues.
Normal hearing in prestin-CreERT2 mice
Prestin KO and prestin nonfunctional mutant mice demonstrate that prestin is vital for OHC function and hearing (Dallos et al., 2008; Liberman et al., 2002). To determine whether the prestin-CreERT2 line maintained normal hearing, auditory brainstem responses (ABRs) were tested at adult ages. Compared with wild-type mice, prestin-CreERT2 +/− and prestin-CreERT2 +/+ mice showed no hearing defects at 4 kHz to 32 kHz at 4–6 weeks (Fig. 4).
FIG. 4.
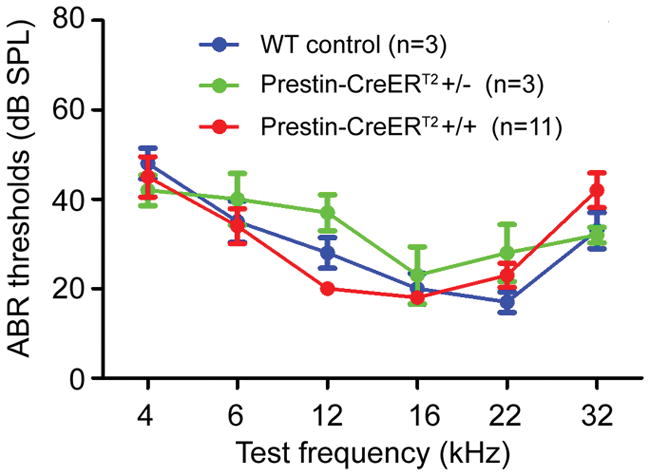
Auditory brainstem response (ABR) thresholds of prestin-CreERT2 +/− and prestin-CreERT2 +/+ and wild-type mice at 4–6 weeks. n, number of mice for ABR testing for each genotype. Bars: standard error of mean.
Inducible Cre activity and hearing threshold of prestin-CreERT2-NN mice
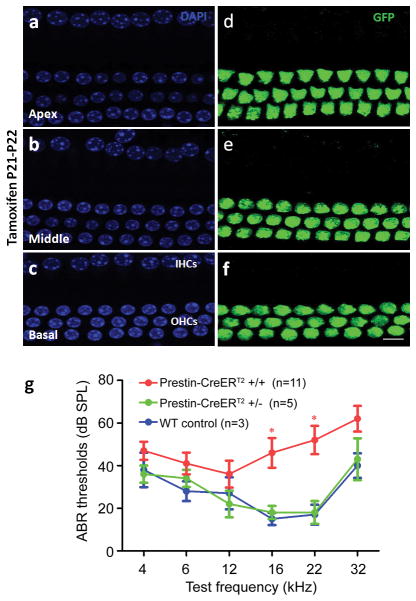
To determine whether the FRT-Neo-FRT cassette insertion had an effect on prestin-CreERT2 expression (Fig. 1a), we removed the FRT-Neo-FRT cassette by crossing prestin-CreERT2 mice with a ACTG-Flpe deletor (Rodriguez et al., 2000) and determined inducible Cre activity in cochleae of the resulting prestin-CreERT2-NN +/− mice that had deleted Neo and had no Flpe. These mice showed inducible Cre activity specific for all OHCs when the same tamoxifen injection protocols were used to prestin-CreERT2 mice (Table 1). All OHCs from Ai6; prestin-CreERT2–NN +/− mice had Cre activity with tamoxifen (9mg/40g body weight) induction at P21–P22 when analyzed at P28 (Fig. 5a–f). ABR measurements of prestin-CreERT2-NN +/− mice showed that they had normal hearing at all frequencies. Although 7 of 11 prestin-CreERT2-NN +/+ mice had threshold elevation 25–30dB at 16 and 22 kHz, the remaining 4 we tested had normal hearing (Fig. 5g). One possible reason for such variable high frequency threshold elevation in prestin-CreERT2–NN +/+ mice is the substantial contribution of C57BL6 strain background from the ACTG-Flpe deletor in the mixed background of prestin-CreERT2–NN +/+ mice (Davis et al., 2007; Rodriguez et al., 2000; Spongr et al., 1997; Zheng et al., 1999). Regardless, most applications use either prestin-CreERT2–or prestin-CreERT2–NN +/− that all display normal hearing.
FIG. 5.
Cre activity and hearing ability of prestin-CreERT2-NN +/− mice. Images of P28 prestin-CreERT2-NN +/−; Ai6 mouse inducted tamoxifen (9mg/40g) at P21–22, DAPI (a, b, c, at the nuclear level) and GFP (d, e, f at the cell body level), in each cochlear turn. Scale bar: 20 μm. (g) ABR thresholds of prestin-CreERT2-NN +/−, prestin-CreERT2-NN +/+ and wild-type mice at 4–6 weeks. N, number of mice for ABR testing for each genotype. Bars: standard error of mean. *: P < 0.05.
In conclusion, our results demonstrate that both prestin-CreERT2 and prestin-CreERT2-NN knockin mouse lines can be useful tools in manipulating gene function in postnatal and mature OHCs. Because of the strong inducible Cre activity in almost all OHCs at postnatal and adult ages, both the prestin-CreERT2 and prestin-CreERT2-NN +/− background display normal hearing, thus suitable for OHC-specific gene manipulations in most applications, and these mice will exhibit no additional hearing defects from the prestin locus. Moreover, when induced at P0–2, the inducible Cre activity of these mice displayed a gradient of reporter expression from the apex to base of the cochlea (Fig. 3a–c‴); such a mosaic gradient pattern of Cre activity could be useful for specific applications of OHC gene function analysis in vivo.
Materials and methods
Generation of prestin-CreERT2 knockin mice
A prestin bacterial artificial chromosome (BAC) was used for modification to generate targeting vector by the highly efficient recombineering-based method (Liu et al., 2003). The prestin-CreERT2 targeting vector was constructed by inserting IRES-CreRT2-FRT-Neo-FRT cassette after the stop coden in exon 20. The targeting vector, constructed in PL253 (Fig. 1a), was electroporated into 129/SvEvTac (129S6) ES cells (TL-1). For prestin-CreERT2 mice PCR genotyping, a PCR assay was developed with 3 primers: 5′-CACAAGTTGTGAATGACCTC -3′, 5′-GTTAAAGAGCGTAATCTGGAACA- 3′, and 5′-TAACTGCTAGCATTTCCCTT- 3′. To remove the neo cassette, prestin-CreERT2 +/− mice were bred with ACTFlpe mice. Prestin-CreERT2–NN indicated the prestin-CreERT2 no neo cassette. Prestin-CreERT2–NN +/− without Flpe mice were obtained by PCR genotyping and inducible Cre activity was further analyzed in these mice. The neo PCR genotyping protocol has been described in Parker et al (Parker et al., 2006).
Ai6, CAG-eGFP conditional reporter, and ACTFlpe mice were obtained from The Jackson Laboratory (Stock# 007906, Stock # 003291, and Stock# 003800, respectively) and PCR genotyping of the reporter mice were performed according to protocols provided by The Jackson Laboratory.
Tamoxifen injection
Tamoxifen (Sigma, St. Louis, MO) was dissolved in corn oil (Sigma) at a concentration of 5 mg/mL or 15 mg/mL at 37°C (approximately 1 h). Tamoxifen was filter-sterilized and stored at 4°C in the dark for up to 7 days. Pups were intraperitoneally injected by a 30G1/2(Thinner 3/10ml) ultrafine needle insulin syringe (Becton Dickinson) and adult mice by a 26G3/8 needle insulin syringe. All different combinations of mice were injected for 2–5 consecutive days with tamoxifen at a dose of 3 mg/40 g body weight or 9 mg/40 g mouse weight (Table 1).
Histologic Analysis and Immunocytochemistry
Mice were anesthetized with avertin and fixed by intracardial perfusion with 2%–4% paraformaldehyde (PFA). Whole-mount immunolabeling was performed as previously described (Liu et al., 2010). Briefly, whole-mount cochlear coils were dissected and labeled with anti-prestin (goat, 1:200, Santa Cruz Biotechnology, sc-22692); anti-myo7a (1:200, Proteus Bioscience, 25-6790). Alexa-conjugated secondary antibodies (Invitrogen) were used at a concentration of 1:1000 (donkey anti-rabbit Alexa Fluor 647, donkey anti-goat Alexa Fluor 568). Samples were mounted in mounting medium with DAPI (Vectashield; Vector Laboratories, Burlingame, CA).
Auditory brainstem response (ABR)
Auditory brainstem response (ABR) measurements were performed as previously described (Wu et al., 2004).
Statistical analyses
GraphPad Prism (GraphPad Software, San Diego, CA) was used for statistical analysis. Data were analyzed using two-way ANOVA, followed by a Student’s t test with a Bonferroni correction.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health (DC006471, DC008800, and CA21765), Office of Naval Research (N000140911014), the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital, and the National Natural Science Foundation of China (NSFC 30540420522). J. Fang is a recipient of Research Award from National Organization for Hearing Research Foundation. J. Zuo is a recipient of The Hartwell Individual Biomedical Research Award.
We thank members of the Zuo lab for discussion and critical comments. We acknowledge Pierre Chambon (Institut Genetique Biologie Moleculaire Cellulaire) for providing the CreERT2.
References
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. doi: 10.1016/s0378-5955(03)00192-8. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci. 2000;20:RC116. doi: 10.1523/JNEUROSCI.20-24-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LM, Tian Y, Weber T, Corbett M, Zuo J, Baker SJ. Inducible Cre recombinase activity in mouse cerebellar granule cell precursors and inner ear hair cells. Dev Dyn. 2006;235:2991–2998. doi: 10.1002/dvdy.20948. [DOI] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RR, Kuo MW, Stanton SG, Canlon B, Krieg E, Alagramam KN. N-Acetyl L-cysteine does not protect against premature age-related hearing loss in C57BL/6J mice: a pilot study. Hear Res. 2007;226:203–208. doi: 10.1016/j.heares.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dymecki SM. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:6191–6196. doi: 10.1073/pnas.93.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Gao J, Wu X, Zuo J. Targeting hearing genes in mice. Brain Res Mol Brain Res. 2004;132:192–207. doi: 10.1016/j.molbrainres.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Henderson D, Hu B, Bielefeld E. Patterns and Mechanisms of Noise-Induced Cochlear Pathology. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection, and Repair. New York: Spinger; 2008. pp. 195–217. [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre K, Safieddine S, Kussel-Andermann P, Petit C, El-Amraoui A. alphaII-betaV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. J Cell Sci. 2008;121:3347–3356. doi: 10.1242/jcs.028134. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Owen T, Zhang L, Zuo J. Dynamic expression pattern of Sonic hedgehog in developing cochlear spiral ganglion neurons. Dev Dyn. 2010;239:1674–1683. doi: 10.1002/dvdy.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Frisina aRD. Age-Related Hearing Loss and Its Cellular and Molecular Bases. In: Schacht Jochen, Poper AN, Fay aRR., editors. Auditory Trauma, Protection, and Repair. New York: Springer; 2008. pp. 145–194. [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Parker LL, Gao J, Zuo J. Absence of hearing loss in a mouse model for DFNA17 and MYH9-related disease: the use of public gene-targeted ES cell resources. Brain Res. 2006;1091:235–242. doi: 10.1016/j.brainres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Talaska AE, Schacht J. Drug-Induced Hearing Loss. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection, and Repair. New York: Springer; 2008. pp. 219–256. [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Tian Y, James S, Zuo J, Fritzsch B, Beisel KW. Conditional and induciblegene recombineering in the mouse inner ear. Brain Res. 2006;1091:243–254. doi: 10.1016/j.brainres.2006.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gao J, Guo Y, Zuo J. Hearing threshold elevation precedes hair-cell loss in prestin knockout mice. Brain Res Mol Brain Res. 2004;126:30–37. doi: 10.1016/j.molbrainres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Yang H, Gan J, Xie X, Deng M, Feng L, Chen X, Gao Z, Gan L. Gfi1-Cre knock-in mouse line: A tool for inner ear hair cell-specific gene deletion. Genesis. 2010a;48:400–406. doi: 10.1002/dvg.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010b;48:407–413. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Long KB, Matsuda KB, Madison LD, Ryan AD, Dallos PD. Genomic characterization and expression of mouse prestin, the motor protein of outer hair cells. Mamm Genome. 2003;14:87–96. doi: 10.1007/s00335-002-2227-y. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]