Abstract
Although vascular invasion is a well-established indicator of poor prognosis for patients with infiltrating ductal adenocarcinoma of the pancreas (PDAC), the histopathologic characteristics of vascular invasion are not well described. Hematoxylin and eosin stained slides from 209 surgically resected infiltrating PDACs were systematically evaluated for the presence or absence of microscopic vascular invasion. For the cases with vascular invasion, we further categorized the histologic pattern of invasion into conventional and pancreatic intraepithelial neoplasia-like (PanIN-like). In addition, several histopathologic factors in the surrounding blood vessels, including lymphocytic infiltration and luminal fibrosis were carefully accessed. Data were compared to clinicopathologic variables, including patient survival. Microscopic vascular invasion was observed in 136 of the 209 PDACs (65.1%). Vascular invasion mimicking pancreatic intraepithelial neoplasia (PanIN-like invasion) was observed in 94 of the 136 cases (69.1%) with vascular invasion. Microscopic vascular invasion was associated with increased tumor size (p=0.04), higher pT classification (p=0.003), lymph node metastasis (p<0.0001), and perineural invasion (p=0.005). Vascular invasion was inversely correlated with neo-adjuvant therapy (p<0.0001). Examination of adjacent blood vessel revealed that peritumoral blood vessels with intimal lymphocytes (p=0.002), intimal (p=0.007) and medial (p=0.001) fibrosis, and cancer cells in vascular wall (p<0.0001), were all highly associated with intraluminal vascular invasion. In univariate analysis, patients whose cancers had microscopic vascular invasion (median survival, 12.9 months) had a significantly worse survival than did patients with carcinomas without vascular invasion (17.6 months; p=0.01, log-rank test). Microscopic vascular invasion is a poor prognostic indicator and can histologically mimic PanIN.
Keywords: Vascular invasion, Pancreatic ductal adenocarcinoma, Pancreatic intraepithelial neoplasia
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDACs) is one of the deadliest forms of cancer. In 2011, an estimated 44,040 Americans will be diagnosed with pancreatic cancer, and approximately 37,660 will die from it.14 At the time of diagnosis, roughly 80% of patients with pancreatic cancer have unresectable disease because their cancer has spread beyond the pancreas.10
Vascular invasion is obviously one route for pancreatic cancer to spread beyond the pancreas and it should not be surprising that vascular invasion has been shown to be a poor prognosticator in a number of studies of surgically resected pancreatic cancer.1, 4, 6,8,9,12,15 The histopathologic characteristics of vascular invasion are not well defined.
Recently, Bandyopadhyay et al. described “isolated solitary ducts” in histological sections of the retroperitoneal resection margin and peripancreatic soft tissue and reported that they were diagnostic of PDAC.3 A small fraction of the isolated solitary ducts they identified were foci in which the neoplastic cells had completely replaced the vascular endothelium in the lumen of a blood vessel, a finding they called “carcino-endothelialization.”3 These “isolated solitary ducts” can mimic pancreatic intraepithelial neoplasia (PanIN) lesions.3 We have also observed pancreatic cancer cells growing inside of muscular vessels and replacing endothelial cells, and wished to determine how common this finding is in pancreatic cancer.
The purpose of this study is to determine the prevalence of microscopic vascular invasion with PanIN-like features in surgically resected PDACs, to determine if this finding has any prognostic significance, and to determine if other histopathologic features are associated with microscopic intravascular invasion.
MATERIALS AND METHODS
Institutional Review Board approval was granted for this study. A series of 209 infiltrating PDAC cases surgically resected from 2004 to 2010 at the Johns Hopkins Hospital was collected and evaluated for vascular invasion. All available slides were re-evaluated by two of the authors (S-MH and RHH). Particular attention was paid to blood vessels containing smooth muscle fibers. Muscular venules, small collecting veins, and medium sized veins were evaluated for microscopic vascular invasion.5 Vascular invasion was defined by presence of neoplastic cells inside of the lumen of blood vessel structures containing circumferential smooth muscle layers in the peritumoral pancreas, intra-tumoral tissue or in fibro-adipose tissues. In order to exclude lymphatic invasion from our analyses, vascular invasion in very small caliber vessels was not scored, nor was vascular invasion of vascular structures without circumferential smooth muscle fibers.
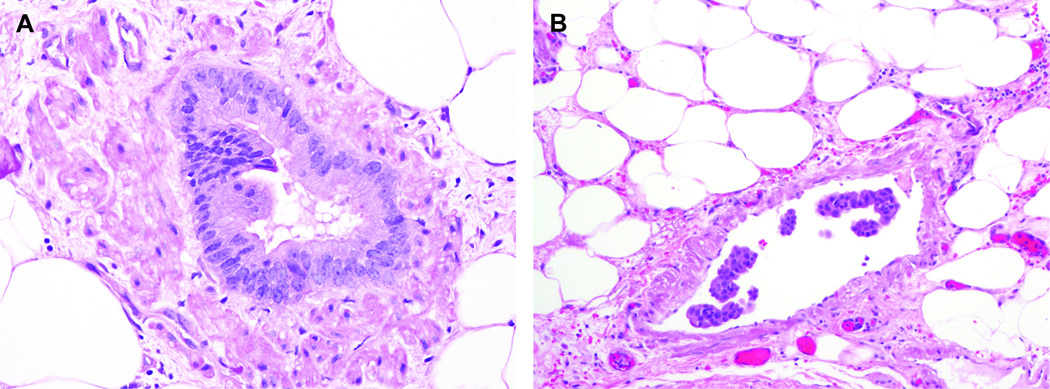
Foci of vascular invasion were then classified into one of two patterns; “vascular invasion with PanIN-like features” and “conventional vascular invasion.” “Vascular invasion with PanIN-like features” was defined as neoplastic cells completely replacing a full circumference of the endothelium and forming a new luminal structure inside of the blood vessel (Figure 1A). “Conventional vascular invasion” encompassed all other histologic forms of intravascular invasion in which the neoplastic cells did not completely replace the endothelium and form a new lumen (Figure 1B). Because vascular invasion with PanIN-like features had not been previously systematically evaluated, when cases showed both PanIN-like and conventional vascular invasion, we counted those cases as having vascular invasion with PanIN-like features.
Figure 1.
Representative images of PanIN-like vascular invasion and conventional vascular invasion. When the invasive neoplastic cells form complete luminal structures inside of the blood vessels, we classified the case as having “PanIN-like vascular invasion” (A). When the neoplastic cells were inside of the blood vessel but did not form complete lumina, we classified the case as having “conventional vascular invasion” (B).
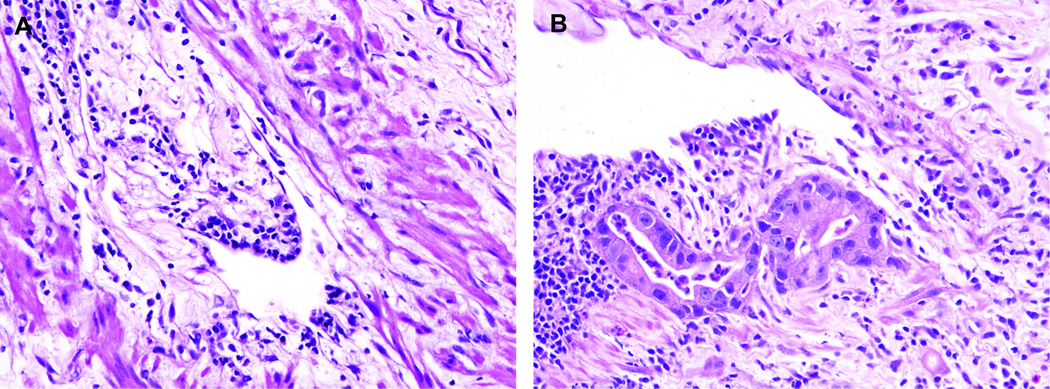
Next, in order to identify histopathologic characteristics associated with intravascular invasion, several other histopathologic features, including lymphocytic infiltration and edema/fibrosis in the vascular lumina, in the intima, and in the media, and invasion of cancer cells in the vascular wall were carefully evaluated in the blood vessels in the peritumoral non-cancerous tissue. More than 5 lymphocytes were required to be in the lumen, intimal layer, or muscular layer for the designation of lymphocytic infiltration (Figure 2).
Figure 2.
Features associated with nearby vascular invasion include (A) lymphocytic infiltration and edema of the intima; and (B) lymphocytic infiltration of the intima, edema/fibrosis of the intima and media, and invasion of cancer cells in the vascular wall.
The presence or absence of vascular invasion as well as PanIN-like features of vascular invasion were compared with other clinicopathologic variables, such as patient gender, age, and ethnicity, tumor size, location, differentiation, pT classification, lymph node status, perineural invasion and neoadjuvant therapy. Data regarding adjuvant radiation-/chemotherapy regimens and response to the same were not collected for our study. Overall survival was the primary endpoint and determined as the time from primary surgery until death of the patients or last patient contact.
Statistical analyses were performed with SPSS version 17 (SPSS Inc, Chicago, IL). Mean values was compared by Student’s t-test or simple ANOVA. Associations between vascular invasion and other clinicopathologic factors were compared using Chi-square and/or Fisher`s exact tests. Overall patient survival was defined as the time from surgical resection of each patient’s pancreatic ductal adenocarcinoma to their death or the date of last follow-up. Survival rates were calculated using the Kaplan-Meier method and statistical significance was evaluated using the log-rank test. The Cox proportional hazards regression models were used to investigate the significance of prognostic factors. Patients who died less than 30 days postoperatively were excluded from outcome analyses. A p-value of < 0.05 was considered statistically significant.
RESULTS
Characteristics of the Cases
The characteristics of the cases are summarized in Table 1. Briefly, the mean age (±std. deviation) of the patients was 66.0±10.5 years. Mean tumor size was 3.3±1.4 cm (range, 1.5–9.5 cm). Of the 209 cases included in this study, 140 (50.2%) were men, 185 (88.5%) were Caucasians, 176 (84%) were located in the pancreatic head, 149 (71.3%) had lymph node metastasis, 93 (44.5%) were >3cm, 137 (65.6%) were T3 classification, 85 (40.7%) were poorly differentiated, 49 (23.4%) had positive margins, and 189 (90.4%) had perineural invasion. Twenty-three of the patients had received neoadjuvant therapy prior to resection. The follow-up period ranged from 0.2 to 73.2 months (mean, 16.3 months). 133 (63.6%) of the 209 patients were followed to death. The overall median survival was 16.7 months and 8.7% of patients were alive after 5 years of follow-up.
Table 1.
Clinicopathologic characteristics of the cases with ductal adenocarcinoma of the pancreas. The table is also stratified based on vascular invasion status.
| Variables | Vascular invasion | P-value | ||
|---|---|---|---|---|
| Absent | Present | |||
| Gender | Male (N=104) | 34 (33%) | 70 (67%) | 0.66 |
| Female (N=105) | 38 (36%) | 66 (64%) | ||
| Ethnicity | Caucasian (N=185) | 9 (38%) | 15 (62%) | 0.82 |
| Non-Caucasian (N=24) | 63 (34%) | 122 (66%) | ||
| Location | Head (N=176) | 56 (32%) | 120 (68%) | 0.07 |
| Body or tail (N=33) | 16 (49%) | 17 (51%) | ||
| Size | <3cm (N=87) | 37 (43%) | 50 (57%) | 0.04* |
| ≥3cm (N=120) | 34 (28%) | 86 (72%) | ||
| Differentiation | Well to moderate (N=124) | 42 (34%) | 82 (66%) | 0.88 |
| Poor (N=85) | 30 (35%) | 55 (65%) | ||
| pT classification | pT1+pT2 (N=64) | 32 (50%) | 32 (50%) | 0.003* |
| pT3+pT4 (N=145) | 40 (28%) | 105 (72%) | ||
| Lymph node metastasis |
pN0 (N=60) | 37 (62%) | 23 (38%) | <0.0001* |
| pN1 (N=149) | 35 (24%) | 114 (76%) | ||
| Perineural invasion |
Absent (N=20) | 13 (65%) | 7 (35%) | 0.005* |
| Present (N=189) | 59 (31%) | 130 (69%) | ||
| Neoadjuvant therapy |
Absent (N=186) | 56 (30%) | 130 (70%) | <0.0001* |
| Present (N=23) | 16 (70%) | 7 (30%) | ||
Significant at the level of p<0.05.
Microscopic Vascular Invasion
Microscopic vascular invasion was observed in 136 (65.1%) of the 209 cases. The associations of microscopic vascular invasion with clinicopathologic characteristics are summarized in Table 1. Microscopic vascular invasion was more frequently observed in patients with larger tumors (≥3cm, 72%, 86/120 cases) than those with smaller tumors (<3cm, 57%, 50/87 cases; p=0.04, Chi-square and Fisher`s exact tests).
Because of the small number of pT1 and pT4 cases, we merged pT1 with pT2 cancers and classified this merged group as localized cancer (pT1+pT2). Similarly, we merged stage pT3 with pT4 cancers under the umbrella term advanced cancer (pT3+pT4). Microscopic vascular invasion was more commonly demonstrated in patients with advanced cancer (pT3+pT4, 72%, 105/145) than those with localized cancer (pT1+pT2, 50%, 32/64; p=0.003). Microscopic vascular invasion was more commonly observed in patients with lymph node metastasis (76%, 114/149 cases) than those without lymph node metastasis (38%, 23/60; p<0.0001) and was more frequent in those with perineural invasion (69%, 130/189 cases) than those without perineural invasion (35%, 7/20 cases; p=0.005). The cancers resected from patients who had received neo-adjuvant therapy were less likely to have microscopic vascular invasion (30%, 7/23 cases) than were those resected from patients who did not receive neoadjuvant therapy (70%, 130/186 cases; p<0.0001). Also, because of the small sample size of well-differentiated cancers, we merged well and moderately differentiated cancers into the group well-moderately differentiated cancer in our analyses of associations between vascular invasion and differentiation. There was no association between microscopic vascular invasion and gender and ethnicity of the patients, location of the tumor, and differentiation.
In addition to identifying microscopic intraluminal vascular invasion, we also examined the histopathologic changes in the adjacent uninvolved blood vessels. These results are summarized in Table 2. When peritumoral blood vessels showed intimal lymphocytic infiltration with more than 5 lymphocytes (80%, 56/70 cases; p=0.002), intimal fibrosis (73%, 92/127 cases; p=0.007) or medial fibrosis (78%, 68/87 cases; p=0.001), or cancer cells within the vascular wall but not within the lumen (86%, 66/77 cases; p<0.0001), these features were highly associated with presence of microscopic vascular invasion elsewhere in the case. Representative examples of each of these features are depicted in Figure 2. Other histologic features, such as fibrous luminal occlusion, more than 5 of lymphocytes within blood vessel lumen or in the medial wall, were not associated with presence of microscopic vascular invasion nearby blood vessels.
Table 2.
Associations between vascular invasion status and histologic characteristics of blood vessels around present vascular invasion
| Histologic factors | Vascular invasion | P-value | ||
|---|---|---|---|---|
| Absent | Present | |||
| Luminal lymphocytes (≥5) |
Absent (N=128) | 50 (39%) | 78 (61%) | 0.10 |
| Present (N=81) | 22 (27%) | 59 (73%) | ||
| Luminal obstruction |
Absent (N=122) | 45 (37%) | 77 (63%) | 0.46 |
| Present (N=87) | 27 (31%) | 60 (69%) | ||
| Intimal lymphocytes (≥5) |
Absent (N=139) | 58 (42%) | 81 (58%) | 0.002* |
| Present (N=70) | 14 (20%) | 56 (80%) | ||
| Intimal fibrosis | Absent (N=83) | 38 (46%) | 45 (54%) | 0.007* |
| Present (N=126) | 34 (27%) | 92 (73%) | ||
| Medial lymphocytes (≥5) |
Absent (N=153) | 57 (37%) | 96 (63%) | 0.19 |
| Present (N=56) | 15 (27%) | 41 (73%) | ||
| Medial fibrosis | Absent (N=122) | 53 (43%) | 69 (57%) | 0.001* |
| Present (N=87) | 19 (22%) | 68 (78%) | ||
| Cancer cells in vascular wall |
Absent (N=132) | 61 (46%) | 71 (54%) | <0.0001* |
| Present (N=77) | 11 (14%) | 66 (86%) | ||
Significant at the level of p<0.05.
PanIN-like Microscopic Vascular Invasion
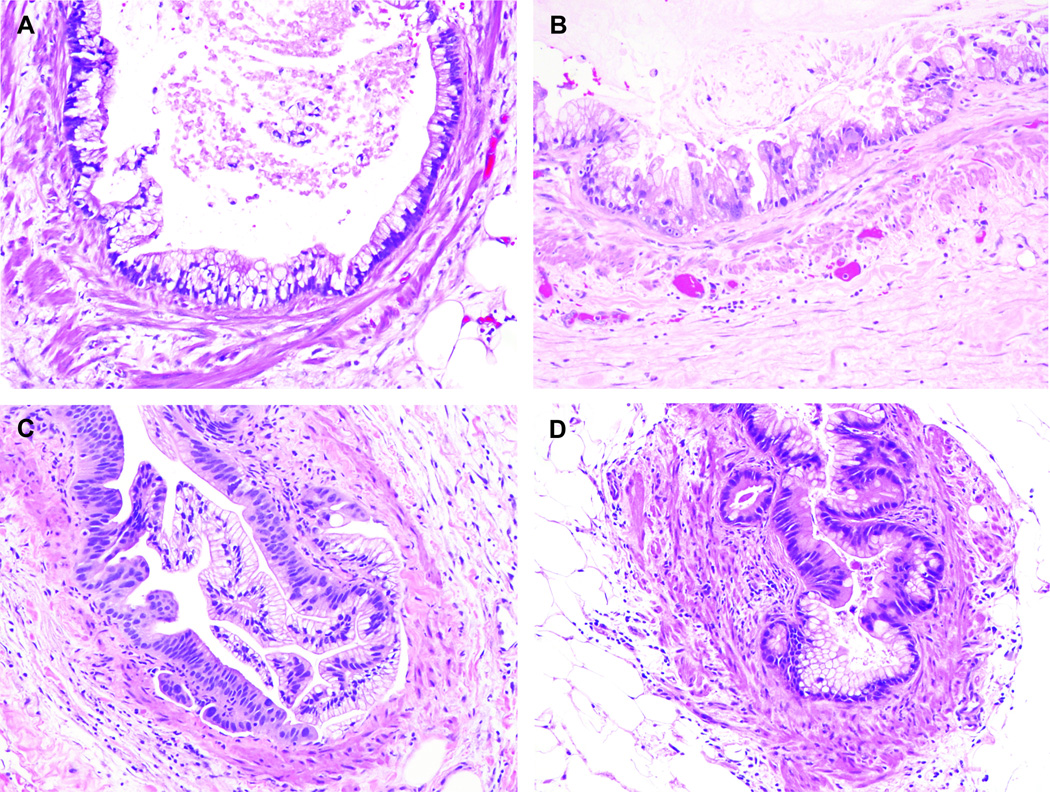
Vascular invasion with PanIN-like features were observed in 94 of 136 cases (69.1%) with vascular invasion. Representative images of vascular invasion with PanIN-like features are depicted in Figure 3. The clinicopathologic characteristics of the patients with microscopic vascular invasion with PanIN-like features are summarized in Table 3. Microscopic vascular invasion with PanIN-like features was more commonly observed in patients with pancreatic head cancer (50.0%, 88/176) than those in body or tail cancer (18.2%, 6/33, p=0.001). Microscopic vascular invasion with PanIN-like features was more commonly demonstrated in patients with advanced cancer (pT3+pT4, 51.0%, 74/145) than those with localized cancer (pT1+pT2, 31.3% 20/64; p=0.01). Microscopic vascular invasion with PanIN-like features was more commonly seen in patients with lymph node metastasis (55.7%, 83/149 cases) than it was in patients without lymph node metastasis (18.3%, 11/60; p<0.0001).
Figure 3.
Representative images of PanIN-like vascular invasion. Like PanIN, the neoplastic cells form papillary structures and have nuclear and cytologic atypia. However, these PanIN-like structures are surrounded by smooth muscle layer. Therefore, these represent PanIN-like vascular invasion.
Table 3.
Vascular invasion with PanIN-like features and clinicopathologic characteristics in pancreatic ductal adenocarcinoma patients
| Variables | Vascular invasion | P-value | ||
|---|---|---|---|---|
| Absent | Present | |||
| Gender | Male (N=104) | 55 (52.9%) | 49 (47.1%) | 0.58 |
| Female (N=105) | 60 (57.1%) | 45 (42.9%) | ||
| Ethnicity | Caucasian (N=185) | 104 (56.2%) | 81 (43.8%) | 0.39 |
| Non-Caucasian (N=24) | 11 (45.8%) | 13 (54.2%) | ||
| Location† | Head (N=176) | 88 (50%) | 88 (50%) | 0.001* |
| Body or tail (N=33) | 27 (82%) | 6 (18%) | ||
| Size | <3cm (N=87) | 51 (59%) | 36 (41%) | 0.32 |
| ≥3cm (N=120) | 62 (52%) | 58 (48%) | ||
| Differentiation | Well to moderate (N=124) | 63 (51%) | 61 (49%) | 0.14 |
| Poor (N=85) | 52 (61%) | 33 (39%) | ||
| pT classification | pT1+pT2 (N=64) | 44 (69%) | 20 (31%) | 0.01* |
| pT3+pT4 (N=145) | 71 (49%) | 74 (51%) | ||
| Lymph node metastasis |
pN0 (N=60) | 49 (81.7%) | 11 (18.3%) | <0.0001* |
| pN1 (N=149) | 66 (44.3%) | 83 (55.7%) | ||
| Perineural invasion |
Absent (N=20) | 15 (75.0%) | 5 (25.0%) | 0.06 |
| Present (N=189) | 100 (52.9%) | 89 (47.1%) | ||
| Neoadjuvant therapy |
Absent (N=186) | 98 (52.7%) | 88 (47.3%) | 0.07 |
| Present (N=23) | 17 (73.9%) | 6 (26.1%) | ||
Significant at the level of p<0.05.
Information regarding tumor size was available from 207 cases
Patients Survival and Vascular Invasion
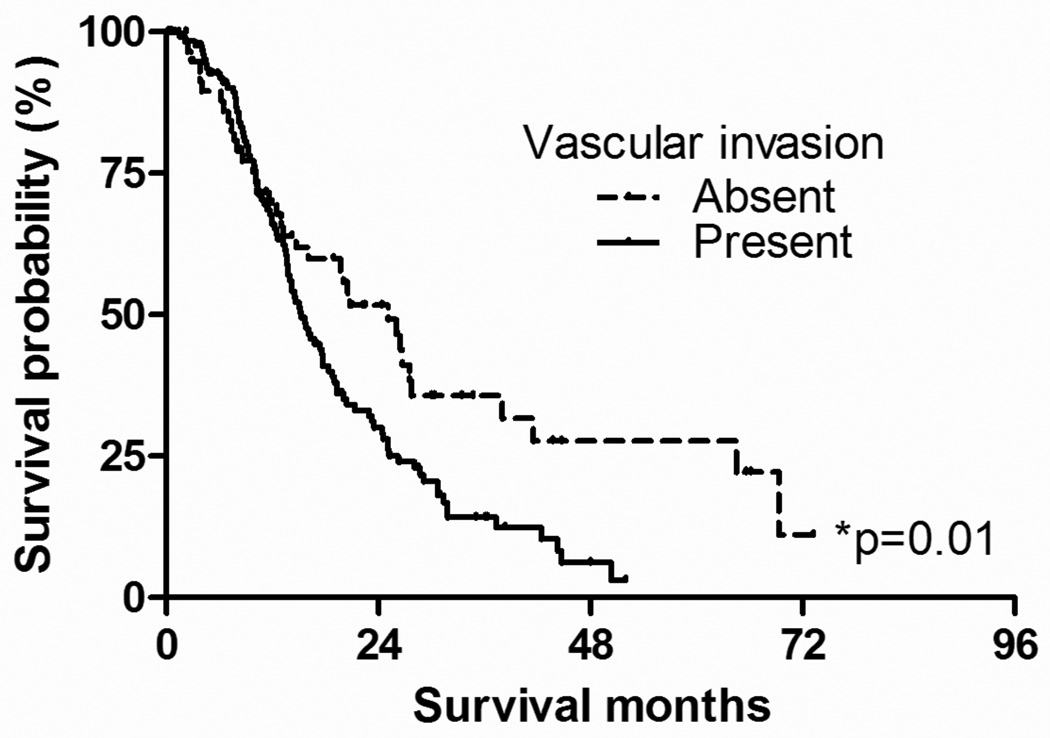
Kaplan-Meyer survival analysis comparing patients with and without vascular invasion is summarized on Figure 4. The median survival for patients with any form of vascular invasion (N=137) was significantly shorter (median survival, 12.9 months; 1-year survival rate, 66.0%; 3-year survival rate, 14.2%) than for patients without vascular invasion (N=72; median survival time, 17.6 months; 1-year survival rate, 67.8%; 3-year survival rate, 31.7%; log-rank test, p=0.01).
Figure 4.
Kaplan-Meyer survival analysis comparing patients with and without vascular invasion. The median survival for patients with vascular invasion (N=137) was significantly shorter (median survival, 12.9 months; 1-year survival rate, 66.0%; 3-year survival rate, 14.2%) than those without vascular invasion (N=72; median survival time, 17.6 months; 1-year survival rate, 67.8%; 3-year survival rate, 31.7%; log-rank test, p=0.01).
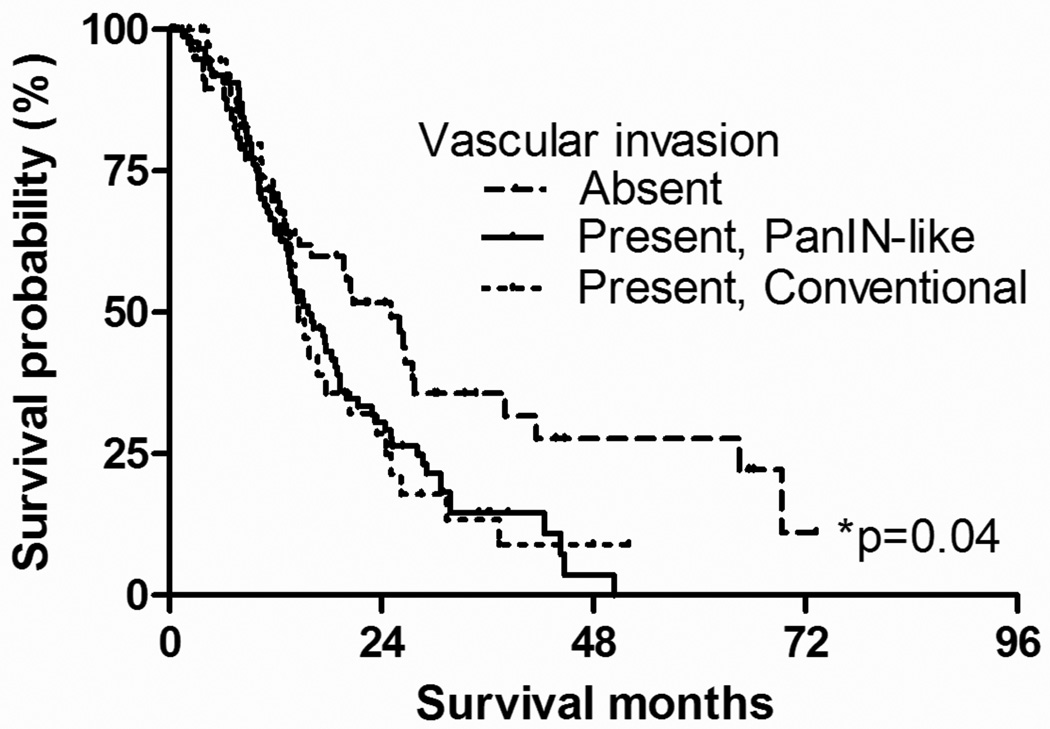
We further compared the survival of the patients with different histologic types of vascular invasion (Figure 5). The median survival for patients with PanIN-like features of vascular invasion was 15.7 months. The median survival for patients with conventional vascular invasion was 14.6 months, while that for patients without vascular invasion was 25.1 months. There was a significant survival difference among 3 groups (p=0.036, log-rank test, overall comparison). When compared in a pair-wise manner, there was a significant survival difference between patients with PanIN-like vascular invasion and those without vascular invasion (p=0.015). There was a marginal significance between patients with conventional vascular invasion and those without vascular invasion (p=0.059). There was no difference in survival between patients with PanIN-like and conventional vascular invasion (p=0.96).
Figure 5.
Kaplan-Meyer survival analysis according to the histologic type of vascular invasion. The median survival for patients with PanIN-like features of vascular invasion was 15.7 months. The median survival for patients with conventional vascular invasion was 14.6 months, while that of patients without vascular invasion was 25.1 months. There was a significant survival difference among 3 groups (p=0.036, log-rank test, overall comparison). When compared in a pair-wise manner, there was a significant survival difference between patients with PanIN-like vascular invasion and those without invasion (p=0.015). There was a marginal significance between patients with conventional vascular invasion and those without invasion (p=0.059). There was no difference between patients with PanIN-like feature and conventional vascular invasion (p=0.96).
Univariate Analysis for Other Clinicopathologic Factors
By univariate survival analysis, the following clinicopathologic factors were associated with shorter patient survival (Table 4): poor tumor differentiation, advanced stage (pT3+pT4), the presence of lymph node metastasis, and the presence of microscopic vascular invasion.
Table 4.
Univariate analysis of clinicopathologic factors
| Variable | Case number | Median survival months | 95% CI | P-value |
|---|---|---|---|---|
| Differentiation | <0.001* | |||
| Well to moderate | 124 | 22.9 | 17.9–27.9 | |
| Poor | 85 | 13.2 | 11.9–14.6 | |
| Location | 0.81 | |||
| Head | 176 | 17.7 | 14.5–20.8 | |
| Body or tail | 33 | 14.6 | 13.9–15.3 | |
| Size† | 0.06 | |||
| <3cm | 87 | 19.9 | 15.3–24.7 | |
| ≥3cm | 120 | 15.0 | 11.7–18.3 | |
| pT classification | 0.002* | |||
| pT1+pT2 | 64 | 25.2 | 16.6–33.8 | |
| pT3+pT4 | 145 | 15.0 | 12.6–17.5 | |
| Lymph node metastasis | 0.03* | |||
| pN0 | 60 | 25.2 | 18.2–32.2 | |
| pN1 | 149 | 14.7 | 12.5–16.8 | |
| Vascular invasion | 0.01* | |||
| Absent | 72 | 25.1 | 17.6–32.5 | |
| Present | 137 | 15.3 | 12.9–17.6 | |
| Perineural invasion† | 0.49 | |||
| Absent | 26 | 20.8 | 11.7–29.8 | |
| Present | 301 | 15.6 | 13.5–17.6 | |
| Resection margin | 0.15 | |||
| Positive | 49 | 16.1 | 11.4–20.8 | |
| Negative | 160 | 17.6 | 13.4–21.7 | |
| Neoadjuvant therapy | 0.85 | |||
| Presence | 23 | 15.8 | 6.8–24.9 | |
| Absence | 186 | 16.8 | 13.5–20.0 |
Significant at the level of <0.05
Information regarding tumor size was available from 207 cases
Multivariate Analysis
Multivariate analyses were performed to assess which factors remained independent predictors of survival after adjusting for factors that were significant by univariate analyses. Differentiation of tumor (p=0.001, Cox regression model) and pT classification were independently prognostic in our model (Table 5). Vascular invasion (p=0.07) showed marginal significance.
Table 5.
Multivariate analysis for prognosis
| Variable | Relative risk | 95% CI | P-value |
|---|---|---|---|
| Differentiation (well to moderate Vs poor) | 1.97 | 1.38 – 2.82 | <0.001* |
| pT classification (pT1+pT2 Vs pT3+pT4) | 1.76 | 1.16 – 2.67 | 0.008* |
| Lymph node metastasis | 1.12 | 0.76 – 1.80 | 0.47 |
| Vascular invasion | 1.46 | 0.96 – 2.23 | 0.07 |
Significant at the level of <0.05
DISCUSSION
Pancreatic intraepithelial neoplasia (PanIN) is a histologically distinct precursor lesion to invasive ductal adenocarcinoma of the pancreas.7 PanINs are common lesions, particularly in the elderly and have been reported in as many as 82% of pancreata harboring an invasive pancreatic cancer.2, 13
We report here a pattern of vascular invasion by ductal adenocarcinoma that histologically mimics PanIN lesions. Although these foci of PanIN-like vascular invasion can be extremely well-differentiated with minimal cytologic and architectural atypia, they can be recognized by the presence of an often subtle circumferential layer of smooth muscle fibers in the wall of the lesion (Figure 3).
These lesions are important to recognize for several reasons. First, one doesn’t want to mistake an invasive carcinoma for a non-invasive precursor on biopsy, or at a surgical margin.11 Second, even if the diagnosis of invasive carcinoma is correctly established, PanIN-like vascular invasion is important to recognize because, just as is true for more conventional vascular invasion, it is a poor prognostic indicator (Figure 5).1,6,8,9,12,15
It should be noted that PanIN-like vascular invasion can appear extremely well-differentiated, even in cases in which the invasive cancer is moderate or poorly differentiated. The absence of significant nuclear and architectural atypia in these lesions can make them extremely hard to recognize, and some are even “PanIN-1A like.” The mechanisms underlying this paradoxical differentiation are unclear but may relate to growth in a pre-existing luminal structure. Certainly it contrasts to the “epithelial-mesenchymal transformation” one might expect based on animal models.
In the present study, we also evaluated several histopathologic features in peritumoral blood vessels in order to identify features that might be suggestive of nearby intraluminal vascular invasion. We observed 3 distinct histologic findings, including intimal lymphocytic infiltration, intimal and medial fibrosis, and cancer cells in the muscular wall of blood vessel that are associated with intraluminal vascular invasion in adjacent vessels. These results suggest that a careful search for vascular invasion is in order when these features are found.
A limitation of this study is that only hematoxylin and eosin stained (H&E) stained slides were examined. We were able to evaluate muscular venules, small collecting veins, and medium sized veins which contain smooth muscle layer. We could not, however, evaluate smaller caliber-venules or venous capillaries which contain few smooth muscle fibers, nor did we evaluate lymphatic invasion, because lymphatics do not contain smooth muscle layers. A previous study performed by Bandyopadhyay et al. described “isolated solitary ducts” in the sections of retroperitoneal resection margin or peripancreatic soft tissue.3 These “isolated solitary ducts” were either cancer cells in lymphatics or in blood vessels, although they did not specify the exact proportions of the lymphatics or vascular invasion. Therefore, in order to evaluate the proportions of these types of vascular invasion in PDACs more precisely, further studies would be needed of larger number of cases supplemented with immunohistochemical labeling with smooth muscle actin, and vascular and lymphatic endothelial antigens would be needed.
In summary, we reviewed a large series of surgically resected pancreatic cancers and found that 1) histologic vascular invasion is a poor prognostic indicator; and 2) that microscopic vascular invasion can mimic PanIN-lesions. It is important that these foci of PanIN-like vascular invasion are not underdiagnosed and misdiagnosed as non-invasive PanIN lesions in resected pancreatic cancer specimens.
Acknowledgements
This work was supported by the Michael Rolfe Foundation and P50CA062924.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors declare no commercial interest.
REFERENCES
- 1.Allema JH, Reinders ME, van Gulik TM, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75:2069–2076. doi: 10.1002/1097-0142(19950415)75:8<2069::aid-cncr2820750807>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay S, Basturk O, Coban I, et al. Isolated solitary ducts (naked ducts) in adipose tissue: a specific but underappreciated finding of pancreatic adenocarcinoma and one of the potential reasons of understaging and high recurrence rate. Am J Surg Pathol. 2009;33:425–429. doi: 10.1097/PAS.0b013e3181908e42. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AI. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 5.Gallagher PJ, van der Wal AC. Blood vessels. In: Mills SE, editor. Histology for pathologists. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 218–240. [Google Scholar]
- 6.Garcea G, Dennison AR, Ong SL, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33:892–897. doi: 10.1016/j.ejso.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–316. [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Gao C, Li H, Juzi JT, Chen H, Hao X. Factors associated with survival after surgical resection in Chinese patients with ductal adenocarcinoma of the pancreatic head. Dig Surg. 2008;25:87–92. doi: 10.1159/000121447. [DOI] [PubMed] [Google Scholar]
- 9.Luttges J, Vogel I, Menke M, Henne-Bruns D, Kremer B, Kloppel G. The retroperitoneal resection margin and vessel involvement are important factors determining survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Virchows Arch. 1998;433:237–242. doi: 10.1007/s004280050242. [DOI] [PubMed] [Google Scholar]
- 10.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthaei H, Hong SM, Mayo SC, et al. Presence of Pancreatic Intraepithelial Neoplasia in the Pancreatic Transection Margin does not Influence Outcome in Patients with R0 Resected Pancreatic Cancer. Ann Surg Oncol. 2011 May 3; doi: 10.1245/s10434-011-1745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura T, Tsuchiya Y, Nashimoto A, et al. Prognostic factors for radical resection of middle and distal bile duct cancer. Hepatogastroenterology. 2009;56:294–298. [PubMed] [Google Scholar]
- 13.Schwartz AM, Henson DE. Familial and sporadic pancreatic carcinoma, epidemiologic concordance. Am J Surg Pathol. 2007;31:645–646. doi: 10.1097/PAS.0b013e31802d6d42. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 15.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]