Abstract
Purpose
Development of mucositis is a frequent side effect of radiotherapy of patients with head and neck cancer. We have recently reported that bacterial flagellin, an agonist of Toll-like receptor 5 (TLR5), can protect rodents and primates from acute radiation syndrome caused by total body irradiation (Burdelya et al., 2008, Science 320: 226-30). Here we analyzed the radioprotective efficacy of TLR5 agonist under conditions of local, single dose or fractionated radiation treatment.
Methods and Materials
Mice received either single-dose (10, 15, 20 or 25 Gy) or fractioned irradiation (cumulative dose up to 30 Gy) of the head and neck area with or without subcutaneous injection of pharmacologically optimized flagellin, CBLB502, 30 minutes prior to irradiation.
Results
CBLB502 significantly reduced the severity of dermatitis and mucositis, accelerated tissue recovery and reduced the extent of radiation induced weight loss in mice after a single dose of 15 or 20 Gy but not from 25 Gy of radiation. CBLB502 was also protective from cumulative doses of 25 and 30 Gy delivered in two (10+15 Gy) or three (3 × 10 Gy) fractions, respectively. While providing protection to normal epithelia, CBLB502 did not affect the radiosensitivity of syngeneic squamous carcinoma SCCVII grown orthotopically in mice. Use of CBLB502 also elicited a radiation independent growth inhibitory effect upon TLR5-expressing tumors demonstrated in the mouse xenograft model of human lung adenocarcinoma A549.
Conclusion
CBLB502 combines properties of supportive care (radiotherapy adjuvant) and anticancer agent, both mediated via activation of TLR5 signaling in the normal tissues or the tumor, respectively.
Keywords: Radiotherapy, flagellin, mucositis, dermatitis, inflammation
INTRODUCTION
Radiation therapy remains one of the most effective approaches to the treatment of various cancers. During radiotherapy, radiation treatment is applied locally and in multiple fractions, the regimens empirically selected to reduce the severity of adverse effects. Nevertheless, damage to normal tissues cannot be avoided and sets the critical limitation for the efficacy of this cancer treatment approach. As an example, the majority of patients undergoing radiotherapy treatment for head and neck cancers develop the highest morbidity from damage to mouth epithelial tissues and salivary glands. Symptoms include: xerostomia, severe mucositis and difficulty with swallowing 1–5. These effects result in significant malnutrition and weight loss. In addition, the irradiated areas of the skin often exhibit acute radiation damage characterized by the onset of erythema, swelling, blisters and ulceration followed by development of chronic inflammation, necrosis, fibrosis and lymphedema 6. These symptoms become the limiting factors during cancer radiotherapy and can force the interruption or termination of the therapeutic course.
Several approaches to mitigate the limiting dose problem are currently under development or in clinical use 7. They include conformal and intensity-modulated radiation and the development of, radioprotective agents based on synthetic thiol compounds, such as amifostine, or natural anti-oxidant compounds 8, 9. Unfortunately, treatment with synthetic thiols is not entirely selective for normal cells and can also involve protection of tumors12. In addition, this course has led to other adverse conditions as demonstrated by the amifostine related toxic epidermal effects observed during head and neck radiotherapy 10, 11. We, and others, have recently demonstrated that bacterial flagellin, ligand and agonist of toll-like receptor 5 (TLR5), and its pharmacologically-optimized derivative, CBLB502, can protect rodents and primates from both hematopoietic and gastrointestinal radiation syndromes induced by total body irradiation 13. In this study, we explore whether CBLB502 can protect mice from dermatitis and mucositis developed as a result of single dose or fractioned local irradiation of the head and neck area in mice.
METHODS AND MATERIALS
Mice
NIH-Swiss (National Cancer Institute, Frederick, MD), C3H/HeJ and athymic nude (Jackson Laboratories, Bar Harbor, Maine) female mice of 10–12 weeks old were used in the study. All described experiments were run strictly under the protocols approved by Institutional Animal Care and Use Committee of Roswell Park Cancer Institute.
Tumor cells
Mouse squamous cell carcinoma SCCVII cells (broadly used as animal model of head and neck cancer) and human lung adenocarcinoma A549 cells were propagated in DMEM media with 10% FBS and antibiotics. SCCVII cells were transduced with lentiviral vector carrying mitochondria-targeted Green Fluorescent -Protein (GFP) to distinguish tumors during imaging procedure. In order to suppress TLR5 expression, A549 cells expressing Firefly luciferase gene under the control of NF-κB promoter (Cellecta, Mountain View, CA) were transduced with lentiviral pLKO1-puro vector expressing shRNA specific to human TLR5 gene [CCG-GCC-TTG-CCT-ACA-ACA-AGA-TAA-ACT-CGA-GTTTAT-CTT-GTT-GTA-GGC-AAG-GTT-TTT-G] or control scrambled shRNA (Sigma-Aldrich, St. Louis, MO). After puromycin selection and testing with luciferase NF-κB reporter assay for inhibition of TLR5 signaling, A549 cells with TLR5 shRNA and control shRNA-expressing constructs were used in the experiments for tumor sensitivity to radiation.
CBLB502 injection, irradiation and histological analysis of mice
Mice were injected s.c. with 100 µl of CBLB502, 1 µg/mouse for fractioned and 2.5 µg/mouse for single radiation treatment, or with 100 µl PBS thirty minutes prior to irradiation. The head and neck areas were irradiated with single doses of 10, 15, 20 or 25 Gy X-ray radiation or in fractions given 24 hours apart with cumulative 25 Gy (10 and 15 Gy) and 30 Gy (3 times 10 Gy) using a Philips RT 250 Orthovoltage X-ray Unit at a dose rate of 1.88 Gy/min under isofluorane inhalation or ketamine injection anesthesia. Mouse survival and body weight were recorded daily or every second day, respectively. Mice which experienced weight loss >25% and limited motility were sacrificed. In the experiments for histological analysis, mice were sacrificed when the difference in body weight loss between the CBLB502 treated and untreated groups was greatest (11–14 days after irradiation). Routine H&E staining of paraffin embedded sections of tongues, lips and skin samples from the frontal neck area of irradiated and non-irradiated control mice was performed and analyzed ‘blindly’ by a core facility pathologist.
Inoculation and treatment of mouse tumors
C3H/HeJ mice were injected s.c. in their necks with 3.5×105 SCCVII-GFP cells. After tumors reached about 5 mm in diameter, 10 or 15 Gy were applied with or without CBLB502 (2.5 µg, s.c.) injected 30 minutes before irradiation. A549 and A549-shTLR5 cells (1×106 per site) were injected s.c. into flanks of athymic nude mice to induce tumors. After tumors reached about 5 mm in diameter, the mice were treated with CBLB502 (1 µg, s.c.) during 3 consecutive days. Tumor volumes were measured every second day and calculated by formula: V=π/6 × d12 × d2, where d1 < d2.
Statistical analysis
Differences in body weight loss (expressed as a percent of initial body weight, n=4–6 mice/group) between groups were analyzed by two-way (time and treatment) repeated measures ANOVA and by Student’s t-test (two-tailed, unequal variances). Significance level was set at P <0.05.
RESULTS
Effects of CBLB502 in mice subjected to single dose local irradiation of head and neck
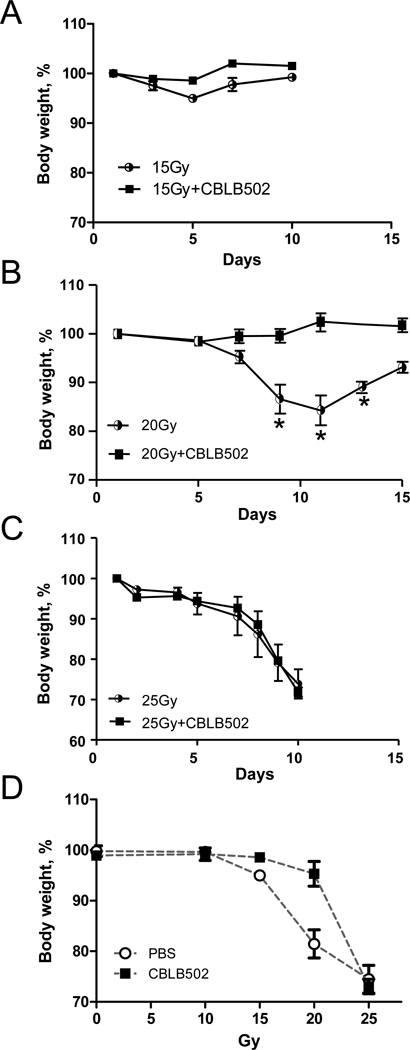
The radioprotective effects of CBLB502 against local radiation induced injury were first studied under the conditions of single or fractioned doses of local irradiation of the head and neck of NIH-Swiss mice. Treatment with CBLB502 alone (without irradiation) produced no effect on body weight relative to untreated mice during the course of experiment (data not shown). Exposure to a single 15 Gy dose of X-ray brought about an approximate five percent loss in initial body weight followed by rapid recovery during the fifth to seventh days (Fig. 1A). Weight change in the group that received CBLB502 30 minutes prior to 15 Gy irradiation was insignificant (1–2 % of initial body weight variation).
Figure 1. The dynamics of mouse body weight changes after radiation treatment of the head and neck area.
Mouse body weight changes after single irradiation with 15 (A, n=4), 20 (B, n=6) or 25 Gy (C, n=6) with and without prior CBLB502 injection (2.5 µg/mouse). Results representative of at least two analogous experiments performed with each of the three irradiation regiments are presented. (D). Dependence of the radioprotective effect of CBLB502 on radiation dose is presented as a minimal body weight observed at any time-point in mice irradiated with indicated doses of irradiation. Available results permit calculating DRF equal 1.3 based on 5% body weight loss. (*) The difference between irradiated groups, with and without CBLB502 treatment, is significant (p<0.05).
In the control group that received 20 Gy, a continuous weight loss until days 11–12 reaching a total loss of more than 15% of initial body weight was observed due to a dramatic reduction in food and water consumption. This was followed by a slow recovery that was still incomplete by day 15 after irradiation. Mice injected with CBLB502 prior to irradiation demonstrated no detectable changes in body weight indicative of protective effect of the drug (results of a representative experiment shown in Fig. 1B).
Increasing the local radiation dose to 25 Gy led to mortality of all mice within 10 days due to weight loss greater than 25% which prompted euthanasia for ethical reasons. As indicated in Figure 1C, pre-treatment with CBLB502 did not alter this outcome. The dependence of radioprotective effect of CBLB502 on radiation dose is shown in Fig. 1D. Although the number of radiation doses analyzed did not allow us to accurately calculate dose reduction factor (DRF) of CBLB502 under applied conditions, it is clear that it is effective at least up to 20 Gy but conferred no protection at 25 Gy.
Effects of CBLB502 in mice subject to fractioned local irradiation of the head and neck
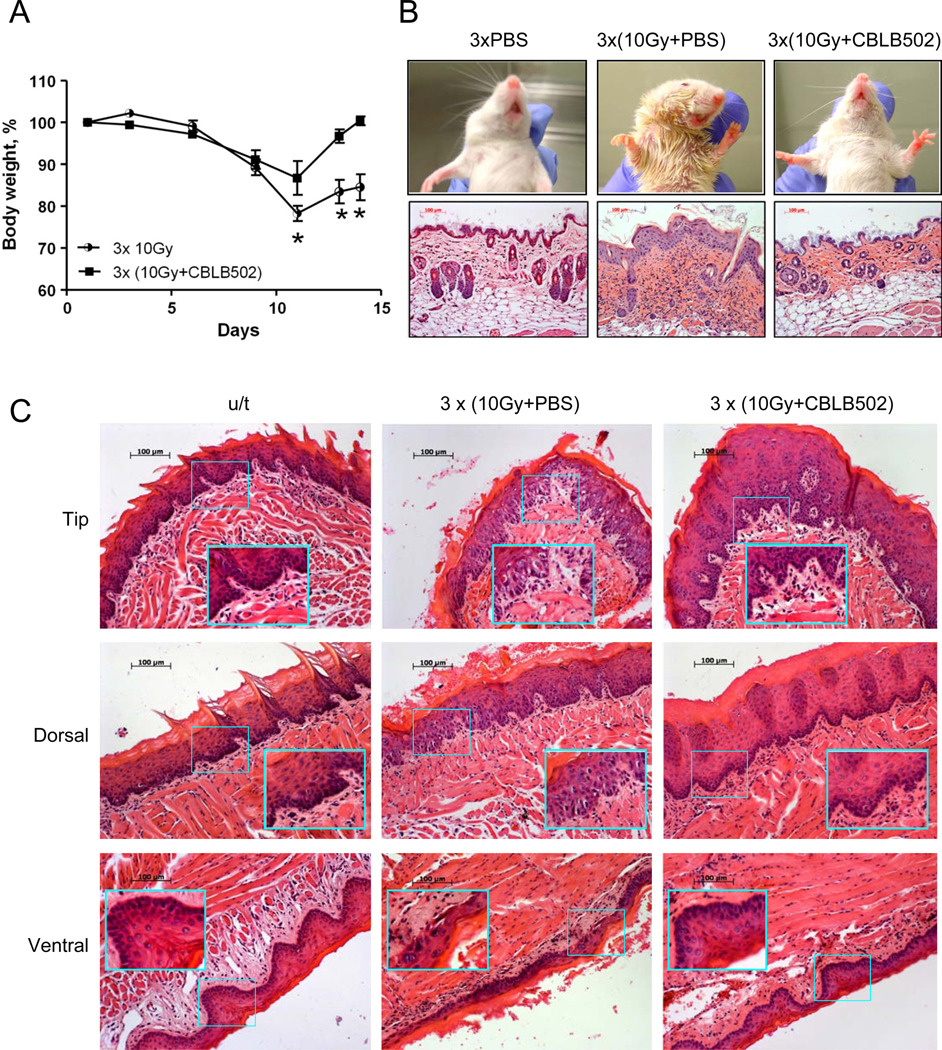
Because normal human radiotherapy practice utilizes fractioned doses of local radiation, we next applied cumulative local X-ray doses to 25 or 30 Gy of X-ray radiation to the head and neck area of NIH-Swiss mice as two (10 and 15 Gy) or three 10 Gy fractions, respectively. CBLB502 was administered at 30 minutes prior to each irradiation dose to the designated mice. Control mice were injected with PBS. Both radiation regimens resulted in substantial (>20%) body weight loss followed by a slow recovery which was still incomplete by day 14 post first irradiation. Animals treated with CBLB502 showed less significant weight loss and complete recovery by day 14 (data for 30 Gy regimen are shown in Fig. 2A).
Figure 2. The protective effect of CBLB502 against radiation damage to tissues in the head and neck area of mice.
(A). Mouse body weight changes after 30 Gy local head and neck radiation treatment, with and without CBLB502 (1 µg/mouse, n=6), given in three 10 Gy fractions over three consecutive days. Results representative of at least two analogous experiments are presented. (*) The difference between irradiated groups, with and without CBLB502 treatment, is significant (p<0.05). (B). Upper row. Photographs of irradiated NIH-Swiss mice with or without CBLB502 pretreatment. Lower row. H&E stained skin sections from the neck area. Mice were locally irradiated with 30 Gy X-ray given in three daily 10 Gy fractions. Images were taken on day 14 after the first radiation treatment. Lower row. H&E stained histological sections of the skin sections. (C). H&E stained histological sections of tip, dorsal and ventral epithelium of mouse tongue obtained 11 days after the first of three 10 Gy fractions of 30 Gy total X-ray irradiation. For B (lower panel) and C, H&E stained tissue sections from healthy untreated (u/t) mice were used as a positive control. The pictures of the untreated mice show stratified squamous epithelium with basal layer of small, dark cells from which the mucosa is constatnly renewed and overlaying larger spinous cells and superficial flattened cells. The irradiated and PBS treated mice reveal atrophic and degenerative changes in the basal and overlaying cells with loss of cells, pyknotic and dysplastic nuclei, erosions and hemorrhage. In contrast, the irradiated and CBLB502 treated mice have mucosa with minimal radiation associated changes and morphology closer to the normal, with better preserved, regular basal and overlaying epithelial cells. Tissues from non-irradiated CBLB502-treated animals were histologically indistinguishable from control non-irradiated vehicle-treated animals (data not shown).
Macrophotographs (Fig. 2B) of the irradiated region taken on day 14 after the first irradiation offer visual evidence of the extensive skin damage and inflammation in the control irradiated vehicle-treated mice. At the histological level, the skin from the irradiated region of control mice showed atrophy of the hair follicles, hyperplasia of the epidermis with papillary thickening of its layers, hyperkeratosis, hyperemia, hemorrhage and infiltration of the inflammatory cells in the underlying derma. CBLB502 administrations dramatically reduced epidermal hyperplasia, hyperkeratosis and atrophy of the hair follicles and dermal inflammation, while some level of hyperemia still persisted.
In the dorsal and ventral epithelium of the tongue of irradiated control animals the hemorrhage, erosion, ulceration, hyperplasia and dysplasia were observed (Fig. 2C). In addition, edema and inflammatory infiltrates, as well as an increased number of mast cells in the buccal and tongue submucosa were observed (not shown). By contrast, irradiated mice treated with CBLB502 the tissue damage, especially erosion, ulceration and epidermis dysplasia and submucosal inflammation were significantly reduced. Observations from all analyzed mice are summarized in Table 1.
Table 1.
Pathomorphological and histological observations in the skin and tongue tissues of mice after local fractioned irradiation of head and neck area, with or without CBLB502 injections
| Treatment | Untreated | 3xCBLB502 | 3x10Gy + PBS | 3x10Gy + CBLB502 |
|---|---|---|---|---|
| Organs | ||||
| Skin: atrophy of hair follicles, hemorrhage, hyperplasia, inflammation, dermatitis | 0/5 | 0/5 | 7/7 | 3/8 |
| Tongue: severe hyperplasia of mucosa, hemorrhage and inflammation | 0/5 | 0/5 | 6/7 | 0/8 |
Effect of CBLB502 on tumor growth and sensitivity to radiotherapy
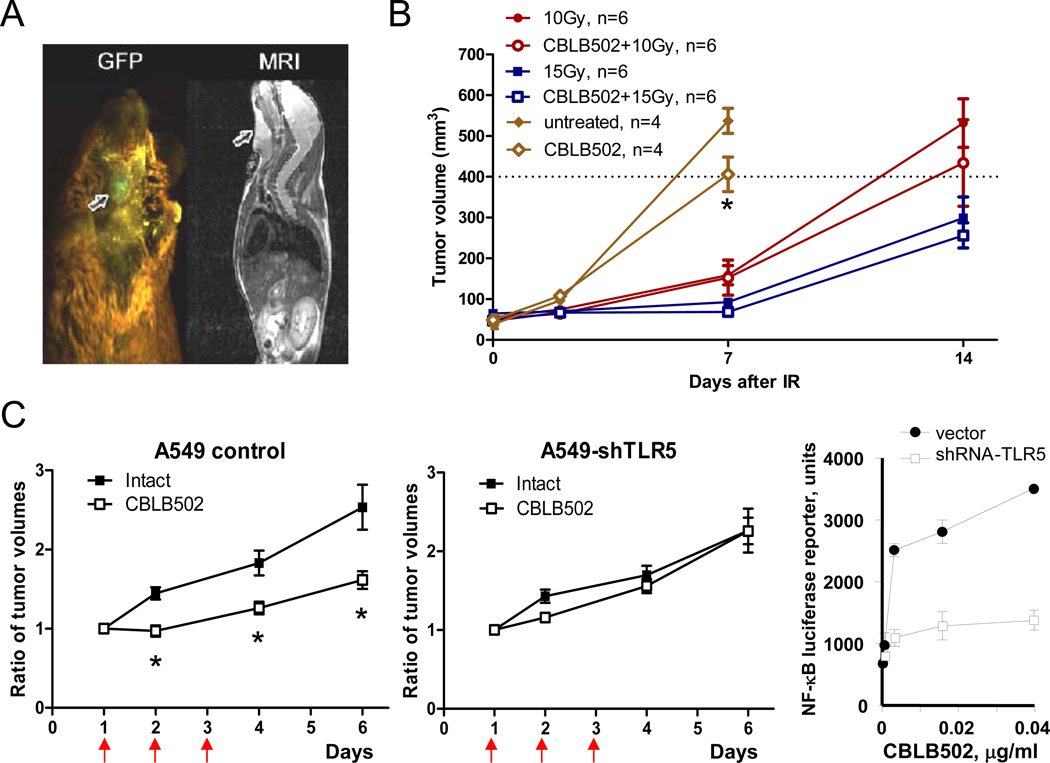
To check whether protective effect of CBLB502 against local irradiation of head and neck area is specific for normal tissues, we used mouse squamous cell carcinoma SCCVII grown s.c. in the neck area in syngeneic C3H mice. (Fig. 3A). Tumor bearing mice were treated with 10 or 15 Gy of local X-ray radiation, with PBS or CBLB502 injection 30 min prior to irradiation. The results shown in Fig. 3B demonstrate that radiation suppressed tumor growth in both PBS and CBLB502 treated groups of mice. Furthermore, administration of CBLB502 alone (no irradiation) led to a reduction in the tumor growth.
Figure 3. Anti-tumor effect of CBLB502 and radiation treatment combined with CBLB502.
(A). Visualization of GFP-expressing SCCVII tumors in the neck area of syngeneic C3H mice two weeks post implantation by whole body fluorescence imaging system (Lightools Research, Encinitas, CA) (left) and MRI (left). Arrows point to the location of the tumor on both images. (B). Changes to SCCVII tumor volume after treatment with CBLB502 and radiation both individually and in combination. Representative results of one out of two analogous experiments are presented, n=6. (C). The dynamics of xenogenic control (control shRNA expressing) A549 cells (left panel) and A549-shTLR5 (middle panel) tumor growth in athymic nude mice was recorded after 3 daily injections of CBLB502 or PBS (vehicle control). The tumor volume data are presented relative to the initial tumor volume, n=10. (*) The difference between tumor volumes in groups with and without CBLB502 treatment is significant (p<0.05). Luciferase reporter assay for NF-kappaB activation in A549 and A549-shTLR5 cells was performed 5 hours after in vitro treatment with different doses of CBLB502 (right panel).
Similar tumor suppressive effect of CBLB502 was seen in another model: A549 lung cancer xenografts (Fig. 3C) grown s.c. in athymic nude mice. This effect was TLR5 dependent since knockdown of TLR5 elicited by lentiviral transduction of shRNA rendered the A549 tumors no longer sensitive to direct antitumor effect of CBLB502. This effect is likely to be exerted via activation of innate immune response, still preserved in nude mice, by CBLB502-mediated induction of TLR5 signaling in the tumor.
DISCUSSION
We previously demonstrated efficacy of TLR5 agonist CBLB502 in reducing severity of and lethality from acute radiation syndrome caused by total body irradiation of mice and Rhesus macaques 13. The mechanism of radioprotection by CBLB502 is multifold and involves NF-κB-mediated suppression of radiation-induced apoptosis in hematopoietic system and gastrointestinal tract, activation of endogenous antioxidants (e.g., superoxide dismutase 2) and induction of a specific set of cytokines contributing to regeneration of damaged tissues (e.g., G-CSF) 13. While total body radiation imitates biodefense scenarios of radiation disaster, the assessment of CBLB502’s efficacy in the context of clinically-relevant scenarios (radiotherapy side effects) requires the use of models that involve local fractioned irradiation. The results presented here demonstrate the efficacy of CBLB502 for protection of dermal and mucosal epithelia from injury resulting from local irradiation.
The choice of a model for this study is prompted by the frequent use of radiotherapy for the treatment of head and neck cancer. For treatment regimens in common use, it is not unusual to encounter adverse effects which predominantly affect the oral mucosa and the severity can be dose limiting 5, 14. The approved supporting care drug for this indication, amifostine, remains underused due to its toxicity 11 and lack of specificity with respect to normal tissue protection12. The availability of quantitative criteria detailing the severity of mucositis associated with radiotherapy of head and neck cancer makes it possible to plan and rapidly complete clinical trials with relatively short-term endpoints. Moreover, the demonstrated strong effect of CBLB502 administration on the development and grade of dermatitis potentially broadens the clinical value of CBLB502 by extending its projected applications to those radiotherapy treatments that are known to be associated with skin toxicity. These include breast cancer patients that frequently suffer from skin burns during radiotherapy15. Evident preservation of hair follicles in CBLB502-treated mice justifies future study aimed at protection against radiation-induced alopecia.
A critical condition for safe clinical use of CBLB502 is the specificity of its radioprotective activity for normal cells. In the present study, we did not see any protective effect of CBLB502 on locally irradiated syngeneic head and neck mouse tumors. Moreover, CBLB502 injection did not show any tumor protection within the context of total body radiation in syngeneic and xenograft mouse tumor models regardless of their TLR5 status (13 and unpublished observations). Furthermore, CBLB502 applied without irradiation had a direct anti-proliferative effect in some tumors which may be explained through the immunostimulatory effect of activation of TLR5 signaling occurring in tumors with functional TLR5, a phenomenon previously reported in other tumor model16. Taken together, the generated results indicate that CBLB502 uniquely combines properties of supportive care (radiotherapy adjuvant) and anticancer agent, both mediated via activation of TLR5 signaling in the normal tissues or the tumor, respectively.
ACKNOWLEDGEMENTS
We thank Patricia Stanhope-Baker and Gary Haderski for help with manuscript preparation. This work was supported by grants from NIH (RC1AI080306, R01AI080446 and RC2AI087616) and Cleveland BioLabs, Inc. to A.V.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Lyudmila Burdelya, Elena Feinstein and Andrei Gudkov are paid consultants of Cleveland BioLabs, Inc., which develops CBLB502 into radiation countermeasure. Corresponding author, Dr. Andrei Gudkov, is a shareholder of Cleveland BioLabs, Inc. This work was funded in part by a research grant from Cleveland BioLabs, Inc. to Roswell Park Cancer Institute (principle investigator – Dr. Andrei Gudkov).
REFERENCES
- 1.Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009;19:29–34. doi: 10.1016/j.semradonc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Vitolo JM, Cotrim AP, Sowers AL, et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Burlage FR, Spijkervet FK, et al. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–225. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 4.Nagler RM, Baum BJ. Prophylactic treatment reduces the severity of xerostomia following radiation therapy for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2003;129:247–250. doi: 10.1001/archotol.129.2.247. [DOI] [PubMed] [Google Scholar]
- 5.Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 6.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Berger ME, Christensen DM, Lowry PC, et al. Medical management of radiation injuries: current approaches. Occup Med (Lond) 2006;56:162–172. doi: 10.1093/occmed/kql011. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinimehr SJ. Trends in the development of radioprotective agen ts. Drug Discov Today. 2007;12:794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 10.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 11.Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol. 2007;25:4084–4089. doi: 10.1200/JCO.2007.11.5816. [DOI] [PubMed] [Google Scholar]
- 12.Grdina DJ, Murley JS, Kataoka Y, et al. Amifostine induces antioxidant enzymatic activities in ormal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys. 2009;73:886–896. doi: 10.1016/j.ijrobp.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs. 2006;22:163–173. doi: 10.1016/j.soncn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008;135:518–528. doi: 10.1053/j.gastro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]