Abstract
Background
Galectin-3 is a soluble ß-galactoside-binding lectin released by activated cardiac macrophages. Elevated levels of galectin-3 have been found to be associated with adverse outcomes in patients with heart failure. We evaluated the association between galectin-3 and long-term clinical outcomes in ambulatory heart failure patients enrolled in the HF-ACTION study.
Methods and Results
HF-ACTION was a randomized controlled trial of exercise training in patients with chronic heart failure due to left ventricular (LV) systolic dysfunction. Galectin-3 was assessed at baseline in a cohort of 895 HF-ACTION subjects with stored plasma samples available. The association between galectin-3 and clinical outcomes was assessed using a series of Cox proportional hazards models. Higher galectin-3 levels were associated with other measures of heart failure severity, including higher NYHA class, lower systolic blood pressure, higher creatinine, higher NTproBNP, and lower maximal oxygen consumption. In unadjusted analysis, there was a significant association between elevated galectin-3 levels and hospitalization-free survival (unadjusted hazard ratio = 1.14 per 3 ng/mL increase in galectin-3, P<0.0001). In multivariable modeling, the prognostic impact of galectin-3 was significantly attenuated by the inclusion of other known predictors and galectin-3 was no longer a significant predictor after the inclusion of NTproBNP.
Conclusions
Galectin-3 is elevated in ambulatory heart failure patients and is associated with poor functional capacity and other known measures of heart failure severity. In univariate analysis, galectin-3 was significantly predictive of long-term outcomes, but this association did not persist after adjustment for other predictors, especially NTproBNP.
Keywords: heart failure, biomarker, prognosis
Heart failure affects over 5 million people in the United States, and there are 500,000 new cases diagnosed each year.1 Despite improvements in medical therapy, outcomes remain poor, with a 5-year mortality approaching 50% in symptomatic patients. Risk stratification of heart failure patients is critical in order to apply potentially costly and invasive therapies (such as cardiac resynchronization therapy or ventricular assist devices) to those patients most likely to benefit.
Biomarkers play an increasingly important role in the risk stratification of heart failure patients.2 The natriuretic peptides b-type natriuretic peptide (BNP) and amino-terminal pro-b-type natriuretic peptide (NTproBNP) are released in response to myocardial stress and have been shown to be powerful markers of prognosis in heart failure.3 There remains a need to identify and evaluate newer biomarkers that may reflect different pathophysiologic mechanisms in order to improve risk stratification and also to individualize therapy. Galectin-3, a soluble ß-galactoside-binding lectin released by activated cardiac macrophages, has been shown to play an important role in pathologic ventricular remodeling in animal models,4 and elevated levels of circulating galectin-3 have previously been found to be associated with adverse outcomes in patients with both undifferentiated dyspnea5 and in heart failure patients prior to discharge from a heart failure hospitalization.6 Few data are available on the relationship between galectin-3 and outcomes in ambulatory heart failure patients.7-9 Accordingly, we sought to evaluate the association of circulating galectin-3 levels with other measures of heart failure status and with long-term clinical outcomes in a cohort of ambulatory heart failure patients enrolled in the HF-ACTION study, a large multicenter randomized trial of exercise training in heart failure.
Methods
Details of the design, rationale, and primary results of the HF-ACTION study have been published elsewhere.10,11 Briefly, HF-ACTION (clinicaltrials.gov, NCT00047437) was a randomized clinical trial evaluating the effect of exercise training versus usual care on long-term morbidity and mortality in patients with chronic heart failure due to left ventricular systolic dysfunction (New York Heart Association [NYHA] class II-IV, left ventricular ejection fraction [LVEF] <0.35). The primary endpoint of HF-ACTION was a composite of all-cause mortality and all-cause hospitalization over a median follow-up of 2.5 years. An independent clinical events committee adjudicated deaths and first cardiovascular hospitalizations. HF-ACTION was approved by local institutional review boards, and all enrolled patients provided written informed consent.
Biomarker Assays
A sub-set of patients enrolled in the HF-ACTION study who agreed to participate in the biomarker substudy underwent plasma collection at baseline, 3 months, and 12 months. Baseline blood samples were obtained on the same day as baseline exercise testing but were obtained prior to exercise. Samples were collected via peripheral vein into EDTA containing tubes and then centrifuged immediately and stored at -70°C for subsequent analysis. Galectin-3 levels were assessed on baseline samples using enzyme-linked immunosorbent assay (ELISA) at an academic core laboratory that was blinded to clinical data.
Statistical Analysis
Baseline characteristics were described using medians and intra-quartile ranges or proportions. The relationship between galectin-3 levels and other baseline variables of interest was analyzed using simple correlations. Additionally, we created a linear regression model with galectin-3 as a dependent variable in order to identify clinical factors most associated with elevations of galectin-3 levels.
The primary outcome variable of interest was time to all-cause hospitalization or all-cause mortality, which was the primary outcome of the HF-ACTION study. We also evaluated the secondary outcomes of cardiovascular death or cardiovascular hospitalization, cardiovascular death or heart failure hospitalization, and all-cause mortality. We analyzed the relationship between galectin-3 and outcomes of interest using a series of Cox proportional hazards models, including adjustment for demographics alone (age, gender, race) as well as for the more comprehensive set of predictors that had been identified in the final adjusted model for the overall HF-ACTION cohort (see Appendix for final adjusted model for each endpoint). Although it had not been part of the modeling process for the overall HF-ACTION trial because it was not available in the entire cohort, we additionally examined each model with and without NTproBNP, given its known strong association with outcomes in chronic heart failure.10 Because the final adjusted model from the overall HF-ACTION dataset included variables from cardiopulmonary exercise testing (CPX), which is not routinely performed in many centers, we examined each adjusted model with and without CPX variables. Covariates were transformed as required to ensure that linearity assumptions were met for survival analysis. After assessment of various methods for handling galectin-3, including log transformation, the best model fit was provided by handling galectin-3 as a continuous variable with truncation at the extreme values (< 8 ng/mL and > 25 ng/mL) to avoid violation of the linearity assumption.
In an additional analysis, we evaluated the relationships between NTproBNP and galectin-3 and the prognostic value of concordant versus discordant values for these 2 biomarkers. For this analysis, we analyzed 4 groups defined by dividing each variable at the median value (galectin-3 low-NTproBNP low, galectin-3 high-NTproBNP low, galectin-3 low-NTproBNP high, and galectin-3 high-NTproBNP high) and evaluating the hazard ratio of each category (compared with reference category of galectin-3 low-NTproBNP low.) A P value ≤0.05 was considered statistically significant for all analyses.
Results
Evaluable baseline plasma samples were available for 895 patients, and baseline characteristics for this study cohort stratified by median galectin-3 levels are shown in Table 1. Median age of the study cohort was 59 years; 64% were Caucasian, and 71% were male. The median NT-proBNP level was 848 pg/ml, and the median LVEF was 24%. Enrolled patients had a high utilization of guideline-based medical therapy for systolic heart failure, with 95% receiving beta-blockers and 74% receiving angiotensin converting enzyme (ACE) inhibitors. The subset of patients with available plasma samples for analysis (n = 895) was broadly similar to the HF-ACTION cohort as a whole (n = 2331, data not shown). The median galectin-3 level in this study cohort was 14.0 ng/mL (interquartile range 11.0, 18.6).
Table 1.
Baseline characteristics by median galectin-3
| Galectin-3
|
||||
|---|---|---|---|---|
| Parameter | Total (n =895) | <14.0 (n =447) | ≥ 14.0 (n = 448) | P Value |
| Age (y) | 59 (51, 68) | 55 (47, 63) | 62 (55, 73) | <0.001 |
| Gender (% female) | 259/895 (29%) | 134/447 (30%) | 125/448 (28%) | 0.494 |
| Race | ||||
| Black | 271/883 (31%) | 159/440 (36%) | 112/443 (25%) | 0.002 |
| White | 567/883 (64%) | 261/440 (59%) | 306/443 (69%) | |
| Other | 45/883 (5%) | 20/440 (5%) | 25/443 (6%) | |
| History of diabetes | 291/895 (33%) | 122/447 (27%) | 169/448 (38%) | 0.001 |
| History of MI | 372/895 (42%) | 152/447 (34%) | 220/448 (49%) | <0.001 |
| History of hypertension | 570/891 (64%) | 272/445 (61%) | 298/446 (67%) | 0.077 |
| Smoking status | <0.001 | |||
| Never | 333/890 (37%) | 181/445 (41%) | 152/445 (34%) | |
| Current | 145/890 (16%) | 89/445 (20%) | 56/445 (13%) | |
| Past | 412/890 (46%) | 175/445 (39%) | 237/445 (53%) | |
| HF hospitalizations in prior 6 months | 0.411 | |||
| 0 | 651/888 (73%) | 330/442 (75%) | 321/446 (72%) | |
| 1 | 181/888 (20%) | 90/442 (20%) | 91/446 (20%) | |
| 2 | 31/888 (4%) | 13/442 (3%) | 18/446 (4%) | |
| 3 | 25/888 (3%) | 9/442 (2%) | 16/446 (4%) | |
| HF etiology | <0.001 | |||
| Ischemic | 454/895 (51%) | 184/447 (41%) | 270/448 (60%) | |
| Non-Ischemic | 441/895 (49%) | 263/447 (59%) | 178/448 (40%) | |
| NYHA class at randomization | <0.001 | |||
| II | 560/895 (63%) | 313/447 (70%) | 247/448 (55%) | |
| III | 323/895 (36%) | 132/447 (30%) | 191/448 (43%) | |
| IV | 12/895 (1%) | 2/447 (1%) | 10/448 (2%) | |
| Mitral regurgitation (moderate or severe) | 96/895 (11%) | 40/447 (9%) | 56/448 (13%) | 0.086 |
| Systolic blood pressure (mmHg) | 112 (100, 126) | 116 (104, 128) | 110 (100, 126) | 0.002 |
| Diastolic blood pressure (mmHg) | 70 (62, 80) | 72 (64, 80) | 68 (60, 78) | <0.001 |
| Heart rate (bpm) | 71 (63, 80) | 71 (64, 80) | 70 (63, 79) | 0.260 |
| Body mass index | 30.4 (26.3, 35.7) | 31.0 (27.0, 36.3) | 30.0 (25.7, 34.9) | 0.019 |
| LVEF (%) | 24 (19, 30) | 25 (20, 30) | 24 (19, 230) | 0.133 |
| Rest ECG ventricular conduction | <0.001 | |||
| Normal | 386/877 (44%) | 221/437 (51%) | 165/440 (38%) | |
| LBBB | 131/877 (15%) | 68/437 (16%) | 63/440 (14%) | |
| RBBB | 30/877 (3%) | 14/437 (3%) | 16/440 (4%) | |
| IVCD | 96/877 (11%) | 47/437 (11%) | 49/440 (11%) | |
| Paced | 234/877 (27%) | 87/437 (20%) | 147/440 (33%) | |
| Creatinine (mg/dL) | 1.2 (1.0, 1.5) | 1.1 (0.9, 1.3) | 1.4 (1.1, 1.8) | <0.001 |
| Blood urea nitrogen (mg/dL) | 21 (15, 28) | 17 (13, 22) | 25 (18, 36) | <0.001 |
| Hemoglobin (g/dL) | 13.3 (12.2, 14.5) | 13.7 (12.6, 14.9) | 12.9 (11.8, 14.2) | <0.001 |
| Pro-BNP (pg/mL) | 848 (359, 1910) | 556 (250, 1155) | 1310 (537, 3079) | <0.001 |
| Exercise duration, CPX test (min) | 9.5 (7.0, 12.0) | 10.5 (8.3, 13.0) | 8.4 (6.0, 10.7) | <0.001 |
| Peak VO2 in mL/kg/min, CPX test | 14.5 (11.6, 17.4) | 15.6 (13.1, 18.8) | 13.20 (10.7, 16.2) | <0.001 |
| 6-minute walk distance (m) | 366 (298, 435) | 387 (321, 456) | 346 (274, 410) | <0.001 |
BNP indicates brain natriuretic peptide; CPX, cardiopulmonary exercise test; ECG, echocardiogram; HF, heart failure; IVCP, inferior vena cava pressure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; RBBB, right bundle branch block.
Association of Galectin-3 with Other Measures of Heart Failure Status
Patients with elevated galectin-3 levels had many other characteristics known to be associated with increased risk, including higher NYHA class, lower systolic blood pressure, higher creatinine, higher NTproBNP, and lower maximal oxygen consumption (Table 1). In linear regression modeling to identify clinical factors associated with elevated galectin-3, the strongest associations were for elevated blood urea nitrogen (BUN) (F = 73.3, P <0.0001), elevated creatinine (F = 31.2, P <0.0001), and older age (F = 14.4, P <0.0002). A variety of other candidate variables, including gender, race, heart failure etiology, NTproBNP, and ejection fraction, were not significant independent predictors of galectin-3 levels. Galectin-3 levels were modestly correlated with measures of exercise capacity such as maximal oxygen consumption (r = -0.25, P <0.001), exercise duration on CPX test (r = -0.27, P <0.001) and 6-minute walk distance (r = -0.23, P <0.001) (Figure 1).
Figure 1.

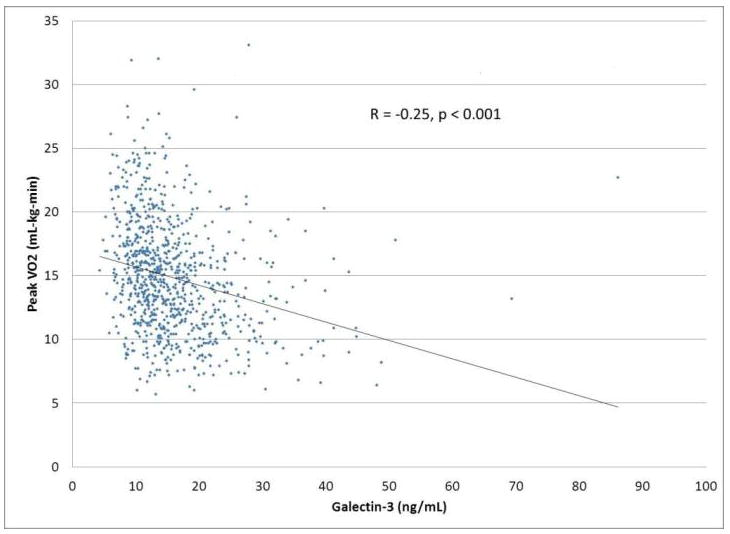
Relationship between galectin-3 levels and functional capacity (A) 6-minute walk distance, and (B) maximal oxygen consumption
Relationship to NTproBNP
Given the strong and consistent relationship between natriuretic peptides and prognosis in heart failure, there is substantial interest in the relationship between novel heart failure biomarkers and the natriuretic peptides. There was a modest correlation between NTproBNP levels and galectin-3 levels (r = 0.3, P <0.001). When evaluated by groupings examining above and below the median for each biomarker, NTproBNP and galectin-3 were discordant for 292 of 815 subjects (36%), divided approximately equally between low-NTproBNP high galectin-3 (n = 144) and high-NTproBNP low galectin-3 (n = 148). Compared with a reference group with low-NT-proBNP low galectin-3, there was a progressively increased hazard for low-NT-proBNP high galectin-3 (hazard ratio [HR] 1.32, P =0.03), high-NT-proBNP low galectin-3 (HR 1.75, P <0.0001), and high-NT-proBNP high galectin-3 (HR 2.19, P <0.0001) (Figure 2).
Figure 2.

Hospitalization-free survival by galectin-3/NTproBNP subgroups Compared with a reference group with low-NT-proBNP low galectin-3, there was a progressively increased hazard for low-NT-proBNP high galectin-3 (P =0.03), high-NT-proBNP low galectin-3 (P <0.0001), and high-NT-proBNP high galectin-3 (P <0.0001)
Galectin-3 and Outcomes
Of 895 patients, 637 (71%) reached the primary outcome of the composite of all-cause hospitalization or all-cause mortality, and 168 of 895 patients (19%) died over a median follow-up period of 32 months. The distribution of galectin-3 levels in patients who did and did not reach the primary outcome is shown in Figure 3. In univariable analysis, there was a significant association between elevated galectin-3 levels and the primary endpoint of all-cause hospitalization or all-cause mortality (unadjusted HR = 1.14 per 3 ng/mL increase in galectin-3, P <0.0001).
Figure 3.

Distribution plot of galectin-3 values by primary outcome
To evaluate the relationship of galectin-3 and outcomes in the context of other known predictors, a series of multivariable models were constructed to determine the relationship between galectin-3 and the primary outcome of all-cause death or rehospitalization (Table 2). Galectin-3 remained predictive of the primary endpoint after adjustment for age, race, and gender (adjusted HR for galectin-3 = 1.13 per 3 ng/mL increase, P <0.001) and after further adjustment for other covariates from the final adjusted model when CPX variables were excluded (adjusted HR for galectin-3 = 1.08 per 3 ng/mL increase, P =0.007). However, for each model the independent association between galectin-3 and outcome no longer reached significance after the inclusion of NTproBNP in the model. In a final model adjusting for all predictors of the primary endpoint in the HF-ACTION dataset as a whole (peak VO2 [as Weber class], gender, region, mitral regurgitation severity, ventricular conduction on echocardiogram [ECG], blood urea nitrogen [BUN], ejection fraction [EF], beta-blocker dose, and Kansas City Cardiomyopathy Questionnaire [KCCQ] symptom stability score) as well as NTproBNP, there was no evidence for an independent association between galectin-3 and outcomes (adjusted HR = 1.00 per 3 ng/mL increase, P =0.90).
Table 2.
Galectin-3 and risk of all-cause death or hospitalization
| HR (per 3 ng/mL increase) | 95% CI | P value | |
|---|---|---|---|
| Univariate | 1.14 | 1.09-1.19 | <0.001 |
| Further adjusted for NTproBNP | 1.03 | 0.98-1.08 | 0.27 |
| Adjusted for age, race, gender | 1.13 | 1.08-1.19 | <0.001 |
| Further adjusted for NTproBNP | 1.05 | 1.00-1.11 | 0.07 |
| Adjusted for final adjusted model covariates* (excluding CPX variables) | 1.08 | 1.02-1.14 | 0.007 |
| Further adjusted for NTproBNP | 1.03 | 0.97-1.09 | 0.39 |
| Adjusted for all final adjusted model covariates including CPX variables* | 1.05 | 0.99 - 1.11 | 0.12 |
| Further adjusted for NTproBNP | 1.00 | 0.94 - 1.07 | 0.90 |
BUN indicates blood urea nitrogen; CI, confidence interval; CPX, cardiopulmonary exercise test; EF, ejection fraction; HR, hazard ratio; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Final adjusted model predictors were peak VO2 (as Weber class), gender, region (US vs non-US), mitral regurgitation severity, ventricular conduction on ECG, BUN, EF, beta-blocker dose (truncated at 50 mg/day carvedilol equivalent), and KCCQ symptom stability score
Similar results were found for other outcomes. For the composite of cardiovascular death or cardiovascular hospitalization, galectin-3 was a significant predictor in univariate analysis (HR = 1.13 per 3 ng/mL increase, P <0.001) but no longer reached statistical significance after multivariable adjustment for final model predictors (adjusted HR = 1.03, P =0.34) and with the further addition of NTproBNP (HR = 0.97, P =0.36). Similar results were seen for the composite of cardiovascular death or heart failure hospitalization (univariate HR = 1.21 per 3 mg/nL increase, P <0.001; adjusted HR in final adjusted model = 1.05 per 3 ng/mL increase, P =0.28; adjusted HR in final adjusted model + NTproBNP = 1.00, P =0.97). For all-cause mortality, galectin-3 was significant in univariate analysis (HR = 1.34 per 3 ng/mL increase, P <0.001) and in the final adjusted clinical model (adjusted HR = 1.13 per 3 ng/mL increase, P =0.027) but not after further adjustment for NTproBNP (adjusted HR = 1.06, P =0.30).
Galectin-3 and Response to Exercise Training
Given the unique nature of the randomized intervention in the HF-ACTION study (exercise training), we examined the interaction between baseline galectin-3 levels and treatment assignment. There was no significant interaction between galectin-3 levels and exercise training for any of the 3 study endpoints (P =0.55 for all-cause death or hospitalization, P =0.91 for cardiovascular death or cardiovascular hospitalization, and P =0.34 for cardiovascular death or heart failure hospitalization.)
Discussion
Galectin-3 is a novel biomarker that has been shown to mediate fibrosis in a variety of organ systems including the heart. In animal models, galectin-3 mediates ventricular remodeling and administration of galectin-3 leads to a phenotype of progressive fibrosis and left ventricular systolic dysfunction.4 These observations have led to interest in galectin-3 as a potential heart failure biomarker that could reflect ongoing ventricular remodeling. In previous studies, galectin-3 has been shown to be predictive of outcome in patients at the time of hospitalization5 or discharge from an acute heart failure hospitalization.6 The current study is the largest analysis to date of the association between galectin-3 and outcomes and has the most comprehensive adjustment of other covariates that have been shown to predict outcome in chronic heart failure, including data from CPX. In our analysis, galectin-3 was significantly associated with outcome in univariate analysis as well as in multivariable analyses that excluded CPX variables and NTproBNP, but this independent relationship did not persist in adjusted models that included CPX variables and/or NTproBNP.
Galectin-3 and NTproBNP
The natriuretic peptides have emerged as the most well validated biomarkers in heart failure and are recommended by current guidelines for both establishing diagnosis in patients with acute dyspnea as well as in risk stratification of patients with chronic heart failure.11 Because elevations in galectin-3 are thought to reflect a different pathophysiologic axis (changes in extracellular matrix leading to ventricular remodeling) than the natriuretic peptides, there is substantial interest in the relationship between galectin-3 and natriuretic peptide measurements. In an analysis comparing 4 groups defined by whether each marker was elevated above the median or not, patients with both markers elevated had a more than2-fold risk (HR = 2.19) of reaching the primary outcome compared to those with both markers below the median. These findings are similar to previous data in patients with both acute heart failure and chronic heart failure.5-7. However, when galectin-3 and NTproBNP were both considered as continuous variables, the inclusion of NTproBNP in any of the multivariable models generally attenuated the prognostic significance of galectin-3, and galectin-3 was not a significant independent predictor of outcome in any model that included CPX variables and/or NTproBNP.
Our results differ from other published findings that have suggested that galectin-3 in an independent predictor of outcome in heart failure. Our findings are unlikely to represent type II error (i.e., inadequate statistical power) because our study is the largest study of galectin-3 to date and has more events (637 events for the primary endpoint) than the other 3 published studies in ambulatory heart failure patients combined.6,7,9 One alternative explanation for these differences is that our analysis had more robust adjustment for other covariates than did prior studies. Given that peak V02 and NTproBNP are among the strongest known predictors of outcome in patients with heart failure, their inclusion in multivariable models makes it more difficult for additional predictors (such as galectin-3) to provide independent prognostic information. Biomarkers may potentially be useful, even if they do not add information, if they can simplify prognostication by eliminating the need for expensive testing or difficult-to-obtain data (such as results from CPX testing). However, our data generally do not suggest significant added prognostic utility for galectin-3 even when CPX variables were excluded from the model, particularly when NTproBNP is included. Prior studies have generally adjusted for a much smaller number of covariates, and have not had data from CPX testing. In a recent study by De Boer, galectin-3 remained significant after adjustment for several covariates but was no longer significant after adjustment for LVEF.6 In an analysis of data from the DEAL study, galectin-3 persisted as a significant predictor after adjustment for age, gender, NTproBNP, and estimated glomerular filtration rate.7
Additionally, the HF-ACTION cohort, which included ambulatory heart failure patients able to be randomized into a structured exercise program, was generally healthier than patients in previous studies. The median galectin-3 level (14.0 ng/mL) was similar to that in the study by Tang et al9 but lower than that in the COACH study (20.0 ng/mL)6 and the DEAL study (17.6 ng/mL).7 Whether the relatively lower galectin-3 levels in our population contributed to the difference in our results compared with other studies is unknown. HF-ACTION was also limited to patients with systolic dysfunction (EF <0.40), and data from de Boer et al suggested it may be a more useful marker in patients with heart failure and preserved systolic function.6 Finally, we studied ambulatory heart failure patients, and the prognostic performance of galectin-3 may differ in those with acute decompensation.5
Although much of the focus in the assessment of new biomarkers in heart failure has focused on diagnosis and risk stratification, other potential indications for biomarkers may be of equal or greater importance. In particular, the development of markers that can aid in guiding therapy would be a major advance in heart failure management. Multiple studies have investigated the role of natriuretic peptides in guiding chronic heart failure therapy,12 but data on other markers to guide therapy are lacking. In animal models, galectin-3 appears to be a mediator of ongoing fibrosis and remodeling of the extracellular matrix, a process that plays a potentially important pathophysiologic role in heart failure progression. Echocardiographic data have correlated galectin-3 levels with measures of diastolic function in some studies13 but not in others.9 This raises the question as to whether assessment of galectin-3 could identify specific subsets of patients who might be more likely to respond to agents targeting fibrosis or, conversely, whether elevated galectin-3 levels could identify individuals with ongoing fibrosis who might be unresponsive to conventional heart failure therapy, as suggested by a recent analysis from the CORONA study.14 These hypotheses require further studies in order to fully elucidate the role of galectin-3 in heart failure management.
Conclusions
In a large well-treated cohort of ambulatory patients with systolic heart failure, galectin-3 was associated with poor functional capacity and other known markers of disease severity. NTproBNP and galectin-3 appeared to provide complementary prognostic information when examined in isolation. However, after multivariable adjustment for a large number of clinical variables, including NTproBNP and data from CPX testing, galectin-3 was no longer a significant predictor of any of the cardiovascular outcomes examined in our study. Whether galectin-3 may have other potentially important uses in heart failure, such as predicting response to therapy, remains unknown.
Supplementary Material
Acknowledgments
Sources of Funding The HF-ACTION study was funded by the National Heart, Lung and Blood Institute. The HF-ACTION Biobank was funded independently by the Duke Clinical Research Institute. This analysis was funded by a grant from BG Medicine.
Disclosures Drs. Felker, Fiuzat, and O’Connor have received research funding from BG Medicine, Critical Diagnostics, and Roche Diagnostics. Drs. Felker and O’Connor have served as consultants for BG Medicine, Critical Diagnostics, and Roche Diagnostics. Dr. Zannad has received research funding from BG Medicine and Roche Diagnostics and served as a consultant for BG Medicine.
Footnotes
None of the other authors report any conflicts.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Biomarkers in heart failure. New England Journal of Medicine. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 3.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does b-type natriuretic peptide predict death and cardiac events in patients with heart failure: Systematic review. British Medical Journal. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 5.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 6.de Boer RA, Lok DJA, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Annals of Medicine. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the deal-hf study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueland T, Aukrust P, Broch K, Aakhus S, Skardal R, Muntendam P, Gullestad L. Galectin-3 in heart failure: High levels are associated with all-cause mortality. Int J Cardiol. 2011;150:361–364. doi: 10.1016/j.ijcard.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 9.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, Klein AL. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108:385–390. doi: 10.1016/j.amjcard.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, Missov ED, Clerico A, Tognoni G, Cohn JN on behalf of the Val-HeFT Investigators. Direct comparison of b-type natriuretic peptide (bnp) and amino-terminal probnp in a large population of patients with chronic and symptomatic heart failure: The valsartan heart failure (val-heft) data. Clin Chem. 2006;52:1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 11.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. European Journal of Heart Failure. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gullestad L, Ueland T, Nymo SH, Kjekshus J, Hulte J, Munthendam P, Adourian A, McMurray JJV, Wikstrand J, Aukrust P. Galectin-3 predicts mortality and response to statin therapy in chronic heart failure. Eur Heart J. 2011;32:297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


