Abstract
Second generation antipsychotics (SGAs) have been linked to metabolic and bone disorders in clinical studies, but the mechanisms of these side effects remain unclear. Additionally, no studies have examined whether SGAs cause bone loss in mice. Using in vivo and in vitro modeling we examined the effects of risperidone, the most commonly prescribed SGA, on bone in C57BL6/J (B6) mice. Mice were treated with risperidone orally by food supplementation at a dose of 1.25 mg/kg daily for 5 and 8 weeks, starting at 3.5 weeks of age. Risperidone reduced trabecular BV/TV, trabecular number and percent cortical area. Trabecular histomorphometry demonstrated increased resorption parameters, with no change in osteoblast number or function. Risperidone also altered adipose tissue distribution such that white adipose tissue mass was reduced and liver had significantly higher lipid infiltration. Next, in order to tightly control risperidone exposure, we administered risperidone by chronic subcutaneous infusion with osmotic minipumps (0.5 mg/kg daily for 4 weeks) in 7 week old female B6 mice. Similar trabecular and cortical bone differences were observed compared to the orally treated groups (reduced trabecular BV/TV, and connectivity density, and reduced percent cortical area) with no change in body mass, percent body fat, glucose tolerance or insulin sensitivity. Unlike in orally treated mice, risperidone infusion reduced bone formation parameters (serum P1NP, MAR and BFR/BV). Resorption parameters were elevated, but this increase did not reach statistical significance. To determine if risperidone could directly affect bone cells, primary bone marrow cells were cultured with osteoclast or osteoblast differentiation media. Risperidone was added to culture medium in clinically relevant doses of 0, 2.5 or 25 ng/ml. The number of osteoclasts was significantly increased by addition in vitro of risperidone while osteoblast differentiation was not altered. These studies indicate that risperidone treatment can have negative skeletal consequences by direct activation of osteoclast activity and by indirect non-cell autonomous mechanisms. Our findings further support the tenet that the negative side effects of SGAs on bone mass should be considered when weighing potential risks and benefits, especially in children and adolescents who have not yet reached peak bone mass.
Introduction
Second-generation antipsychotics (SGAs) are used to treat major psychiatric disorders in part due to their lower incidence of extrapyrimidal side effects compared to first-generation antipsychotics (FGAs) [1]. Risperidone is one such SGA that is currently indicated for use in adolescents (as young as 10) and adults with schizophrenia and bipolar disorders, although it is widely used for attention deficit disorders. Risperidone is also approved for use in children as young as 5 years old for treatment of irritability associated with autism. Even though SGAs are highly prescribed, they have been linked to metabolic disorders including obesity, hyperglycemia and dyslipidemia [2–4]. The mechanism of metabolic changes associated with antipsychotics is unknown, but it is clear that a single dose (intravenous or intracerebroventricular) of certain SGAs (olanzapine, clozapine, and to a lesser degree risperidone) can dramatically reduce insulin sensitivity in the liver and this effect continues after multiple doses [5]. Additionally, SGA-linked obesity and type 2 diabetes mellitus is more prevalent in children and adolescents [6–8].
The relationship among energy metabolism, insulin signaling and bone remodeling is becoming more apparent, so it is likely that drugs that alter energy metabolism will ultimately affect bone mineral density [9]. Indeed, clinical studies demonstrate reduced bone mineral density and increased fracture risk in patients treated with risperidone and other SGAs, although these studies are confounded by indication (since the underlying disorders treated with antipsychotic medication can be associated with reduced bone mineral density in some cohorts) [10–16]. Risperidone can induce hyperprolactinemia by dopamine receptor blockade and this can lead to hypothalamic hypogonadism, which has been suggested as a possible mechanism of bone loss [11, 12, 15, 17]. However, a recent study demonstrated that less than half of the patients using risperidone developed hyperprolactinemia [11]. In addition, the increase in prolactin serum levels due to risperidone treatment is often a temporary event [18]. Moreover, unlike FGAs that bind mainly dopamine (D2) receptors, SGAs bind to multiple targets, including serotonin (5-HT), histamine, and D2 receptors, and therefore can affect additional organ systems that indirectly impact skeletal remodeling.
Despite some evidence that SGAs have a deleterious effect on bone, rodent studies involving SGA administration have focused on prolactin and the major metabolic consequences including weight gain, fat redistribution and glucose intolerance. However, no studies have examined whether risperidone is capable of causing direct deleterious effects on bone, nor have any studies been reported in mice. Here, we examined the effects of risperidone on bone and adipose tissue metabolism, and found significant trabecular and cortical bone loss independent of weight gain and overt metabolic dysfunction using two methods of risperidone administration (orally in food and by subcutaneous infusion). Reduced trabecular bone mass in mice fed a diet containing risperidone was due to increased resorption, an effect that can be recapitulated by direct risperidone administration to primary bone marrow derived osteoclasts in vitro. On the other hand, chronic infusion of risperidone suppressed bone formation, and showed a trend toward increased resorption, indicating that the mode of delivery and age of mice are important variables. Despite these differences, we have clearly established a negative role for risperidone on bone metabolism through both direct and indirect mechanisms.
Materials and Methods
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Maine Medical Center Research Institute. The animals were maintained on 12 h light/12 h dark cycles. The mice had free access to water and food.
Mice – oral risperidone administration
Male C57BL/6J (B6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 3 weeks of age. Mice were fed normal laboratory chow until 3.5 weeks of age, when they were switched to wet mash supplemented with risperidone such that the dose delivered was 1.25 mg/kg/day or control wet mash. Food intake was monitored twice per week to ensure correct dosing. Mice were maintained on risperidone or control diet for five (n=8 control, 8 risperidone) or eight weeks (n=8 control, 8 risperidone), at which time mice were euthanized and tissues were collected after an overnight fast. Livers were stained for lipid deposition with Oil Red O.
Mice – risperidone infusion
Female C57BL6/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age. At 7 weeks of age, mice were implanted with Alzet Osmotic Minipumps (Durect Corporation, Cupertino, CA) containing either 0.5 mg/kg/day risperidone (Sigma, St. Louis, MO) or vehicle in the subcutaneous region dorsal to the right hip. Concentration of risperidone was based on the average weight of C57BL/6 female mice from 7–11 weeks of age. After four weeks of risperidone or vehicle infusion mice were euthanized and tissues collected after an overnight fast.
Dual-energy X-ray absorptiometry (DXA)
Control and risperidone treated mice were measured at the time of harvest for lean muscle mass, fat, and bone mineral density using the PIXImus dual-energy X-ray densitometer (GE-Lunar, Madison, WI, USA). The PIXImus was calibrated daily with a mouse phantom provided by the manufacturer. Mice were placed ventral side down with each limb and tail positioned away from the body. Full body scans were obtained, and X-ray absorptiometry data were gathered and processed with the manufacturer's supplied software (Lunar PIXImus 2, version 2.1). The head was specifically excluded from all analyses due to concentrated mineral in skull and teeth.
Micro-computed tomography (µCT)
Micro-architecture of the distal trabecular bone and midshaft cortical bone of the femur were analyzed by µCT (resolution 10 µm, VivaCT-40, Scanco Medical AG, Bassersdorf, Switzerland). Bones were scanned at energy level of 55kVp, and intensity of 145 µA. The VivaCT-40 is calibrated weekly using a phantom provided by Scanco. Trabecular bone volume fraction and micro-architecture were evaluated in the secondary spongiosa, starting proximately at 0.6 mm proximal to the distal femoral growth plate, and extending proximally 1.5 mm. Approximately 230 consecutive slices were made at 10.5 um interval at the distal end of the growth plate and extending in a proximal direction, and 180 contiguous slices were selected for analysis. Measurements included bone volume/total volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th.) and trabecular separation (Tb.Sp.). Scans for the cortical region were measured at the mid-point of each femur, with an isotropic pixel size of 21 µm and slice thickness of 21 µm, and used to calculate the average bone area (BA), total cross-sectional area (TA), bone area/total area (BA/TA), and cortical thickness (Ct.Th.). For mid-shaft analysis, the cortical shell was contoured by user-defined threshold and iterated across the 50 slices. All scans were analyzed using manufacturer software (Scanco, version 4.05).
Histology and Quantitative Histomorphometry
Qualitative histologic analysis and quantitative static and dynamic histomorphometry were performed as described previously [19]. To examine bone-formation rates, calcein label (20 mg/kg) and demeclocycline label (20 mg/kg) were injected intraperitoneally at 9 and 2 days prior to euthanization, respectively. Immediately after euthanization, tibias for histomorphometry were placed in 70% ethanol and maintained in the dark at 4°C. Histomorphometric measurements were performed on the secondary spongiosa of the proximal tibia metaphysis using an OsteoMeasure morphometry system (Osteometrics, Atlanta, GA, USA). For dynamic histomorphometry, mineralizing surface per bone surface (MS/BS, %) and mineral apposition rate (MAR, µm/day) were measured in unstained sections under ultraviolet (UV) light and used to calculate bone-formation rate with a surface referent (BFR/BS, µm3/µm2/year), volume referent (BFR/BV, %/year) and tissue referent (BFR/TV, %/year). Static measurements included BV/TV (%), trabecular thickness (Tb.Th, µm), trabecular number (Tb.N, /mm), trabecular separation (Tb.Sp, µm), eroded surface per bone surface (ES/BS, %), osteoid surface per bone surface (OS/BS, %), osteoid volume per tissue volume (OV/TV, %), osteoid thickness (O.Th, µm), osteoblast surface per bone surface (Ob.S/BS, %), osteoclast surface per bone surface (Oc.S/BS, %), osteoblast number per bone perimeter (N.Ob/B.Pm, /mm), osteoblast number per tissue area (N.Ob/T.Ar, /mm2), osteoclast number per bone perimeter (N.Oc/B.Pm, /mm), osteoclast number per tissue area (N.Oc/T.Ar, /mm2), and adipocytes per total area (/mm2). Terminology and units followed the recommendations of the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research [20].
Magnetic Resonance Imaging
Axial and coronal images were acquired in a 7.0T Bruker PharmaScan magnet. The animals were anesthetized with inhaled isoflurane 1%, 0.4 l/min. The infused volume was adjusted, occasionally, depending on animal respiration frequency. The acquisition method used for body fat was: TR 600 ms, TE 87 ms, FOV 40×40×10 mm, matrix size 256×256×32, slice thickness10 mm, 1 slice, no fat suppression, 4 average, with total scan time of about 11 minutes. For single pulse spectroscopy: TR 1000 ms, no fat suppression, 400 averages, with total scan time of about 7 min. Spectroscopy was carried out by using the following parameters: TR= 2500 msec, TE= 20 msec, voxel= 0.75×7×0.75 mm, no fat suppression, 400 averages, with total scan time about 17 minutes.
Serum Measurements
Serum adiponectin was measured with the Mouse Adiponectin Assay Kit (Meso Scale Discovery, Gaithersburg, MD). Serum prolactin was measured with the Mouse/Rat Prolactin Kit (Calbiotech, Spring Valley, CA). Serum P1NP and CTx were measured with the Rat/Mouse P1NP EIA and the RatLaps EIA, respectively (Immunodiagnostic Systems, Scottsdale, AZ).
Insulin and Glucose Tolerance
For the insulin tolerance test (ITT), mice were fed ad libitum and injected I.P. with insulin at a dose of 1 U/kg. Glucose levels were then measured at 0, 20, 40, 60, 100, 120 and 150 min post injection. For the glucose tolerance test (GTT), mice were placed in a clean cage with water and fasted overnight (16 hr). A 1 g/kg dose of glucose was administered I.P. and blood glucose levels were measured at 0, 20, 40, 60, 80, 100, 120, 150 and 180 min post injection. Glucose levels were measured using the OneTouch Ultra Glucometer (LifeScan, Inc., Milpitas, California, USA) per manufacturer's instructions.
Mass Spectrometry
Risperidone and the active metabolite 9-OH risperidone levels were measured by mass spectrometry in serum as previously shown [21].
Osteoclast Culture
Whole bone marrow was isolated from 8 week old female C57BL6/J mice and plated in 96-well plates at a density of 2 × 106 cells/well and cultured in the presence of RANKL and M-CSF as previously described [22]. Five replicates were performed per experiment. Risperidone (0, 2.5 or 25 ng/ml) was added with each media change at day 0, 3 and 6. Osteoclasts were harvested at day 7 by fixation with 2.5% glutaraldehyde and stained for TRAP (Sigma Kit, St. Louis, MO). TRAP positive osteoclasts with three or more nuclei were counted.
Statistical Analyses
All data is presented as mean ± standard error. Statistical significance was determined using a two-tailed Student’s t-test (alpha = 0.05) with Microsoft Excel (Microsoft, Redmond, WA).
Results
Oral Risperidone Administration
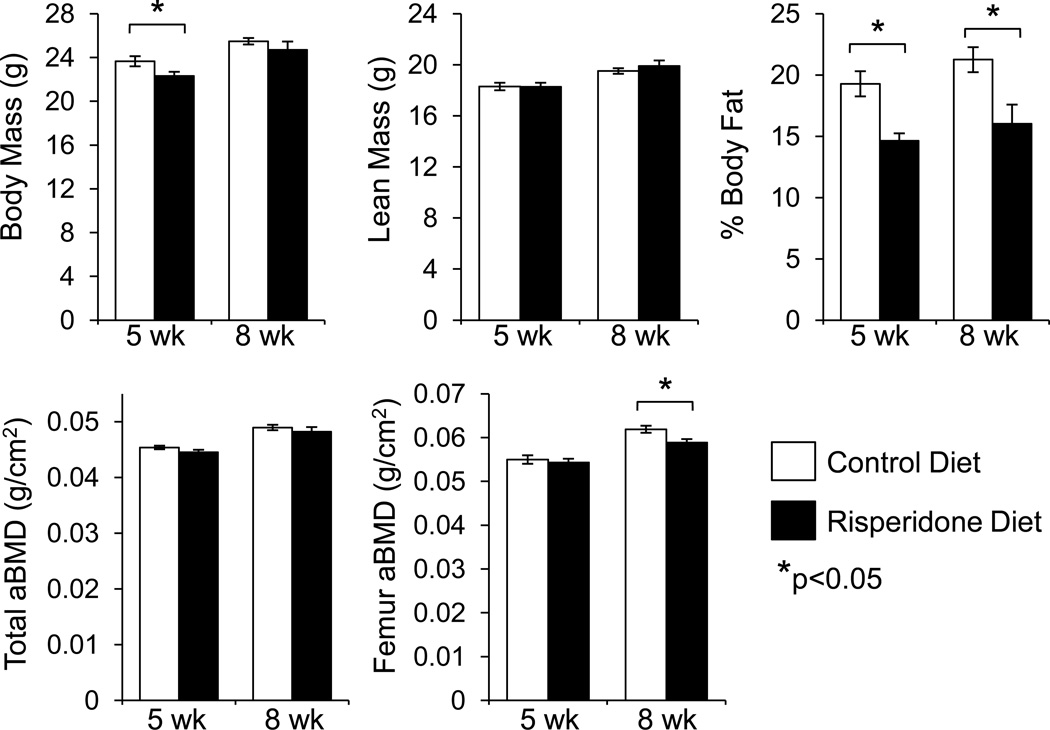
Risperidone was administered to male 3.5 week old B6 mice at a dose of 1.25 mg/kg per day in the food to examine its effects on bone metabolism. Risperidone fed mice had significantly lower body weights and lower body fat compared to controls after five weeks of treatment (Figure 1). After eight weeks, the lower body fat remained statistically significant but there was no significant difference in body mass. Although there was no detectable difference in total aBMD by DXA at either time point, risperidone-fed mice had significantly lower femur aBMD after eight weeks of treatment compared to untreated controls.
Figure 1.
Reduced body fat and femur areal bone mineral density after risperidone administration. Risperidone was administered to male B6 mice orally in the food for five or eight weeks, starting at 3.5 weeks of age. Lean mass, fat mass, total and femur areal BMD were measured by DXA. Bars represent mean ± standard error of untreated (white) and risperidone treated mice (black). N=8. *p<0.05 by Student’s t-test.
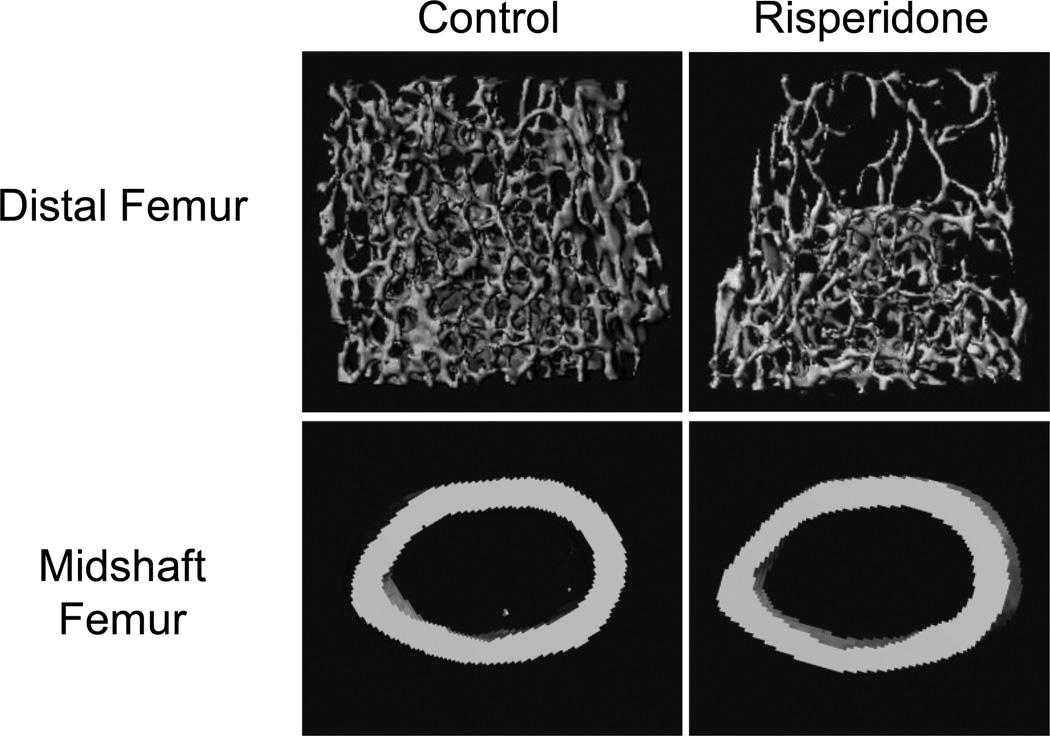
After eight weeks of risperidone administration, distal femur trabecular bone volume fraction (BV/TV) was 23% lower (p<0.01) than that of untreated control mice (Figure 2, Table I). At this time point, trabecular number and connectivity density were significantly lower while trabecular separation was significantly higher than control. By the fifth week of treatment, risperidone treated mice had reduced femur midshaft cortical thickness, which can be attributed to a reduction in periosteal circumference compared to controls (Table I). After eight weeks, however, the difference in periosteal circumference was no longer evident, although risperidone-treated mice continued to have significantly lower percent Ct.Ar/T.Ar (Table I).
Figure 2.
Reduced trabecular and cortical bone mass in the femur after eight weeks of risperidone administration. Shown are representative three-dimensional µCT images from control and risperidone treated mice.
Table I.
Male femur µCT after five and eight weeks of risperidone treatment.
| Five Week Treatment | Eight Week Treatment | |||
|---|---|---|---|---|
| Control (n=8) | Risperidone (n=8) | Control (n=8) | Risperidone (n=8) | |
| Trabecular | ||||
| BV/TV (%) | 8.1 ± 0.6 | 7.7 ± 0.8 | 9.5 ± 0.4 | 7.3 ± 0.6** |
| Tb.N (1/mm) | 4.5 ± 0.1 | 4.3 ± 0.2 | 4.6 ± 0.1 | 4.2 ± 0.1* |
| Tb.Th (mm) | 0.0379 ± 0.0010 | 0.0378 ± 0.0012 | 0.0401 ± 0.0007 | 0.0383 ± 0.0005 |
| Tb.Sp (mm) | 0.219 ± 0.007 | 0.233 ± 0.011 | 0.207 ± 0.004 | 0.232 ± 0.007* |
| Conn.D (1/mm3) | 100 ± 12 | 92 ± 15 | 130 ± 12 | 82 ± 12* |
| Cortical | ||||
| Tt.Ar (mm2) | 1.96 ± 0.03 | 1.84 ± 0.02** | 1.95 ± 0.02 | 1.98 ± 0.04 |
| Ma.Ar (mm2) | 1.12 ± 0.02 | 1.07 ± 0.01 | 1.05 ± 0.01 | 1.09 ± 0.02 |
| Ct.Ar (mm2) | 0.84 ± 0.02 | 0.77 ± 0.01** | 0.90 ± 0.01 | 0.89 ± 0.02 |
| Ct.Ar/Tt.Ar (%) | 42.9 ± 0.3 | 41.7 ± 0.5* | 46.1 ± 0.3 | 44.9 ± 0.4* |
| Ct.Th (mm) | 0.191 ± 0.003 | 0.180 ± 0.002** | 0.204 ± 0.002 | 0.201 ± 0.002 |
p<0.05,
p<0.01 compared to age-matched control by two-tailed Student’s t-test.
Abbreviations: BV/TV, bone volume/total volume; Tb., trabecular; N, number; Th, thickness; Sp, separation; Conn.D, connectivity density; Tt., total; Ar, area; Ma, medullary; Ct., cortical.
Static and dynamic histomorphometry was performed on trabecular bone of the proximal tibia in order to determine how risperidone altered remodeling. Consistent with femur µCT results, after eight weeks of treatment, trabecular BV/TV and trabecular number were significantly lower in the risperidone treated group compared to untreated controls (Table II). Additionally, the reduction in BV/TV was also statistically significant in the tibia by 5 weeks of treatment, which was not apparent in the femur µCT. Neither treatment period significantly altered measurements of mineralization, bone formation, or osteoblast numbers. However, risperidone treated mice did have significantly elevated bone resorption parameters, at both five and eight weeks of treatment, including percent of bone surface in contact with osteoclasts and number of osteoclasts per unit bone surface. Erosion surface was also 2.5-fold higher in mice treated with risperidone for five weeks compared to control mice. This difference was not statistically detectable at the eight-week time point. Bone marrow adipocyte number was not significantly different after five or eight weeks of oral risperidone administration vs control treated animals (not shown).
Table II.
Male static and dynamic tibia histomorphometry after five and eight weeks of risperidone treatment.
| Five Week Treatment | Eight Week Treatment | |||||
|---|---|---|---|---|---|---|
| Control (n=6–8) |
Risperidone (n=8) |
p value | Control (n=7–8) |
Risperidone (n=8) |
p value | |
| BV/TV (%) | 8.8 ± 0.4 | 7.3 ± 0.5* | 0.03 | 9.9 ± 1.1 | 6.9 ± 0.7* | 0.03 |
| Tb.Th (µm) | 29.9 ± 1.2 | 27.2 ± 1.0 | 0.10 | 31.3 ± 1.2 | 28.5 ± 0.9 | 0.09 |
| Tb.N (/mm) | 2.95 ± 0.12 | 2.66 ± 0.12 | 0.11 | 3.12 ± 0.22 | 2.38 ± 0.19* | 0.03 |
| Tb.Sp (µm) | 313 ± 15 | 353 ± 16 | 0.09 | 301 ± 24 | 415 ± 42* | 0.03 |
| MS/BS (%) | 27.7 ± 4.2 | 23.2 ± 2.1 | 0.32 | 26.3 ± 2.5 | 27.6 ± 2.3 | 0.71 |
| MAR (µm/day) | 2.03 ± 0.09 | 2.11 ± 0.18 | 0.72 | 1.18 ± 0.08 | 1.02 ± 0.07 | 0.15 |
| BFR/BS (µm3/µm2/year) | 199 ± 24 | 180 ± 22 | 0.57 | 113 ± 14 | 103 ± 13 | 0.61 |
| BFR/BV (%/year) | 1301 ± 150 | 1273 ± 146 | 0.90 | 706 ± 61 | 741 ± 113 | 0.78 |
| BFR/TV (%/year) | 117 ± 17 | 92 ± 10 | 0.21 | 76 ± 14 | 49 ± 7 | 0.13 |
| Ob.S/BS (%) | 5.2 ± 1.1 | 6.5 ± 1.3 | 0.43 | 2.3 ± 0.6 | 3.0 ± 1.0 | 0.53 |
| N.Ob/T.Ar (/mm2) | 26.2 ± 4.9 | 28.9 ± 4.9 | 0.70 | 12.2 ± 3.2 | 11.2 ± 3.0 | 0.81 |
| N.Ob/B.Pm (/mm) | 4.6 ± 1.0 | 5.4 ± 0.8 | 0.57 | 2.1 ± 0.5 | 2.4 ± 0.7 | 0.78 |
| OV/TV (%) | 0.044 ± 0.011 | 0.047 ± 0.011 | 0.89 | 0.012 ± 0.004 | 0.015 ± 0.006 | 0.63 |
| OS/BS (%) | 3.2 ± 0.8 | 3.3 ± 0.7 | 0.89 | 0.8 ± 0.3 | 1.6 ± 0.6 | 0.24 |
| O.Th (µm) | 2.1 ± 0.3 | 2.5 ± 0.2 | 0.35 | 2.7 ± 0.4 | 1.6 ± 0.4 | 0.08 |
| Oc.S/BS (%) | 2.5 ± 0.6 | 5.7 ± 0.9* | 0.01 | 1.5 ± 0.3 | 2.9 ± 0.5* | 0.02 |
| N.Oc/T.Ar (/mm2) | 6.4 ± 1.6 | 11.8 ± 1.2* | 0.02 | 3.3 ± 0.6 | 5.2 ± 0.9 | 0.11 |
| N.Oc/B.Pm (/mm) | 1.1 ± 0.3 | 2.3 ± 0.3* | 0.01 | 0.6 ± 0.1 | 1.1 ± 0.1* | 0.01 |
| ES/BS (%) | 0.98 ± 0.20 | 2.56 ± 0.38* | <0.01 | 0.53 ± 0.14 | 0.93 ± 0.27 | 0.21 |
p<0.05 by two-tailed Student’s t-test.
Abbreviations: BV, bone volume; TV, tissue volume; Tb., trabecular; N, number; Th, thickness; Sp, separation; MS, mineralizing surface; BS, bone surface; MAR, mineral apposition rate; BFR, bone formation rate; Ob.S, osteoblast surface; N.Ob, number of osteoblasts; T.Ar, tissue area; B.Pm, bone perimeter; OV, osteoid volume; OS, osteoid surface; O.Th, osteoid thickness; Oc.S, osteoclast surface; N.Oc, number of osteoclasts; ES, eroded surface.
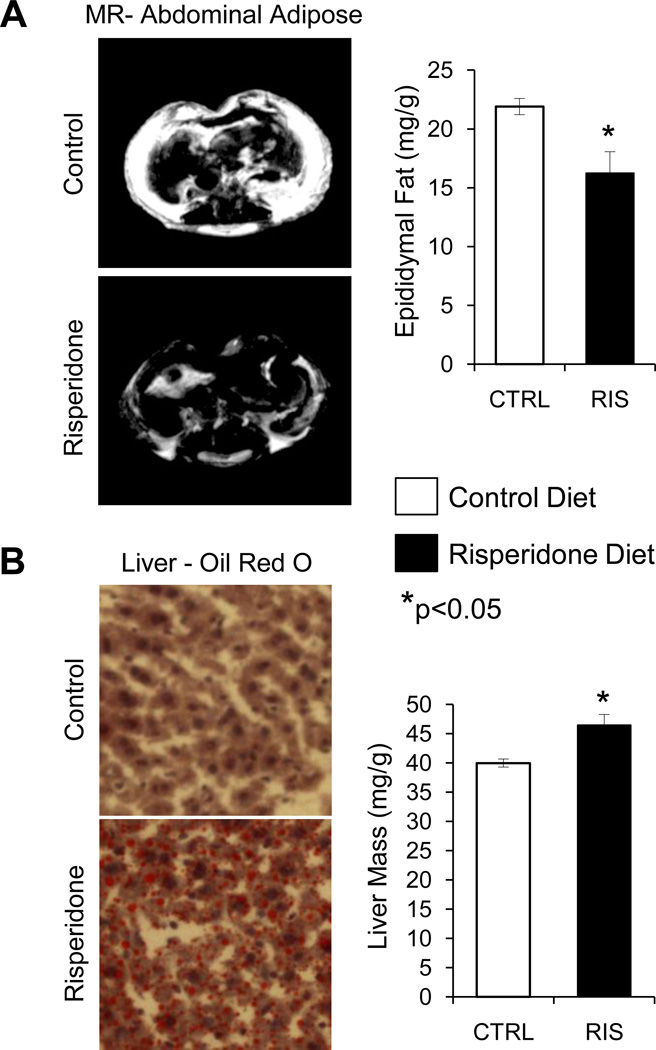
In order to further investigate adipose tissue changes observed by DXA, we examined abdominal and subcutaneous fat by MRI. Representative MR images demonstrate that both subcutaneous and visceral fat was reduced after five weeks of risperidone treatment (Figure 3A). This reduction in mass of normal white adipose tissue depots was coupled with an increase in liver adiposity as measured by Oil Red O staining and by weight measurements (Figure 3B).
Figure 3.
Reduced white adipose tissue and increased liver adiposity after five weeks of risperidone treatment. (A) Representative abdominal MR images and epididymal fat mass. (B) Liver Oil Red O staining (red stain indicates presence of lipids) and liver mass. Bars represent mean ± standard error of untreated (white) and risperidone treated mice (black). N=8. *p<0.05 by Student’s t-test.
Given the potential variability due to food supplementation, we measured risperidone and 9-OH risperidone levels in the serum of mice at each time point. Although risperidone levels were low to undetectable by mass spectrometry (likely due to the overnight fast and therefore no new drug exposure), the more stable 9-OH risperidone was detectable at levels of 7.9 ± 3.5 ng/ml (range 1.6–24.1 ng/ml) for the five week treatment and 6.0 ± 0.8 ng/ml (range 4.6–9.3 ng/ml) for the eight week treatment, which are within the range obtained in human patients [23–25].
Subcutaneous Risperidone Infusion
Due to variability in the amount of risperidone delivered per mouse by food supplementation, we administered risperidone (0.5 mg/kg per day) to 7-week old C57BL/6J mice via subcutaneous osmotic minipumps for 28 days. In the literature, female mice are more likely to have a weight-gain side effect of SGA administration; therefore we used female mice instead of males to try to exclude weight loss as a confounding factor in the bone effects we observed with male mice. At two and four weeks after pump implantation, serum risperidone concentration was 5.9 ± 0.7 ng/ml (range 5.0–7.9 ng/ml) and 1.3 ± 0.3 ng/ml (range 0.8–2.3 ng/ml), respectively. The concentration of 9-OH risperidone was 3.8 ± 0.5 ng/ml (range 2.8–5.7 ng/ml) at two weeks and 2.4 ± 0.3 ng/ml (range 1.5–3.1 ng/ml) at four weeks, which was considerably less variable than levels measured during oral administration.
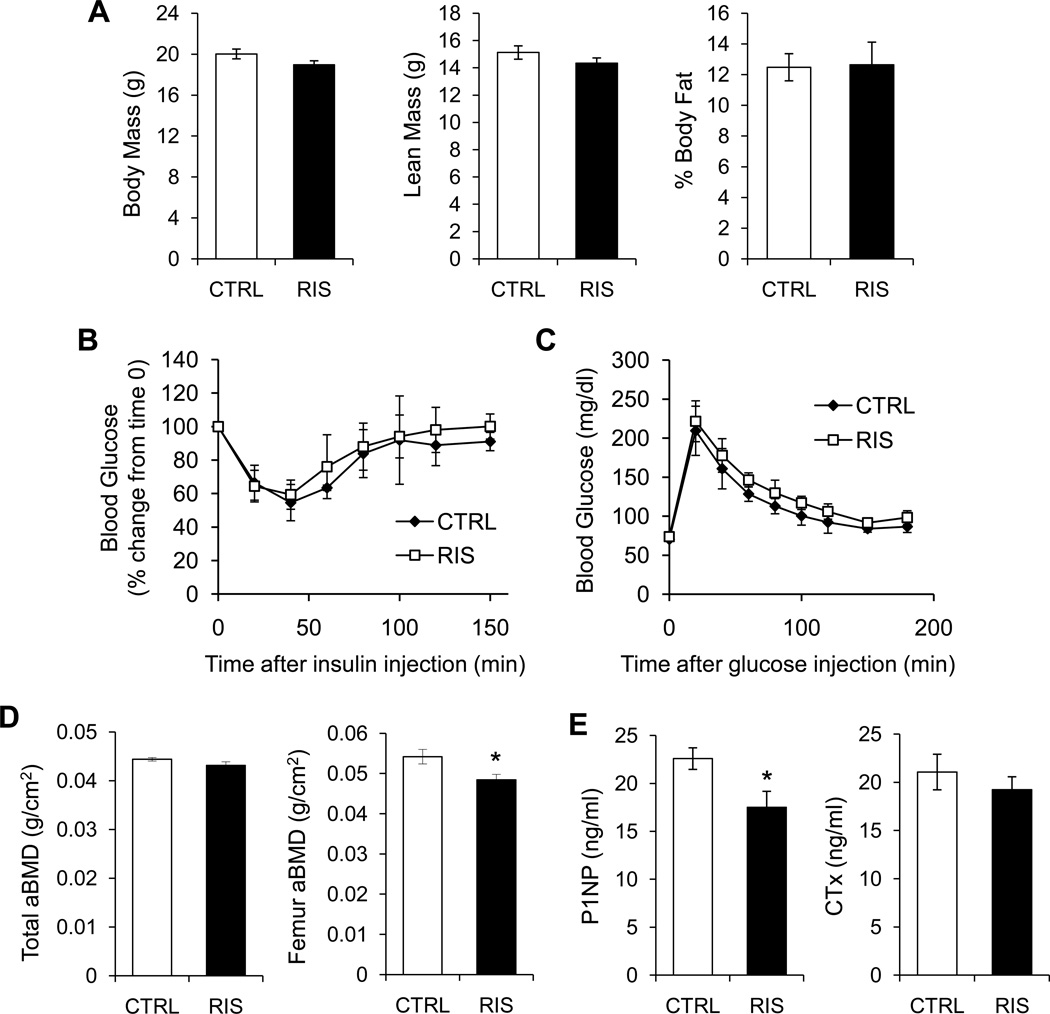
Mice did not have significantly altered body mass, fat mass or lean mass after infusion of risperidone (Figure 4A). Additionally, insulin tolerance (Figure 4B) and glucose tolerance (Figure 4C) were not different between treatment groups. Similar to oral administration, whole body aBMD was not different between groups. Despite the shortened treatment duration and lower dose, femur aBMD was lower in mice treated with risperidone compared to control (Figure 4D). Both distal femur trabecular BV/TV and connectivity density were significantly reduced (44% and 64%, respectively) (Table III). Cortical thickness, cortical area and percent of the total area were also lower in the femur of risperidone treated mice compared to control.
Figure 4.
Subcutaneous infusion of risperidone did not alter total body, lean or fat mass, but did reduce femur areal BMD and serum P1NP. Risperidone was administered to female B6 mice by subcutaneously implanted osmotic minipump for four weeks, starting at 8 weeks of age. (A) Lean mass and fat mass were measured by DXA. (B) serumInsulin tolerance test. (C) Glucose tolerance test. (D) Total and femur aBMD were measured by DXA. (E) Serum P1NP and CTx were measured after an overnight fast. Bars represent mean ± standard error of untreated (white) and risperidone treated mice (black). N=5. *p<0.05 by Student’s t-test.
Table III.
Female tibia µCT after four weeks of risperidone infusion.
| Osmotic Minipumps |
p value |
||
|---|---|---|---|
|
Saline (n=5) |
Risperidone (n=5) |
||
| Trabecular | |||
| BV/TV (%) | 4.3 ± 0.6 | 2.4 ± 0.3* | 0.02 |
| Tb.N (1/mm) | 3.1 ± 0.2 | 2.9 ± 0.1 | 0.07 |
| Tb.Th (mm) | 0.036 ± 0.002 | 0.033 ± 0.001 | 0.15 |
| Tb.Sp (mm) | 0.322 ± 0.017 | 0.359 ± 0.007 | 0.08 |
| Conn.D (1/mm3) | 28 ± 7 | 10 ± 2* | 0.03 |
| Cortical | |||
| Tt.Ar (mm2) | 1.73 ± 0.01 | 1.67 ± 0.03 | 0.14 |
| Ma.Ar (mm2) | 0.98 ± 0.01 | 0.99 ± 0.02 | 0.56 |
| Ct.Ar (mm2) | 0.75 ± 0.01 | 0.68 ± 0.02* | 0.03 |
| Ct.Ar/Tt.Ar (%) | 43.2 ± 0.5 | 40.6 ± 0.5* | 0.02 |
| Ct.Th (mm) | 0.182 ± 0.003 | 0.168 ± 0.004* | 0.02 |
p<0.05 by two-tailed Student’s t-test.
Abbreviations: BV/TV, bone volume fraction; Tb., trabecular; N, number; Th, thickness; Sp, separation; Conn.D, connectivity density; Tt., total; Ar, area; Ma, medullary; Ct., cortical.
Serum P1NP, a marker of bone formation, was lower in risperidone treated mice while there was no difference in CTx, a marker of resorption (Figure 4E). Consistent with these data, histomorphometric analysis of the tibia in 7 week old mice treated with subcutaneous risperidone infusion revealed significantly suppressed MAR (decreased 18%) and BFR/BV (decreased 34%). In addition, osteoblast number was markedly reduced (p=0.05) and there was a 53% increased in osteoclast number although it did not reach statistical significance (p=0.12) (Table IV).
Table IV.
Female static and dynamic histomorphometry after four weeks of risperidone infusion.
| Osmotic Minipumps | |||
|---|---|---|---|
|
Saline (n=4–5) |
Risperidone (n=4–5) |
p value | |
| BV/TV (%) | 5.1 ± 1.1 | 3.7 ± 0.9 | 0.34 |
| Tb.Th (µm) | 32.7 ± 2.5 | 30.4 ± 1.9 | 0.49 |
| Tb.N (/mm) | 1.50 ± 0.26 | 1.15 ± 0.24 | 0.34 |
| Tb.Sp (µm) | 775 ± 217 | 1230 ± 494 | 0.42 |
| MS/BS (%) | 30.4 ± 3.1 | 27.4 ± 2.8 | 0.49 |
| MAR (µm/day) | 2.96 ± 0.04 | 2.42 ± 0.10* | <0.01 |
| BFR/BS (µm3/µm2/year) | 329 ± 32 | 241 ± 25 | 0.08 |
| BFR/BV (%/year) | 2181 ± 241 | 1436 ± 167* | 0.04 |
| BFR/TV (%/year) | 95 ± 20 | 58 ± 18 | 0.22 |
| Ob.S/BS (%) | 6.6 ± 1.5 | 2.0 ± 1.4 | 0.05 |
| N.Ob/T.Ar (/mm2) | 15.5 ± 5.2 | 4.8 ± 3.1 | 0.12 |
| N.Ob/B.Pm (/mm) | 5.4 ± 1.4 | 1.5 ± 1.0 | 0.05 |
| OV/TV (%) | 0.003 ± 0.003 | 0.002 ± 0.001 | 0.82 |
| OS/BS (%) | 0.26 ± 0.26 | 0.46 ± 0.29 | 0.62 |
| O.Th (µm) | 0.69 ± 0.69 | 0.75 ± 0.46 | 0.95 |
| Oc.S/BS (%) | 2.9 ± 0.7 | 4.4 ± 0.9 | 0.19 |
| N.Oc/T.Ar (/mm2) | 3.1 ± 0.8 | 4.2 ± 1.1 | 0.43 |
| N.Oc/B.Pm (/mm) | 1.1 ± 0.2 | 1.7 ± 0.2 | 0.12 |
| ES/BS (%) | 1.49 ± 0.42 | 1.86 ± 0.39 | 0.53 |
| N.Ad/T.Ar (/mm2) | 7.9 ± 1.5 | 32.5 ± 10.0 | 0.07 |
p<0.05 by two-tailed Student’s t-test.
Abbreviations: BV, bone volume; TV, tissue volume; Tb., trabecular; N, number; Th, thickness; Sp, separation; MS, mineralizing surface; BS, bone surface; MAR, mineral apposition rate; BFR, bone formation rate; Ob.S, osteoblast surface; N.Ob, number of osteoblasts; T.Ar, tissue area; B.Pm, bone perimeter; OV, osteoid volume; OS, osteoid surface; O.Th, osteoid thickness; Oc.S, osteoclast surface; N.Oc, number of osteoclasts; ES, eroded surface; N.Ad, number of adipocytes.
Direct effect of risperidone on osteoclast differentiation
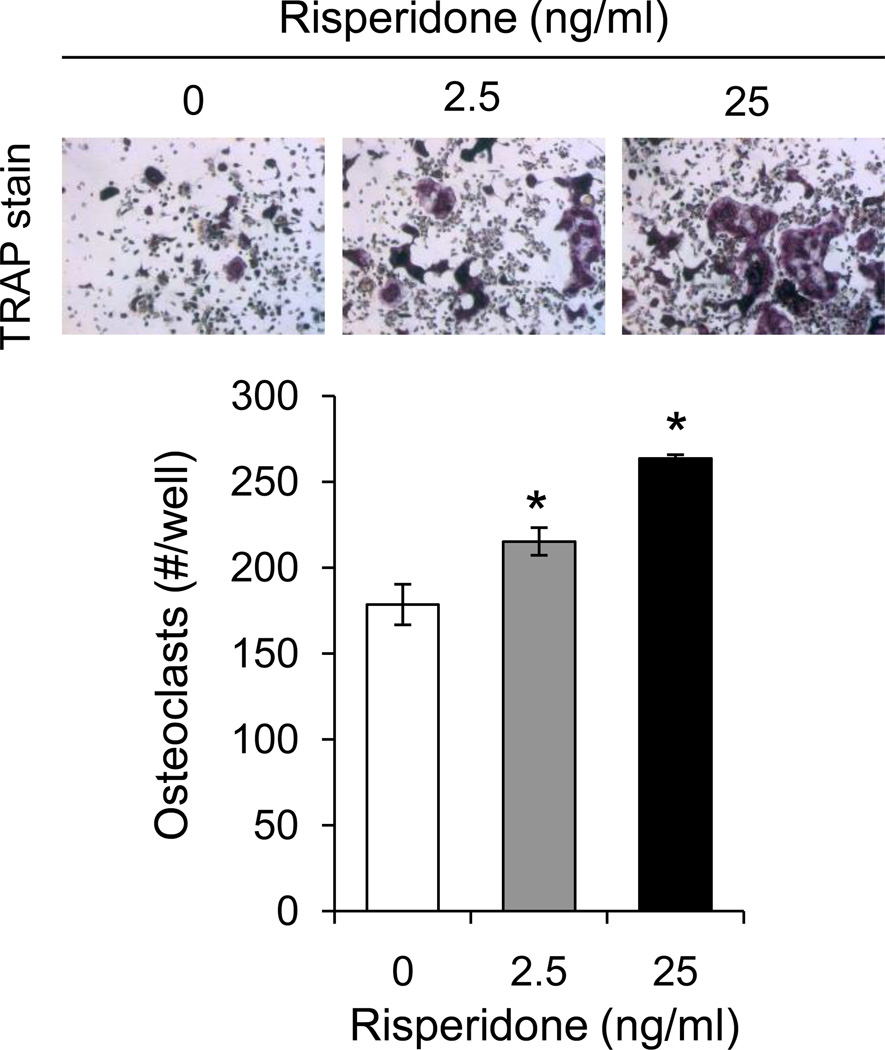
In order to determine whether the effects of risperidone on resorption parameters could be due to direct interaction with osteoclasts, we cultured primary osteoclasts from bone marrow in the presence or absence of risperidone. Addition of both 2.5 and 25 ng/ml risperidone with each media change increased TRAP positive osteoclast number in a dose dependent manner (Figure 5). On the other hand, when bone marrow stromal cells were exposed to risperidone in vitro, there were no differences in osteoblast proliferation or differentiation compared to untreated cells (data not shown).
Figure 5.
Risperidone induces osteoclastogenesis in vitro. Whole bone marrow was cultured in the presence of M-CSF and RANKL to induce osteoclastogenesis. From day 0, wells were also treated with 0, 2.5 or 25 ng/ml risperidone with each media change. Cells were fixed on day 6 and stained for TRAP. Osteoclasts (positive for TRAP and containing 3 or more nuclei) were counted. Bars represent mean ± standard error. N=3. *p<0.05 by Student’s t-test.
Discussion
In this paper we have clearly established that risperidone has a negative impact on bone mass in mice and that this effect may be due to both direct and indirect mechanisms. Using both male and female mice, and both oral administration and subcutaneous infusion, we found that risperidone reduces both trabecular and cortical bone mass, and that this effect is likely due to imbalanced bone remodeling (Table II). Interestingly, both modes of administration of risperidone reduced bone mass but histomorphometric analyses revealed there were different mechanisms of action for risperidone. For example, oral risperidone treatment was associated with a primary increase in bone resorption with little change in bone formation, whereas subcutaneous infusion of risperidone resulted in a reduction in P1NP (Figure 4B) and suppression of both bone formation and mineral apposition rates. These data suggest there may be both cell autonomous (i.e. on osteoclasts) and non-cell autonomous effects (i.e. on osteoblasts) since in vitro studies could not demonstrate significant changes in the pace of osteoblast differentiation in response to risperidone.
However, it should be noted that only 7-week-old female mice were administered subcutaneous risperidone whereas only 3.5-week-old male mice were treated with the oral form. At 7 weeks of age, females B6 mice are near their peak in trabecular number and trabecular BV/TV in the distal femur [26]. Thereafter, trabecular BV/TV in the distal femur declines due to enhanced resorption relative to formation. Risperidone treatment accelerated trabecular bone loss in B6 females by further suppressing bone formation. Male B6 mice gain trabecular BV/TV and number up to 8 weeks of age, thus during the first treatment period (3.5 to 8.5 weeks of age) normal modeling favors bone formation, whereas during the second period (8.5 to 11. 5 weeks of age) modeling tends to favor resorption [26]. Therefore, from 3.5 to 8.5 weeks of age, trabecular bone mass in the risperidone treated animals is likely unable to reach its peak due to elevated resorption compared to B6 controls. During the remainder of the treatment time (i.e. 8.5 to 11.5 weeks), formation and resorption begin to decrease, but resorption in the risperidone treated mice does not decrease to the level of the control mice. This effect, coupled to the inability of risperidone administered mice to reach peak trabecular bone mass, contributes to the greater % trabecular BV/TV reduction observed in the mice with longer risperidone exposure. Thus, gender differences, age differences, levels of risperidone achieved, as well as the direct and indirect effects of the drug on bone cells contribute to the skeletal response to this drug. Notwithstanding these confounding variables, it is clear that bone loss occurs with risperidone treatment of mice, independent of weight gain.
At a first glance, hyperprolactinemia would be another mechanism of bone loss secondary to risperidone, which is a dopamine receptor antagonist [11, 12]. However, there is controversy about risperidone-induced hyperprolactinemia and we were unable to show an elevation in serum prolactin with either type of risperidone administration. Furthermore, female mice subjected to risperidone treatment did not show uterine atrophy that would be expected with hypogonadism (not shown). While there are data suggesting that high PRL levels are a long-term complication of risperidone treatment [27, 28], others indicate that its occurrence is a transient side effect [18]. Therefore, an alternative mechanism likely exists for the profound alterations in bone turnover that we observed.
In addition to its actions as a dopaminergic antagonist, risperidone also has anti-serotonergic effects. Recently, serotonin was found to be a crucial intermediate molecule in the regulation of bone mass by leptin [29, 30]. Leptin modulates bone metabolism indirectly through the hypothalamus by activating the sympathetic nervous system (SNS), thereby suppressing bone formation while stimulating bone resorption via skeletal beta adrenergic receptors on osteoblasts [31, 32]. Leptin-deficient ob/ob mice show increased cancellous spine bone mass and overt obesity in spite of hypogonadism and elevated cortisol levels [33]. The pattern of bone changes in leptin-deficient mice may be reproduced by a chemical lesion of neurons in the region of the ventromedial hypothalamus [34]. However, another component of the central action of leptin emerged recently, when it was demonstrated that leptin receptor in hypothalamic ventromedial neurons is not essential to trigger leptin action [35]. Likely, leptin acts in other sites of the brain to regulate bone remodeling. Serotonergic neurons of the brainstem are a reasonable candidate to mediate this action, particularly since the consequences of leptin deficiency on bone turnover can be reversed by hampering serotonin production in the brainstem. Mice deficient in brain-derived production of serotonin (Tph2−/−) exhibit the opposite phenotype of leptin-deficient mice. Notably, Tph2−/− mice have decreased bone mass associated to low bone formation and increased bone resorption, albeit they exhibit normal levels of leptin [36]. Thus, it is plausible that the sympathetic nervous system activity may be elevated during risperidone administration and this may contribute to the non-cell autonomous effects on osteoblasts observed with subcutaneous administration.
Ota, et al. also demonstrated that rats treated with risperidone are resistant to weight gain [37, 38]. These authors attributed this observation to increased energy expenditure as suggested by the enhanced gene expression of Ucp1 in brown adipose tissue, and Ucp3, β1 adrenergic receptor and lipolytic enzymes in white adipose tissue. In accordance with the Ota results, our risperidone-treated mice did not show an obesity phenotype. Moreover, the present study shows that risperidone treatment was associated with increased fat deposition within the liver. This occurrence might reflect increased lipolysis in visceral adipose tissue, which promptly releases free fatty acids into the portal system, or may be due to a generalized redistribution of fat mass. However, we only demonstrated a non-significant increase in marrow adiposity in the subcutaneously infused mice (p=0.07) and no change in marrow adiposity in the orally administered mice. Taken together, the evidence from histomorphometry, bone turnover markers and µCT in risperidone treated mice prevent us from excluding a non-cell autonomous effect on bone remodeling due to increased sympathetic nervous system activity.
In our study and other rodent SGA studies, risperidone treated mice did not gain weight as is often observed in human clinical trials. However, it is necessary to take into account that the circumstances of our experiments are different from those observed in clinical investigation. Different psychiatric disorders (i.e., anorexia nervosa, schizophrenia and depression) have diverse impacts on body weight and eating behavior, probably reflecting typical alteration on brain physiology [30, 39, 40]. Thus, it can be hypothesized that the impact of drugs, with actions on neuromodulators, or on eating behavior in individuals can be different from that observed in conditions of psychiatric disorders.
Despite clear evidence for a mechanism of risperidone affecting bone metabolism indirectly through the central nervous system, we did observe increased osteoclast differentiation with direct risperidone administration in vitro (Figure 5). Osteoclasts express serotonin receptors and others that are potential targets for risperidone. Moreover, the in vitro changes in osteoclast differentiation are consistent with the in vivo histomorphometric data when mice are administered oral risperidone. However, as noted, we cannot exclude that there is a non-cell autonomous effect of risperidone on osteoblasts that stimulates RANKL and subsequently causes increased bone resorption. One possibility is that this is sympathetically mediated. Another possible mechanism is that risperidone activates PPARγ which can suppress bone formation and enhance osteoclastogenesis. Interestingly, in the mice treated by minipump, there was a four-fold increase in marrow adipocytes (p=0.07) that was not seen with oral administration.
In conclusion, the present data show that risperidone impacts bone remodeling in mice by acting on resorption and formation in both a cell autonomous and non-cell autonomous manner. Consequently, we observed decreased bone mass and altered bone microarchitecture. In addition to reduced fat mass, risperidone therapy was associated with enlarged fatty liver. Thus, in addition to risperidone therapy creating conditions that could affect the central regulation of bone mass, the present study mandates that further clinical studies be focused on its direct effects on bone turnover, particularly in adolescents when peak bone mass is being acquired.
Highlights.
Risperidone reduced trabecular and cortical bone mass in mice
Risperidone elevated bone resorption and suppressed bone formation
Risperidone induced osteoclast (but not osteoblast) differentiation in vitro
Weight gain did not contribute to risperidone-induced bone loss
Acknowledgments
The authors thank Phuong Le, Victoria DeMambro, Deborah Barlow, Marilena Preda and Terry Henderson for technical assistance. This work was funded by Grant Number F32AR061932 to K.J.M from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases; Grant Number 0102-09-1 to I.D.P. from CAPES, Brazil; MMCRI Intramural Award to A.E.M.; Grant Number 201650/2008-8 to F.J.A.P. from the National Council for Scientific and Technological Development (CNPq), Brazil; and ARRA Grant Number AR054604 to C.J.R. from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. This work was supported by NIH grants P20 RR18789 and P20 RR15555 to Don M. Wojchowski and P30 RR030927 to Robert Friesel, and by institutional support from Maine Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–1124. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19:101–109. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komossa K, Rummel-Kluge C, Schwarz S, Schmid F, Hunger H, Kissling W, Leucht S. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006626.pub2. CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, Lieberman JA. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289–297. doi: 10.1038/sj.npp.1301209. [DOI] [PubMed] [Google Scholar]
- 6.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goeb JL, Marco S, Duhamel A, Kechid G, Bordet R, Thomas P, Delion P, Jardri R. Metabolic side effects of risperidone in early onset schizophrenia. Encephale. 2010;36:242–252. doi: 10.1016/j.encep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 Suppl 1:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 9.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 10.Halbreich U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21:641–657. doi: 10.2165/00023210-200721080-00003. [DOI] [PubMed] [Google Scholar]
- 11.Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA. A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry. 2010;71:338–347. doi: 10.4088/JCP.08m04595gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker D, Liver O, Mester R, Rapoport M, Weizman A, Weiss M. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64:761–766. doi: 10.4088/jcp.v64n0704. [DOI] [PubMed] [Google Scholar]
- 13.Liperoti R, Onder G, Lapane KL, Mor V, Friedman JH, Bernabei R, Gambassi G. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: results of a case-control study. J Clin Psychiatry. 2007;68:929–934. doi: 10.4088/jcp.v68n0616. [DOI] [PubMed] [Google Scholar]
- 14.Dore DD, Trivedi AN, Mor V, Friedman JH, Lapane KL. Atypical antipsychotic use and risk of fracture in persons with Parkinsonism. Mov Disord. 2009;24:1941–1948. doi: 10.1002/mds.22679. [DOI] [PubMed] [Google Scholar]
- 15.Meaney AM, O'Keane V. Bone mineral density changes over a year in young females with schizophrenia: relationship to medication and endocrine variables. Schizophr Res. 2007;93:136–143. doi: 10.1016/j.schres.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto T, Watanabe K, Shimada N, Makita K, Yagi G, Kashima H. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry. 2008;69:385–391. doi: 10.4088/jcp.v69n0307. [DOI] [PubMed] [Google Scholar]
- 17.Bushe C, Yeomans D, Floyd T, Smith SM. Categorical prevalence and severity of hyperprolactinaemia in two UK cohorts of patients with severe mental illness during treatment with antipsychotics. J Psychopharmacol. 2008;22:56–62. doi: 10.1177/0269881107088436. [DOI] [PubMed] [Google Scholar]
- 18.Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry. 2003;64:1362–1369. doi: 10.4088/jcp.v64n1113. [DOI] [PubMed] [Google Scholar]
- 19.de Paula FJ, Dick-de-Paula I, Bornstein S, Rostama B, Le P, Lotinun S, Baron R, Rosen CJ. VDR Haploinsufficiency Impacts Body Composition and Skeletal Acquisition in a Gender-Specific Manner. Calcif Tissue Int. 2011 doi: 10.1007/s00223-011-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 21.Cabovska B, Cox SL, Vinks AA. Determination of risperidone and enantiomers of 9-hydroxyrisperidone in plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:497–504. doi: 10.1016/j.jchromb.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc Natl Acad Sci U S A. 2010;107:10508–10513. doi: 10.1073/pnas.1000788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knegtering R, Baselmans P, Castelein S, Bosker F, Bruggeman R, van den Bosch RJ. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162:1010–1012. doi: 10.1176/appi.ajp.162.5.1010. [DOI] [PubMed] [Google Scholar]
- 24.Riedel M, Schwarz MJ, Strassnig M, Spellmann I, Muller-Arends A, Weber K, Zach J, Muller N, Moller HJ. Risperidone plasma levels, clinical response and side-effects. Eur Arch Psychiatry Clin Neurosci. 2005;255:261–268. doi: 10.1007/s00406-004-0556-4. [DOI] [PubMed] [Google Scholar]
- 25.Verma SK, Tan CH, Chan YH, Chong SA. Plasma risperidone levels and clinical response in patients with first-episode psychosis. J Clin Psychopharmacol. 2005;25:609–611. doi: 10.1097/01.jcp.0000186242.26050.1e. [DOI] [PubMed] [Google Scholar]
- 26.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 27.Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31:577–588. doi: 10.1016/j.psyneuen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Roke Y, van Harten PN, Boot AM, Buitelaar JK. Antipsychotic medication in children and adolescents: a descriptive review of the effects on prolactin level and associated side effects. J Child Adolesc Psychopharmacol. 2009;19:403–414. doi: 10.1089/cap.2008.0120. [DOI] [PubMed] [Google Scholar]
- 29.Yadav VK, Oury F, Tanaka KF, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar KK, Tung S, Iqbal J. Bone loss in anorexia nervosa: leptin, serotonin, and the sympathetic nervous system. Ann N Y Acad Sci. 2010;1211:51–65. doi: 10.1111/j.1749-6632.2010.05810.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 33.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 35.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota M, Mori K, Nakashima A, Kaneko YS, Fujiwara K, Itoh M, Nagasaka A, Ota A. Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin Exp Pharmacol Physiol. 2002;29:980–989. doi: 10.1046/j.1440-1681.2002.t01-1-03755.x. [DOI] [PubMed] [Google Scholar]
- 38.Ota M, Mori K, Nakashima A, Kaneko YS, Takahashi H, Ota A. Resistance to excessive bodyweight gain in risperidone-injected rats. Clin Exp Pharmacol Physiol. 2005;32:279–287. doi: 10.1111/j.1440-1681.2005.04184.x. [DOI] [PubMed] [Google Scholar]
- 39.Baranowska B, Baranowska-Bik A, Bik W, Martynska L. The role of leptin and orexins in the dysfunction of hypothalamo-pituitary-gonadal regulation and in the mechanism of hyperactivity in patients with anorexia nervosa. Neuro Endocrinol Lett. 2008;29:37–40. [PubMed] [Google Scholar]
- 40.Saarni SE, Saarni SI, Fogelholm M, Heliovaara M, Perala J, Suvisaari J, Lonnqvist J. Body composition in psychotic disorders: a general population survey. Psychol Med. 2009;39:801–810. doi: 10.1017/S0033291708004194. [DOI] [PubMed] [Google Scholar]