Abstract
Objective
Neuroprotection by hypothermia has been an important research topic over last two decades. In animal models of spinal cord injury (SCI), the primary focus has been assessing effects of hypothermia on behavioral and histological outcomes. While a few studies have investigated electrophysiological changes in descending motor pathways with motor evoked potentials recorded during cooling, we report here, hypothermia induced increased electrical conduction in the ascending spinal cord pathways with somatosensory evoked potentials (SSEPs) in injured rats. In our experiments these effects lasted long after the acute hypothermia and were accompanied with potential long term improvements in motor movement.
Design
Laboratory Investigation.
Setting
University Medical School.
Subjects
21 Female Lewis Rats.
Interventions
Hypothermia.
Measurements and Main Results
All animals underwent spinal cord contusion, with the NYU-Impactor, by a 12.5mm weight drop at thoracic vertebra T8. A group (n=10) was randomly assigned for a systemic 2hr. hypothermia episode (32±0.5°C) initiated ~2.0hrs post-injury. 11 rats were controls with post-injury temperature maintained at 37±0.5°C for 2hrs. The two groups underwent pre-injury, weekly post-injury (up to 4wks) SSEP recordings and standard motor behavioral tests (BBB). Three randomly selected rats from each group were euthanized for histological analysis at post-injury Day 3 and Day 28.
Compared to controls, the hypothermia group showed significantly higher SSEP amplitudes post-injury; with longer latencies. The BBB scores were also higher immediately after injury and 4 weeks later in the hypothermia group. Importantly, specific changes in the BBB scores in hypothermia group (not seen in controls) indicated regained functions critical for motor control. Histological evaluations showed more tissue preservation in hypothermia group.
Conclusions
Post-SCI, early systemic hypothermia provided significant neuroprotection weeks after injury via improved sensory electrophysiological signals in rats. This was accompanied by higher motor behavioral scores and more spared tissue in acute and post-acute periods after injury.
Keywords: Spinal Cord Injury (SCI), Hypothermia, Neuroprotection, Somatosensory Evoked Potentials (SSEP), Rat Model, BBB Score
INTRODUCTION
Approximately 12,000 Americans sustain and survive after spinal cord injury (SCI) each year with around 259,000 currently living with SCI [1]. SCI is immediately followed by axonal disruption and vascular and metabolic changes [2]. Following the initial trauma, secondary injury cascades into extensive damage due to inflammation, adverse immune reactions, apoptosis-induced cell death, necrosis and further nerve and axonal damage, which are also due to demyelination [2–5]. Among other effects, these responses lead to increased production of free radicals and endogenous opioids and excessive release of excitatory neurotransmitters. Cell death around injury epicenter further promotes Wallerian degeneration and demyelination in somatosensory as well as motor pathways. The CNS is known to have a limited regenerative capacity and as a result, the secondary damages due to SCI significantly reduce the likelihood of long term recovery. Therefore, a critical aspect of post-SCI therapeutic intervention is to either prevent or reduce the secondary injury and recent evidence suggests that this is critical during the first few hours after injury [2, 4–7]. For example, a recent retrospective clinical study by Levi et al. compared small groups of patients with and without post-SCI intravascular modest hypothermia (48 hrs, 33°C). The study suggested that early systemic cooling may have long term rehabilitation benefits measured by AIS scores [7].
There have been numerous reports on the potential beneficial effects of hypothermia after injury to the central nervous system (CNS) [4, 6–11]. While most recent clinical reports have focused on the use of cooling for head injury, stroke, cardiac arrest, cardiac surgery, and cerebral and aortic aneurysm repair [12–18], increasing interest has developed for its potential use after SCI in animal models as well as clinical investigations [4, 6, 19–25]. Many of these studies reported beneficial effects of hypothermia with gait improvement and reduction in histopathological damage to gray and white matter structures. Although a few studies have reported on the descending pathway with the monitoring of motor evoked potentials, the ramifications of acute cooling on the somatosensory function have not been reported in details. Importantly, the afferent somatosensory pathway is an integral part of the complete sensory-motor network and its integrity is essential for successful locomotion. Thus the electrophysiological consequences of injury to these pathways are critical to determining the neuroprotective role of hypothermia in the spinal cord. Here, we describe the effects of acute, systemic hypothermia on contused spinal cord using a rat model. We present evidence that a single, acute administration of hypothermia can potentially provide a long-term functional benefit as measured by somatosensory electrophysiological measurements as well as increasing motor behavior scores [26–27]. Our results indicate that the neuroprotection provided by 2 hours of early moderate hypothermia initiated within ~2 hours after injury leads to sustained improvements in somatosensory conduction indicated by SSEP waveforms, higher BBB scores and reduced tissue damage up to 4 weeks following injury. Preservation of sensory pathways early after injury suggests that acute hypothermia may be potentially beneficial for long-term recovery.
MATERIALS AND METHODS
Animals
A total of 21 adult female Lewis rats (200–220gms; Charles River Laboratories, Germantown, MD) were used for the contusion injury model. The animals were housed individually in cages and had free access to food and water throughout the experimental period. All the procedures were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University.
Anesthesia
The anesthesia for all surgical procedures, except for SSEP recordings, was a mixture of 30.4mg/kg of Ketamine, 4.3mg/kg of Xylazine, and 0.9mg/kg Acepromazine Maleate administered via intra-peritoneal injection (~0.12ml i.p.).
To induce general anesthesia for SSEP monitoring and recording, the rat was held in a transparent chamber, with a mixture of 3% isofluorane gas and room air, until the onset of drowsiness. The rat’s mouth and nose was then placed within an anesthesia mask with a well-fitting rodent size diaphragm. A mixed flow influx of 1.5% isofluorane, 80% oxygen and room air was delivered to the mask at a rate of 2 L/min. This mask was connected to a C-Pram circuit designed to deliver and evacuate the gas through one tube. An adequate level of anesthesia was determined by monitoring hindlimb withdrawal to painful stimuli and the corneal reflex. Rats continued spontaneous breathing and anesthesia depth was maintained at a constant level throughout the entire recording. The rats were also placed on a homeothermic blanket system (Harvard Apparatus Ltd., Kent, UK) to maintain their body temperature at 37±0.5°C, as measured by a rectal probe, throughout the entire experiment. Lacrilube ophthalmic ointment (Allergan Pharmaceuticals, Irvine, CA) was applied to the rats’ eyes to prevent drying.
Contusion Spinal Cord Injury
Following intra-peritoneal injection to induce general anesthesia, the back region of the rat was shaved and aseptically prepared with Chlorhexidine (Phoenix Pharmaceuticals, Inc., St. Joseph, MO). A midline incision was made along the thoracic vertebrae and the skin was opened. The paravertebral muscles at the region of interest (T6–T12) were retracted. A laminectomy was performed at thoracic vertebra T8 to expose the dorsal surface of the spinal cord, without opening the dura mater. The spinous processes of the vertebrae at T6 and T12 were secured in stabilization clamps to reduce the motion of the spinal column during impact. The exposed dorsal surface of the spinal cord at the T8 level was then contused with the NYU weight-drop device by dropping a 10g rod with a flat circular impact surface from pre-calibrated height of 12.5 mm. Biomechanical parameters such as the impact velocity of the rod, the distance of cord compression, the cord compression rate, and the dynamic force applied to the cord were precisely monitored using the computer to make sure consistency among all rats. There was less than 0.05% variation among rats..
Acute Hypothermia
A group of n=10 rats was randomly assigned after the injury for hypothermia treatment. Following contusion SCI, anesthesia was maintained and rectal temperature was monitored with an anal probe digital thermometer (Physitemp Instruments, Clifton, NJ) and maintained at 37 ± 0.5 °C with the help of an electric heating pad for two hours after the time of contusion (Figure 1). Two hours after contusion, general hypothermia was induced by spraying the rat with alcohol mist (70% ethanol) and using an electric fan until the rectal temperature dropped to 32 ± 0.5° C. The typical cooling duration was 20 ± 5 mins. The temperature was then maintained at 32 ± 0.5° C for the next 2hrs. After hypothermic exposure, rats were gradually re-warmed to 37 ± 0.5°C using the heating pad. This rewarming period typically lasted 28 ± 5 mins, and was followed by a 90 min observation period without heating pad to ensure that the animals regained the capability to regulate body temperature on their own. The injured rat was then returned to its cage and was given easy access to food and water.
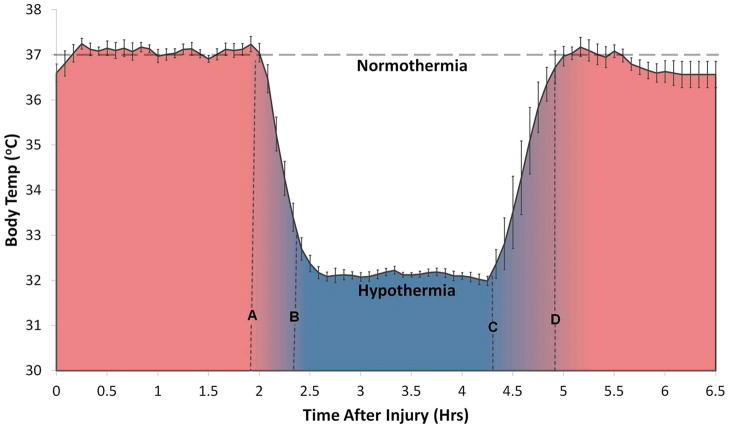
Figure 1.
Recorded rectal temperature (Mean ± SEM) in a group of n = 10 rats after induction of spinal cord contusion. The temperature control was initiated (t = 0 in the plot) within ~30 mins of a contusive spinal cord injury (T8) with 12.5 mm weight drop using the NYU impactor. For the first ~ 2 hrs, (shaded red) the temperature was held constant (37 ± 0.5°C). Systemic hypothermia was induced with cooling down to 32 ± 0.5 °C (A–B) after which the temperature was again held constant at 32 ± 0.5°C for ~2 hrs (B–C). This was followed by gradual warming (C–D) back to 37 ± 0.5 °C and then removal of the heating pad beyond line D. A normothermia group (n = 11) with constant temperature 37 ± 0.5 °C was used as controls.
Post-Injury Care
After injury, the muscles were sutured in layers using absorbable 2-0 suture, and the skin was closed with 4-0 suture. After the hypothermia induction in rats under that group, all rats were allowed to recover in a warmed cage and food and water was kept easily accessible. The antibiotic Gentamycin (5 mg/kg, intramuscular; Abbott Laboratories, Abbott Park, IL) was administered immediately post-surgery and then daily for 7 days. The analgesic, Buprenex (0.01 mg/kg of 0.3 mg/ml, intramuscular; Reckitt Benckiser Pharmaceuticals, Inc., Richmond, VA) was delivered post-surgery and daily for 4 days. After surgery, the rat’s bladders were expressed 2 times a day for the first 4 days or until they regained control of the urination. There were no complications or other infections to report. No signs of autotomy or autophagy were observed. The rats were housed for 4 weeks after injury, and thereafter anesthetized and euthanized by transcardial perfusion with 4% formaldehyde.
SSEP Recording
Electrode Implantation
One week prior to injury, the rats were anesthetized and their head region was shaved and aseptically prepared with Chlorhexidine (Phoenix Pharmaceuticals, Inc., St. Joseph, MO). A local anesthetic of 2% Lidocaine HCl (Abbott Laboratories, North Chicago, IL) was injected under the skin, and a minute later an incision was made along the midline. The cranium bone was cleaned by removing the tissue under the skin. A standard dental drill (Fine Science Tools, North Vancouver, BC, Canada) was used to drill five burr holes into the exposed part of the cranium. Four holes were located on the somatosensory cortex corresponding to the hind and forelimbs in each hemisphere. On each hemisphere, the forelimb recording sites were located 0.2 mm posterior to bregma and 3.8 mm laterally from the bregma, and the hindlimb recording sites were located 2.5 mm posterior to bregma, and 2.8 mm laterally from the bregma. A fifth hole drilled on the right frontal bone, situated 2 mm from both the sagittal and coronal sutures, served as the intracranial reference. Transcranial screw electrodes (E363/20, Plastics One, Inc., Roanoke, VA) were then screwed into the holes such that they made very light contact with the dura mater, without causing compression of the brain tissue. The distal end of each electrode was inserted into one of the slots of an electrode pedestal (MS363, Plastics One Inc., Roanoke, VA). In order to secure the electrodes for long-term recording of cortical SSEPs, carboxylate dental cement (Durelon Carboxylate Cement, 3M ESPE, St. Paul, MN) was used to hold the screw electrodes and the electrode pedestal in place. After hardening of the cement, the skin incision was closed with a 4-0 suture.
SSEP Acquisition
Sub-dermal needle electrode pairs (Safelead F-E3-48, Grass Technologies, West Warwick, RI) were used to electrically stimulate the tibial nerves of both left and right hind limbs, without direct contact with the nerve bundle. An isolated constant current stimulator (DS3, Digitimer Ltd., Hertfordshire, England) was used for the electrical stimulation of the limbs. A Windows-Microsoft based personal computer was interfaced with the stimulator. A neurological monitoring system (Tucker-Davis Technologies, Alachua, FL) was used to set the stimulation parameters and trigger the stimulator. Positive current pulses of 3.5mA magnitude and 200 μsec duration at a frequency of 1Hz were used for limb stimulation, which sequentially stimulated each of the four limbs at a frequency of 0.25 Hz using a custom demultiplexer. Cortical SSEPs from the transcranial electrodes were amplified by an optically isolated biopotential amplifier (Tucker-Davis Technologies, Alachua, FL). The analog signal from each hemisphere was transferred to a personal computer via an optical data acquisition system with four input channels at a sampling rate of 5KHz. The electroencephalogram (EEG) of each hemisphere, containing the SEP for the respective hemisphere, the stimulation pulse signal and the stimulated limb number, was recorded on separate channels for post-operative data analysis. Contralateral SSEP recordings were used for analysis. All signal processing was performed using custom algorithms developed with MATLAB7.4 (MathWorks Inc, Natick, MA). The signal to noise ratio was improved by ensemble averaging of 100 stimulus-locked sweeps. The first 5 msec in the SEP signals were generally corrupted by the stimulation artifact and were discarded from the analysis. Further, only the SEP signal between 5 ms and 30 ms was considered for analysis since a response beyond 30 ms has negligible amplitude and is corrupted with noise.
SSEP Analysis
In the post experimental SSEP analysis, the signal-to-noise ratio (SNR) was improved by detrending, subtracting the waveform mean and applying a 60Hz notch filter for all waveforms for stimulation of each limb. A custom peak detection algorithm was developed for computing the peak-to-peak mean amplitude and the latency within a window between 5 and 30ms after the stimulation artifact, a window that corresponds to the nervous systems response to the stimulus. The mean amplitudes and latencies for both the experimental groups were computed for each day of the recording and were subject to statistical comparisons.
Behavioral Tests
Locomotor function was assessed using the BBB locomotor rating scale before injury and every three days post-injury. This scoring scale is sensitive to rat lower limbs joint moviments, hindlimb motion, trunk position and stability, coordination, stepping, paw placement, and tail position; and the details of the scoring scheme can be found in [26–28]. The increasing BBB scores correspond to an increasing level of locomotor recovery, and can be categorized into three phases: early, intermediate, and late. Rats were placed individually in a 90 cm plastic open field. Two examiners observed and scored each rat for exactly 4 min, giving a separate score for each hindlimb. The final given score for each limb was the minimum between the two observers. The rats were scored in a range 0–21, 0 signifying no hindlimb movement and 21 signifying perfect hindlimb movement with extensive joint movements, including anckle, knee and hip of hindlimbs, consistent plantar stepping, consistent toe clearance, no rotation in stepping, and tail consistently up [26–28].
Histology
On the post-injury day 3 and day 28, three rats from each group (normothermia and hypothermia) were randomly chosen for histological analysis. These animals were euthanized via transcardial perfusion with DPBS (14190, GIBCO, Grand Island, NY), and with paraformaldehyde (PFA) solution (4%; 15713-S, Electron Microscopy Sciences, Hatfield, PA). The spinal cord was then carefully extracted from the vertebrae column and post-fixed in 4% PFA, followed by 30% sucrose solution for 24 hours and embedded in paraffin for sectioning. Tissue sections were stained with hematoxylin and eosin (H&E) to assess the morphology of the site of injury.
Statistical Analysis
All data reported are presented as Mean±SEM. Statistical analysis was implemented using a commercial software (Stata, StataCord LP, College Station, TX). For each rat, BBB scores from each hindlimb were averaged to yield one score per test session. BBB scores and mean amplitudes and latencies for SSEPs in the hyporthermia and control groups were compared at each time point using the Student’s t-test (two-tailed; unpaired). Differences were considered statistically significant at p<0.01 to adjust for multiple comparisons.
RESULTS
As described above, we employed SSEP monitoring, motor behavioral testing and histological examination to study the effects of acute hypothermic treatment on the progress of contusive spinal cord injury in rats. Rats with normothermia were used as controls.
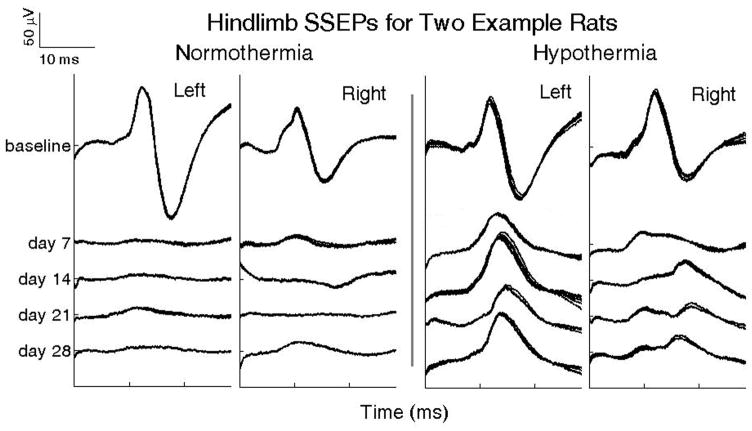
Figure 2 shows example SSEP sweeps from two rats illustrating the effects of spinal cord contusion on controls and rats with acute hypothermia immediately following injury. Each cluster of waveforms is composed of five SSEP sweeps overlaid, where each is a moving average of individual time-locked 20 epochs. The baseline SSEP is characterized by latency following stimulus and a peak-to-peak amplitude in both experimental groups. Following injury, SSEPs were measured on days 4, 7, 14, 21 and 28. The left panel shows the effects of spinal cord injury on the SSEPs from an untreated rat (normothermia). The SSEP is virtually absent on day 7 and shows little to no recovery thereafter up to 28 days following injury. The right panel shows an example rat exposed to hypothermia immediately following spinal cord injury. After exposure to hypothermia, this rat showed a partial retention of somatosensory conduction one week after injury, as illustrated by the greater amplitude of the SSEP compared to the normothermic rat; this difference continued over the 4 wk observation period.
Figure 2.
Panels show changes over 4 weeks, in somatosensory evoked potentials (SSEP) upon hindlimb stimulation in two representative rats after a thoracic spinal cord contusion (T8) with a NYU impactor (12.5 mm impact height). Left: A rat with normothermia; Right: A rat subjected to an acute 2 hr systemic hypothermia (32 ± 0.5°C) initiated 2hrs after the contusion.
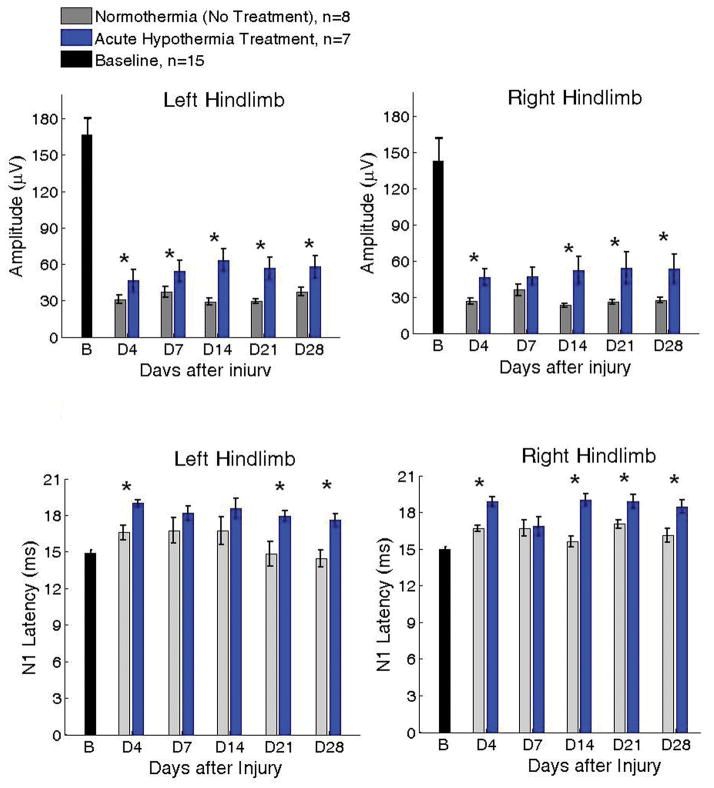
Figure 3 shows the group results for weekly variation in SSEP amplitudes and latencies (Mean ± SEM) for a total of n = 15 rats. The two panels correspond to the SSEP amplitudes recorded upon stimulation of the two hindlimbs. The colors correspond to the two experimental groups (Gray: Normothermia, n = 8; Blue: Hypothermia, n = 7). All rats underwent baseline recordings prior to injury. Following the baseline recording, the rats were randomly divided into two experimental groups for hypothermia treatment n = 10, and normothermia control n = 11. Three randomly chosen rats from both groups were euthanized for acute histological assessments on day 3. Thus the weekly SSEP was monitored for 15 rats (n=7 in hypothermia group and n=8 in normothermia group).
Figure 3.
SSEP amplitudes (top panels) and N1 peak latencies (bottom panels) upon stimulation of the two hindlimbs for two groups of rats after SCI at T8 with impact height 12.5 mm. Blue: acute, 2 hr hypothermia (n = 7; 32 ± 0.5 °C); Gray: Normothermia (n = 8; 37 ± 0.5 °C). B = Baseline; D4–D28 denote Days 4, 7, 14, 21, 28 after injury. It is important to note that no deleterious effects from hypothermia were detected in any rat as would have been indicated by decreases in SSEP amplitude (*p<0.01 between treatment groups; Mean ± SEM).
Four days following injury, the mean SSEP amplitudes for normothermia group decreased from 166.38 ± 13.85 μV to 31.05 ± 3.73 μV; and 143.13 ± 18.02 μV to 26.63 ± 7.25 μV for the left and right hindlimbs, respectively. The hypothermia group showed a comparatively smaller decrease in amplitude from baseline to day 4 after injury, to 46.54 ± 9.13 μV and 46.49 ± 6.90 μV for the left and right limbs. The difference between the two groups was statistically significant (p<0.01). In the follow up period, the mean SSEP amplitudes from the hypothermia group were consistently higher than the normothermia group. The difference between the two groups was found statistically significant for most weekly recordings (p<0.01). As the bottom panels in figure 3 show, the SSEP latencies were longer in hypothermia group compared to controls and this difference was found statistically significant on most days between both groups (p<0.05). These results may be indicative of potentially long term changes in conduction speed along neural fibers due to acute hypothermia. However, a detailed neuro-biological understanding of lasting increases in SSEP latencies months following acute hypothermia needs further investigation.
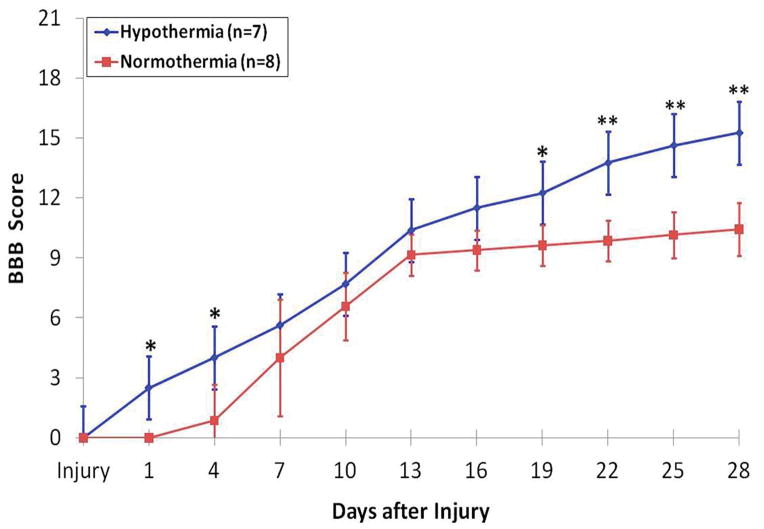
In addition to SSEP recording, standard BBB motor scoring was performed every 3 days following injury. Figure 4 shows the mean BBB scores for the two experimental groups over the time-course of the experiment. All rats were healthy prior to surgery and exhibited BBB scores of 21 (data not shown). Both groups showed motor function improvement one day after injury, yet the hypothermia treated group exhibited better total recovery by day 28, scoring an average of 15.2 ± 2.1 points, than did the control group, scoring only 11.1 ± 1.9 points (p<0.0001). It is important to note that significant improvements in locomotor activity and balance occur from 11–14 points on the BBB score scale [26–27]. A score of 10 indicates only occasional weight supported plantar steps and no front-hind limb coordination while a score of 14 means consistent weight-supported plantar steps and consistent front-hind limb coordination [27]. Thus, it is especially interesting to note that the hypothermia group and not the control group exceeded this threshold.
Figure 4.
Variation in BBB scores (Mean ± SEM) over a period of 28 days in the two groups, normothermia (n = 8; red) and hypothermia (n=7; blue). It should be noted that a change in the BBB score from 11 to 14 is a critical milestone in recovery. It is the difference between an animal having almost no front-hind limb coordination with only occasional weight supported plantar steps and a consistent coordinated front-hind limb motion with consistent plantar steps. The hypothermia group exceeded this benchmark in recovery while the normothermia group did not. (*p<0.002;**p<0.00004).
In addition, the two groups showed a significant difference in motor activity 1–4 days after injury with higher SSEP amplitudes in the hypothermia group (p < 0.002). This is consistent with the SSEP data and suggests that hypothermia promotes recovery from the initial or acute impact of the injury itself.
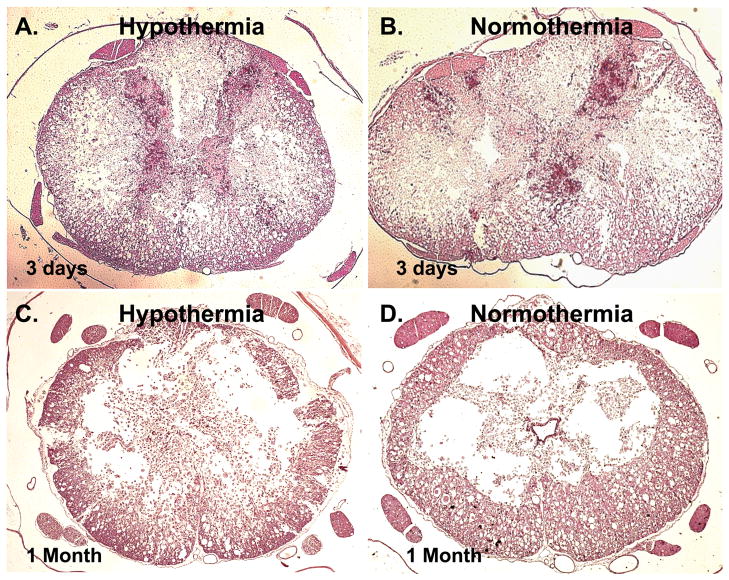
On the post-injury day 3 and day 28, three rats from each group (normothermia and hypothermia) were randomly chosen for histological analysis as described in the methods. Figure 5 shows representative examples of the acute and post-acute effects of hypothermia on the rat spinal cord. Results showed that rats with early post-injury hypothermia showed a significantly lower damage on day 3 compared to controls. The dorsal and ventral horns of the gray matter in these rats was preserved to a large extent. In contrast, the normothermia group showed a near complete destruction of the dorsal and ventral horns of the spinal cord. These were clear indications of lower secondary damage in hypothermic rats. The benefits of hypothermia was less discernible on day 28, but the extent of preserved gray matter was still greater in the hypothermia group than that seen in the normothermia group as shown in the two bottom panels in Figure 5. This is consistent with the normal progression of SCI where the greatest extent of secondary damage occurs within the first week following injury. Thus, the initial benefit of hypothermia on gray matter preservation does not completely alleviate all of the effects of secondary damage in the cord the weeks following injury.
Figure 5.
Four representative spinal cord histological sections at the site of injury of each treatment group at 3 days (A–B) and at 1 month (C–D), stained with H&E. At 3 days, better preservation of morphology was observed in the hypothermia-treated rat (A) than in the untreated rat (B). Tissue analysis showed that preservation of gray matter at 3 days post-injury (e) was significantly better in the hypothermia-treated rat than in the untreated rat. At 1 month, there were no significant difference in observable morphology between the hypothermia-treated rat (C) and the untreated rat (D), which is reflected in the analysis of gray and white matter tissue sparing.
DISCUSSION AND CONCLUSIONS
Hypothermic treatment after CNS injury has been used successfully as a neuroprotective intervention for both spinal cord and brain trauma in rodents [3, 21, 29–32] and in man [4, 7, 9, 23]. However the mechanisms underlying the neuroprotection have eluded researchers. Most of the animal model research in this context has focused on the two components of motor function, usually assessed by behavioral testing and histologically identified tissue preservation. Few studies have reported the descending tract electrophysiology after hypothermia in rats after SCI with the motor evoked potentials as the outcome measure [3, 23, 31], which focus on the integrity of the descending pathways from the brain to the periphery. In contrast, we have reported here the electrophysiological effects of acute hypothermia after SCI by SSEPs that assess the functional integrity of ascending sensory pathways from the peripheral nerves to the somatosensory cortex.
We presented the potential long-term benefits of acute hypothermia after contusion spinal cord injury and its effect on preservation of the ascending somatosensory pathway using SSEP monitoring. Our results showed improvements lasting for several weeks in SSEP amplitudes. This was also accompanied with increased motor behavioral scores and histological preservation, indicative of neuroprotection. Importantly, early enhancements of SSEP amplitudes in hypothermia-treated rats indicated that the benefits lie in the preservation of somatosensory conductivity following injury and reducing the secondary damages.
Interestingly, hypothermia treated rats also demonstrated longer latencies. It is possible that longer latency is a direct result of slower somatosensory conduction post-SCI. Another possibility is change in the neural connectivity and compensation indicative of different plastic responses post-hypothermia. In a diversity of contexts, few previous studies have explored the effects of moderate or severe cooling on the somatosensory evoked potentials; for example, in healthy rats [33–34]; in rats immediately after compressive SCI [35]; in patients undergoing open heart surgery [36]; and in rats after cardiac arrest [16]. All of these studies have reported acute elongation of SSEP latencies resulting from hypothermia. Given that the SSEP stimulus travels through the neuronal axons and synapses in a serial manner and the effects of cooling on these are additive [35]; the effects of cooling on SSEP may be due to changes in conductivity as well as connectivity.
These results necessitate future studies to determine the mechanisms of hypothermia after SCI in the SSEP pathways. Indeed, studies in other systems have shown that hypothermia tends to reduce the overall inflammatory response in tissues [37–38]. This is consistent with other studies of hyperthermia where body temperatures are elevated after injury. In these cases, posttraumatic hyperthermia was evaluated after lower thoracic SCI [39] which demonstrated worsened behavioral and histopathological outcomes compared to normothermia suggesting a role of body temperature in SCI recovery.
Therefore, it seems reasonable to suggest that at the cellular level hypothermia may reduce redox and heat-shock induced proteolytic activity that causes cell mediated apoptosis. However, it is not known at this time which cells or which tracts of the spinal cord are preserved or “targeted” by hypothermia. Morino et al. have suggested that hypothermic treatment is effective for the amelioration of delayed motor dysfunction via inhibition of microglial inflammatory responses [37]. Our results demonstrate that like the motor tracts the sensory tracts are affected by hypothermic treatment. This is important since sensory functionality is a vital and essential part of the complete sensory-motor functional circuitry. Without recovery of the sensory functionality, motor commands via the descending pathways alone will not be sufficient for successful locomotion.
Many experimental SCI studies have reported the benefits of local as well as systemically administered hypothermia on SCI specifically focusing on the behavioral tests and demonstrations of tissue preservation by histopathology (Reviewed in [3, 5]). For example, Yu and colleagues [21] showed benefits of early, systemic hypothermia (33°C) in improving motor function assessed by the open field locomotor test [26–27]. Also, compared with normothermic SCI rats (37°C), the degree of gray and white matter damage was significantly reduced in cooled rats. These initial results emphasized the beneficial effects of mild cooling on white matter integrity and long tract function. Most hypothermia studies have however relied on the open field behavioral test, which may be subjective and highly variable in many types of SCI [40]. Moreover, BBB testing while assessing locomotor activity does not necessarily correlate with the functional integrity and neurological conduction of the spinal cord. Unlike in rodents, these are the processes that define recovery in clinical settings with patients [23]. Therefore, the importance of our findings demonstrates how short-term hypothermic treatment after SCI can help preserve the sensorimotor tract immediately after injury and that this benefit persists one month afterwards in terms of stabilized SSEP signals and significant improvements in BBB scores. Preservation of SSEP pathways early after injury also suggests that acute hypothermia may be beneficial for long-term recovery as well as provide a unique window of opportunity for other interventions such as stem cell therapies for remyelination of neuronal axons or regeneration of neurons.
Acknowledgments
Sources of Support:
The authors would like to thank Neil O’Donnell and Nikta Pashai of JHU for their help with the behavioral scoring of animals and animal care, Amir Pourmorteza of JHU for insightful discussions and Liye Zhou of JHU for histological preparations. The financial support for this work came from the Maryland Stem Cell Research Fund (MSCRF Grant #2007-MSCRFII-0159-00 and Grant #2009-MSCRFII-0091-00 awarded to AHA) and National Institute of Health (NIH Grant #R21HD057487-01A1 awarded to CLK).
Footnotes
Study Location:
The Johns Hopkins University School of Medicine, Biomedical Engineering Department
The authors have not disclosed any potential conflicts of interest.
References
- 1.NSCISC. National Spinal Cord Injury Statistical Center Annual Statistical Report. University of Alabama; Birmingham: 2009. [Google Scholar]
- 2.Gupta R, Bathen ME, Smith JS, Levi AD, Bhatia NN, Steward O. Advances in the management of spinal cord injury. The Journal of the American Academy of Orthopaedic Surgeons. 2010;18:210–22. doi: 10.5435/00124635-201004000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. Journal of neurotrauma. 2009;26:301–12. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich W, Cappuccino A, Cappuccino H. Systemic Hypothermia for the Treatment of Acute Cervical Spinal Cord Injury in Sports. Current Sports Medicine Reports. 2011;10(1):50. doi: 10.1249/JSR.0b013e318205e0b3. [DOI] [PubMed] [Google Scholar]
- 5.Kwon BK, Sekhon LH, Fehlings MG. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine. 2010;35:S263–70. doi: 10.1097/BRS.0b013e3181f3286d. [DOI] [PubMed] [Google Scholar]
- 6.Marion D, Bullock M. Current and future role of therapeutic hypothermia. Journal of neurotrauma. 2009;26(3):455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- 7.Levi AD, Casella G, Green Ba, Dietrich WD, Vanni S, Jagid J, Wang MY. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66:670–7. doi: 10.1227/01.NEU.0000367557.77973.5F. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich WD. Therapeutic hypothermia for spinal cord injury. Critical care medicine. 2009;37:S238–42. doi: 10.1097/CCM.0b013e3181aa5d85. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35:E57–62. doi: 10.1097/BRS.0b013e3181b9dc28. [DOI] [PubMed] [Google Scholar]
- 10.Kao CH, Chio CC, Lin MT, Yeh CH. Body Cooling Ameliorating Spinal Cord Injury May Be Neurogenesis-, Anti-inflammation- and Angiogenesis-Associated in Rats. The Journal of trauma. 2010;XX:1–9. doi: 10.1097/TA.0b013e3181e7456d. [DOI] [PubMed] [Google Scholar]
- 11.Batchelor PE, Kerr NF, Gatt AM, Aleksoska E, Cox SF, Ghasem-Zadeh A, Wills TE, Howells DW. Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. Journal of neurotrauma. 2010;27:1357–68. doi: 10.1089/neu.2010.1360. [DOI] [PubMed] [Google Scholar]
- 12.Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Mayberg MR, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32(8):1847. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- 13.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. 2002:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbacher J, Siderys H, Shahriari A. Preservation of renal function utilizing hypothermic circulatory arrest in the treatment of distal thoracoabdominal aneurysms (types III and IV) Annals of vascular surgery. 2007;21(2):204–207. doi: 10.1016/j.avsg.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Madhok J, Iyer S, Thakor N, Maybhate A. Characterization of neurologic injury using novel morphological analysis of Somatosensory Evoked Potentials. in Engineering in Medicine and Biology Society (EMBC). Annual International Conference of the IEEE; 2010; Beunos Aires, Argentina: IEEE; 2010. [DOI] [PubMed] [Google Scholar]
- 16.Madhok J, Maybhate A, Xiong W, Koenig MA, Geocadin RG, Jia X, Thakor NV. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: Prognostication of functional outcomes. Critical Care Medicine. 2010;38:1709. doi: 10.1097/CCM.0b013e3181e7dd29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters JH, Morley PT, Nolan JP. The role of hypothermia in post-cardiac arrest patients with return of spontaneous circulation: A systematic review. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Callaway CW. Refining the use of therapeutic hypothermia after cardiac arrest*. Critical Care Medicine. 2011;39(1):201. doi: 10.1097/CCM.0b013e3181ffe264. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Arizala A, Green B. Hypothermia in spinal cord injury. Journal of neurotrauma. 1992;9:S497. [PubMed] [Google Scholar]
- 20.Westergren H, Yu W, Farooque M, Holtz A, Olsson Y. Systemic hypothermia following spinal cord compression injury in the rat: axonal changes studied by -APP, ubiquitin, and PGP 9.5 immunohistochemistry. Spinal Cord. 1999;37(10):696–704. doi: 10.1038/sj.sc.3100920. [DOI] [PubMed] [Google Scholar]
- 21.Yu WR, Westergren H, Farooque M, Holtz a, Olsson Y. Systemic hypothermia following spinal cord compression injury in the rat: an immunohistochemical study on MAP 2 with special reference to dendrite changes. Acta neuropathologica. 2000;100:546–52. doi: 10.1007/s004010000206. [DOI] [PubMed] [Google Scholar]
- 22.Inamasu J, Nakamura Y, Ichikizaki K. Induced hypothermia in experimental traumatic spinal cord injury: an update. Journal of the neurological sciences. 2003;209:55–60. doi: 10.1016/s0022-510x(02)00463-x. [DOI] [PubMed] [Google Scholar]
- 23.Kwon BK, Mann C, Sohn HM, Hilibrand AS, Phillips FM, Wang JC, Fehlings MG. Hypothermia for spinal cord injury. The spine journal : official journal of the North American Spine Society. 2008;8:859–74. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Levi AD, Green Ba, Wang MY, Dietrich WD, Brindle T, Vanni S, Casella G, Elhammady G, Jagid J. Clinical application of modest hypothermia after spinal cord injury. Journal of neurotrauma. 2009;26:407–15. doi: 10.1089/neu.2008.0745. [DOI] [PubMed] [Google Scholar]
- 25.Lo TP, Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, Stagg M, Abril RM, Patel S, Dietrich WD, Pearse DD. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. The Journal of comparative neurology. 2009;514:433–48. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- 26.Basso DM, Beattie MS, BRESNAHAN JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 27.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Experimental neurology. 1996;139(2):244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 28.All AH, Agrawal G, Walczak P, Maybhate A, Bulte JWM, Kerr DA. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurological Sciences. 2010;31:595–601. doi: 10.1007/s10072-010-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsala M, Galik J, Ishikawa T, Yaksh TL. Technique of selective spinal cord cooling in rat: methodology and application. Journal of neuroscience methods. 1997;74:97–106. doi: 10.1016/s0165-0270(97)02270-x. [DOI] [PubMed] [Google Scholar]
- 30.CHATZIPANTELI K, YANAGAWA Y, MARCILLO A, KRAYDIEH S, YEZIERSKI R, DIETRICH W. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. Journal of neurotrauma. 2000;17(4):321–332. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]
- 31.Dimar JR, Shields CB, Zhang YP, Burke Da, Raque GH, Glassman SD. The role of directly applied hypothermia in spinal cord injury. Spine. 2000;25:2294–302. doi: 10.1097/00007632-200009150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Molecular Brain Research. 2005;138(2):124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Budnick B, McKeown K, Wiederholt W. Hypothermia-induced changes in rat short latency somatosensory evoked potentials. Electroencephalography and Clinical Neurophysiology. 1981;51(1):19–31. doi: 10.1016/0013-4694(81)91506-6. [DOI] [PubMed] [Google Scholar]
- 34.Oro J, Haghighi SS. Effects of altering core body temperature on somatosensory and motor evoked potentials in rats. Spine. 1992;17(5):498. doi: 10.1097/00007632-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Jou I. Effects of core body temperature on changes in spinal somatosensory-evoked potential in acute spinal cord compression injury: an experimental study in the rat. Spine. 2000;25(15):1878. doi: 10.1097/00007632-200008010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Markand ON, Warren C, Mallik GS, King RD, Brown JW, Mahomed Y. Effects of hypothermia on short latency somatosensory evoked potentials in humans. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1990;77(6):416–424. doi: 10.1016/0168-5597(90)90002-u. [DOI] [PubMed] [Google Scholar]
- 37.Morino T, Ogata T, Takeba J, Yamamoto H. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord. 2008;46(6):425–431. doi: 10.1038/sj.sc.3102163. [DOI] [PubMed] [Google Scholar]
- 38.Kao CH, Chio CC, Lin MT, Yeh CH. Body Cooling Ameliorating Spinal Cord Injury May Be Neurogenesis-, Anti-inflammation- and Angiogenesis-Associated in Rats. The Journal of trauma. 2011;70(4):885–893. doi: 10.1097/TA.0b013e3181e7456d. [DOI] [PubMed] [Google Scholar]
- 39.Yu CG, Jagid J, Ruenes G, Dietrich WD, Marcillo AE, Yezierski RP. Detrimental effects of systemic hyperthermia on locomotor function and histopathological outcome after traumatic spinal cord injury in the rat. Neurosurgery. 2001;49(1):152. doi: 10.1097/00006123-200107000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Barros Filho TEP, Molina AEIS. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics. 2008;63:103–108. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]