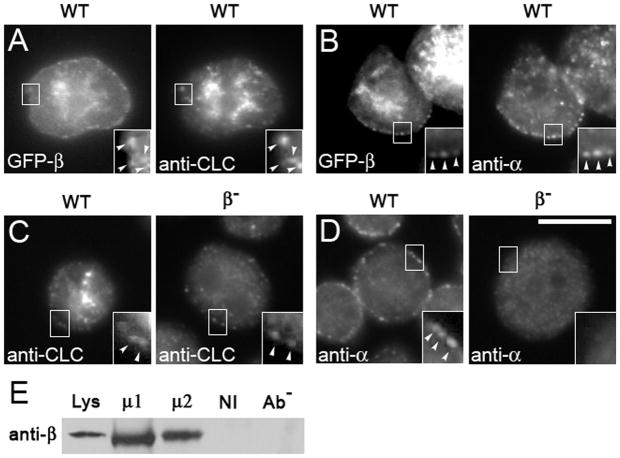
Figure 3. APβ1/2 co-localizes with and influences the localization of clathrin and alpha adaptin.
Cells transformed with GFP-tagged β1/2 (GFP-β) were immunostained. (A–B) GFP-β co-localizes with clathrin (A) and with punctae of AP2α at the cell periphery (B). (C–D) Gene disruption of APβ1/2 (β-) leads to the loss of clathrin (C) and loss of AP2α (D) at the cell periphery. Scale bar, 10μm. Cell lysates from wild-type cells were immunoprecipitated with either anti-μ1 (μ1) or anti-μ2 (μ2) and, as controls, non-immune serum (NI) and no antibody (Ab−). (E) Immunoprecipitates were assessed on Western blots probed with anti-APβ1/2 (anti-β) to detect co-immunoprecipitation of APβ1/2. APβ1/2 co-immunoprecipitates specifically with both μ1 and μ2.