Abstract
Although carbon monoxide (CO) is known to be toxic due to its ability to interfere with oxygen delivery at high concentrations, mammalian cells endogenously generate CO primarily via the catalysis of heme by heme oxygenases (HO). Recent findings have indicated that HO and generation of CO serve as a key mechanism to maintain the integrity of the physiological function of organs, and supported the development of a new paradigm that CO, at low concentrations, functions as a signaling molecule in the body and exerts significant cytoprotection. Consequently, exogenously delivered CO has been shown to mediate potent protection in various injury models through its anti-inflammatory, vasodilating, and anti-apoptotic functions. Ischemia/reperfusion (I/R) injury associated with organ transplantation is one of the major deleterious factors limiting the success of transplantation. I/R injury is a complex cascade of interconnected events involving cell damage, apoptosis, vigorous inflammatory responses, microcirculation disturbance, and thrombogenesis. CO has a great potential in minimizing I/R injury. This review will provide an overview of the basic physiology of CO, preclinical studies examining efficacy of CO in I/R injury models, and possible protective mechanisms. CO could be developed to be a valuable therapeutic molecule in minimizing I/R injury in transplantation.
Keywords: carbon monoxide, kidney transplantation, ischemia/reperfusion injury, inflammation, apoptosis
INTRODUCTION
Carbon monoxide (CO), a diatomic gas, is an invisible, chemically inert, colorless, non-irritant, and odorless gas. It is a chemically stable molecule with a molar mass of 28.0 and vapor density of 0.967 relative to air, slightly lighter than air. CO is commonly viewed as a poison in high concentrations; however, recent findings that the body endogenously produces CO to regulate neural and vascular functions have changed the view of CO from an environmental poison to an endogenous regulatory gaseous molecule. During the last decade, CO has received great attention as a biological regulator that can be used as a therapeutic tool, and the current review focuses on the use of CO to prevent ischemia/reperfusion (I/R) injury particularly in the setting of transplantation.
1. History of CO: environmental poison to endogenous regulator
The early history of CO is difficult to trace; however, environmental CO is readily produced by the incomplete combustion of carbon-containing materials (e.g. coal, wood, natural gas), and the presence of noxious fume has been known since the Greek and Roman era. During the 18th century, the chemical composition of CO was determined, and in 1857, Bernard demonstrated that CO could cause asphyxia by reversibly displacing oxygen from hemoglobin and forming carboxyhemoglobin (COHb) (1). During the following years, CO’s toxicity has been extensively studied (2, 3); however, it also became apparent that some level of CO is present in the blood of normal man and animals. The origin of CO in blood was initially assumed to be exogenous and absorbed from the environment; however, there was an assumption that some levels of CO might be formed endogenously. Actual experimental evidence was obtained around 1950 demonstrating that CO is indeed endogenously formed in our body (4–6). Further experiments showed that CO can be generated by oxidation of hemoglobin and heme, in particular the α-methene bridge carbon atom of the heme porphyrin ring and suggested that CO might be a catabolic byproduct of hemoglobin in the body under abnormal conditions (7, 8). Finally, in 1968 Tenhunen and colleagues identified heme oxygenase (HO) as the enzyme that catalyzes heme and endogenously produces CO (9).
Although endogenous production of CO was confirmed by HO, the biological implications of endogenous CO had been unrevealed for long time. Based on the conventional understanding of this gas, CO was considered as an endogenous waste of heme metabolism. The view of CO was dramatically changed after recognition of nitric oxide (NO) as a body’s gaseous messenger instead of a poison in the conventional understanding. The biological and physiological functions of endogenous CO were experimentally demonstrated in 1993 by Verma and colleagues; using primary olfactory neuron culture and HO inhibitors, they have shown that CO is an endogenous neural transmitter and regulates cGMP through guanylyl cyclase (GC) (10).
Subsequent studies, mostly using HO inhibitors and/or exogenous CO, have provided piling evidence those endogenous CO functions as a membrane/receptor-independent gaseous regulator of vascular tone; CO is shown to regulate the nonadrenergic/noncholinergic intestinal relaxation (11, 12), intrahepatic vascular resistance (13), pulmonary vascular resistance (14), and relaxation of tail artery tissues (15).
Based on these studies showing endogenous CO as a neuromessenger and a regulator of vascular tone, subsequent studies have actively used exogenous CO to treat various experimental disease conditions. Exogenously delivered CO has exerted potent protective function in numerous experimental models of inflammation, sepsis/endotoxicemia, hemorrhagic shock, autoimmune diseases, and fibrosis. Preclinical experimental conditions, in which CO can be applicable as a therapeutic choice are rapidly growing, and reviewed by recent article by Motterlini (16). In the field of transplantation, CO has been shown to inhibit acute and chronic allograft rejection (17–21), and xenograft rejection (22). However, CO’s potent protective effects are well documented in controlling I/R injury associating with transplantation, and these laboratory works on CO indicate a therapeutic potential of CO to minimize I/R injury in clinical transplantation.
2. Heme oxygenase and CO
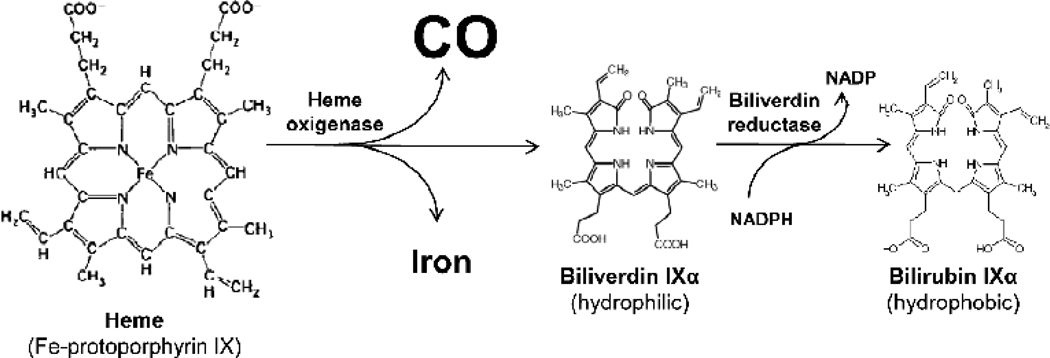
As mentioned above, HO is the rate-limiting step in heme degradation and regulates endogenous CO generation. Oxidative cleavage of the α-mesocarbon bridge of b-type heme molecules by HO yields equimolar quantities of biliverdin (BV)-IXα and CO, while the central iron is released (Figure 1). Three isozymes, HO-1 (23, 24), HO-2 (25, 26), and HO-3 (27), have been identified. HO-3 is recently cloned and has a substantially lower catalytic activity, and significant biologic functions come from HO-1 and HO-2. Although HO-1 and HO-2 catalyze the same reaction, they differ in molecular weight and are immunologically distinct in regulation and expression patterns in various tissues. HO-2, “constitutive” isozyme, is constitutively expressed in most tissues and abundant in brain, testis and liver, and responsible for particularly high HO activity in these organs during the steady (unstimulated) condition (28). In contrast, the “inducible” isoform, HO-1, is a ubiquitous heat shock protein (HSP32) that is highly induced by diverse stress-related conditions. It is upregulated in response to oxidative stress, hyperthermia, and proinflammatory stimuli in a variety of tissues, and has been shown to exert potent cytoprotective and anti-apoptotic properties, providing generalized endogenous anti-inflammatory protection against oxidative stress (29, 30). Three byproducts generated during the heme catabolism have been suggested as potential protective mediators (31–33), and CO has been shown to provide significant protection against various types of injury. Ultimately, CO is exhaled by the lung and analysis of exhaled CO can serve to assess stress-induced HO activity in vivo in critical illness (34, 35).
Figure 1. Heme degradation and endogenous CO generation.
Heme oxygenases catalyze heme into biliverdin with the release of CO and ferric iron. Biliverdin is further converted to bilirubin by biliverdin reductase.
3. Molecular targets of CO
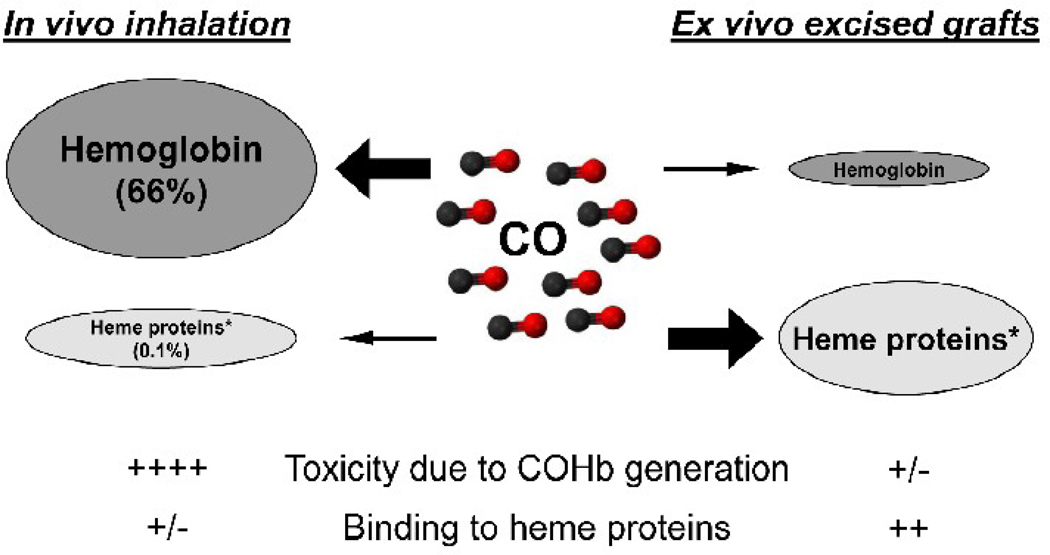
CO is chemically stable and does not appear to involve membrane receptors to exert the function. However, CO has a remarkable affinity for transition metals, suggesting that the major molecular targets of CO most likely are transition metals. Indeed, metal-containing proteins are key molecules in gas transport, gas generation and gas sensing in the body. Iron is the most abundant element of the body's transition metals. The body iron is found in hemoglobin (66%), myoglobin (3%), heme enzymes (0.1%), intracellular storage as ferritin (30%) or labile iron (chelatable iron, 1%), and extracellular transferrin (0.1%). These heme-containing proteins play important roles in regulating cellular functions in oxygen delivery and mitochondrial respiration to signal transduction. In particular, heme-containing enzymes play crucial roles in regulating cellular function, and include catalase, cyclooxygenase, cytochrome c, cytochrome P450, cytochrome c oxidase, GC, NADPH oxidase, NO synthases, prostaglandin endoperoxide synthase, peroxidases, tryptophan dioxygenase, and pyrrolases (36). In vitro studies suggest that CO interferes with these enzymes and alter their functions; for example, CO is shown to activate bovine GC (37, 38), and inhibit cytochrome c oxidase (39–41) and cytocrome P450 (42). Accordingly, in vivo function of CO might be mediated by the binding to heme-containing enzymes. As hemoglobin and myoglobin are the largest pool of the body iron, in vivo inhaled gaseous CO most likely binds to them, and the binding of CO to heme enzymes may be limited. However, when CO is used in the bloodless condition to treat excised grafts, CO might efficiently bind to heme-containing enzymes (see later, Figure 2). In rat kidney grafts exposed to CO during cold storage in CO-containing UW solution, renal I/R injury was mitigated via the inhibition of cytochrome P450 degradation and detrimental intracellular free heme increases. The study suggests that the binding of CO to renal cytochrome P450 would stabilize and prevent degradation of cytochrome P450 during cold I/R injury (43).
Figure 2. CO application for transplantation.
CO can be applied in transplantation by in vivo inhalation or ex vivo graft treatment. Ex vivo application of CO to excised bloodless grafts by cold preservation solution containing CO could avoid CO binding to hemoglobin and COHb generation. At the same time, during ex vivo exposure to CO, the efficient binding of CO to graft heme proteins could take place.
* Crucial heme proteins: catalase, cyclooxygenase, cytochrome c, cytochrome P450, guanylyl cyclase, NADPH oxidase, NO synthases, peroxidases, prostaglandin H synthase, tryptophan dioxygenase.
NO and hydrogen sulphide also are important physiological gaseous mediators in the body. Because of chemical properties as a radical species, NO has a stronger affinity to metal irons; an approximately 3000-fold higher binding ability to hemoglobin (44–47) and 50-fold greater ability to activate sGC (37, 48), compared to CO. Thus, as NO could promptly interact with hemoglobins or other heme-containing proteins and lose function, exogenous delivery of NO is difficult. In contrast, CO’s moderate affinity to target heme enzymes makes it more feasible to use this gas for therapeutic tool.
4. CO toxicity
CO’s major acute toxicity is due to its avid binding to hemoglobin and formation of COHb with an affinity significantly (210–300 times) higher than that of oxygen. This results in an interference with the oxygen-carrying capacity of the blood and consequent tissue hypoxia. COHb levels of 10–30% can cause headache, shortness of breath and dizziness, and higher levels (30–50%) produce deleterious toxicity, such as severe headache, vomiting, syncope and arrhythmia (49, 50).
In addition, due to the affinity of CO for metal atoms, CO could bind and form complexes with other numerous heme-containing proteins in the body. CO binding to the central iron group contained within these metalloproteins potentially influences the biological activity of hemeproteins. This could be the mechanisms of CO in mediating beneficial effects; however, the same process also could induce adverse outcomes. It has been suggested that CO also has a mortal toxicity independent of hemoglobin, as the toxicity does not correlate to COHb levels and CO induces late toxicity after COHb elimination (51). These toxic effects of CO could count for CO binding to hemeproteins, such as mitochondrial cytochromes and myoglobin. Although relevant cellular enzymes such as cytochrome c (a–a3) have a greater affinity for O2 than CO, and the binding of CO to cytochromes may be limited, some levels of interaction with cytochrome c and other hemeproteins may occur in the absence of tissue hypoxia (52). Cytochrome P450 (CYP) represent a large family of hemeenzymes that catalyze the oxidation of endogenous and exogenous compounds, including drugs, antioxidants, fatty acids, steroids, eicosanoids, lipid hydroperoxides, and amino acids. CYP3A1/2 metabolizes immunosuppressive drugs and is important in clinical care of transplant recipients. Therapeutic concentrations of inhaled CO in experimental animals (250 ppm for 24 hrs) do not significantly alter the CYP3A1/2 activity in the liver (unpublished data). Thus, it remains to be explored if CO preferentially binds to specific hemeproteins according to the affinity, protein location, or protein abundance.
CO is a ubiquitous pollutant found in outdoor air pollution caused by automobile exhaust and industrial emissions, and chronic exposure to low concentrations of CO also has effects on human health. In remote areas, natural background CO levels average 0.04 ppm. In contrast, CO levels in major cities could reach ~50 ppm and further increase in tunnels and garages. COHb levels in normal individual are reported to be 0.4–0.96% due to endogenous CO generation, and occupational exposure to CO in traffic polices, coal miners, and transportation mechanics, results in COHb levels ~5% (49). Chronic exposure to cigarette smoke also increases COHb levels to 2–18%. The National Institute for Occupational Safety and Health has established a recommended exposure limit for CO of 35 ppm as an 8-hour time-weighted average and 200 ppm as a ceiling for short-term exposure, based on the risk of elevated COHb levels exceeding 5%.
5. CO delivery
5-1. Inhalation of gaseous CO
Donor and/or recipient treatment with inhaled gaseous CO could be a straightforward delivery method to apply CO to treat I/R injury associating with transplantation. Low concentrations of CO could be easily used during surgery and early post-transplant period while patients are intubated. Monitoring, adjustment, and control of CO inhalation can be technically achieved with blood COHb monitoring. A brief CO inhalation at low concentrations (~250 ppm) with COHb levels 10~20% does not associate with noticeable morbidity in animal transplant experiments. Normal healthy volunteers exposed to 500–1000 ppm CO for 1 hr with COHb levels of 7–8% do not exhibit adverse effects (53, 54). However, exposure to ~500 ppm CO (COHb levels ~6%) to subjects with coronary artery disease has been reported to induce electrocardiographic changes (55–57). CO’s toxic effects might be different in transplant patients with end stage organ diseases, and in vivo inhalation delivery of gaseous CO to transplant donors/recipients will require additional precautions.
5-2. CO in liquid phase in preservation solution for excised graft
Several previous have studies used CO in the fluid phase by bubbling gaseous CO into solutions and showed beneficial effects. Typically, in ex vivo perfusion circuits, perfusion of the lung and liver with CO-equilibrated solution induces vasorelaxation in the pulmonary (58) and hepatic vasculature (13, 59). Further, culturing pancreatic islets in medium pre-saturated with CO before transplantation significantly improves islet function after transplantation (60) and enhances islet allograft survival in mice (61).
We have extended the use of CO to preservation solution by simply bubbling gaseous CO into UW solution. Ex vivo delivery of CO to excised organ grafts by storing in CO-containing UW solution conferred protective effects relevant to those seen with low-dose CO inhalation (43, 62–65) (see below). Although the solubility of gaseous CO in aqueous media is known to be poor (66), clinically effective levels of soluble CO can be achieved in UW solution after bubbling compressed gaseous CO. Ex vivo application of CO in cold storage solution has two significant potential advantages; 1) the strategy decreases the concerns of adverse effects due to COHb possibly induced during in vivo CO inhalation treatment, and 2) during ex vivo CO exposure to grafts in cold preservation solutions, CO could function effectively by binding to crucial hemeproteins in excised bloodless grafts without interference by hemoglobin.
Alternatively, transition metal carbonyls have been used as a carrier to develop CO-releasing molecules (CORM) to carry and deliver controlled amounts of CO into biological systems (67, 68). A wide range of CO carriers are currently available and include manganese (CORM-1), ruthenium (CORM-2 and -3), boron (CORM-A1) and iron (CORM-F3). Water-soluble complexes with tricarbonyl dichlororuthenium (II) dimer (CORM-3) liberate CO under physiological conditions and have been shown to elicit biological activities similar to those seen with gaseous CO, when they are used in cold preservation solution (69–71) and ex vivo perfusion solutions (72–74).
6. I/R injury in organ transplantation
I/R injury is considered one of major deleterious factors limiting the success of transplantation particularly in the current organ shortage era with an increasing use of organs from extended criteria donors. In the immediate posttransplant period, I/R injury increases risks of delayed or primary-non-function of transplanted grafts and complicates posttransplant recipient management. Furthermore, I/R injury has been shown to accelerate alloantigen specific and non-specific immune reactions, and identified as a key risk factor for developing graft degenerative changes/fibrosis and for poor long-term graft survival (75, 76).
The development of I/R injury and the application of CO in transplantation could be divided into 3 stages: initial donor stage, second cold preservation period, and final reperfusion stage. The currently available preclinical data on CO therapy in I/R injury are reviewed below according to these stages of transplantation (Table 1).
Table 1.
Application of CO to donor, graft, and recipient in mitigating I/R injury
| Species/ organ |
Model | CO delivery method | Specific protective effects |
Proposed molecular mechanisms | Ref |
|---|---|---|---|---|---|
|
DONOR Rat, heart |
CS 24 hrs and Syn Tx |
CO inhalation donor (recipient) 400 ppm UW solution saturated with CO gas 1% |
Anti-apoptosis |
NA |
(79) |
| Rat, lung | CS 6 hr and Syn Tx | CO inhalation donor 1000 ppm | Anti-inflammatory Anti-thrombosis |
ERK inhibition, Egr-1 inhibition sGC activation |
(81) |
| Rat, lung | CS 6 hr ind Syn Tx | CO inhalation donor/recipient 250 ppm |
Anti-inflammatory | p38 MAPK activation ERK1/2 inhibition |
(80, 139) |
|
GRAFT Rat, Kidney |
CS 24 hrs and Syn Tx |
UW solution saturated with CO gas 5% (40 µM) |
Anti-inflammatory |
Inhibition of cytochrome P450 degradation and intracellular heme release |
(43) |
| Rabbit, Kidney |
CS 24 hrs and ex vivo perfusion |
CORM-A1, CORM-3 in Celsior solution 50 µM |
Vasodilatation | sGC activation | (69) |
| Pig, Kidney | CS 48 hrs and Auto Tx |
UW solution saturated with CO gas 5–10% (40–100 µM) |
Anti-inflammatory Anti-fibrotic |
NA | (64) |
| Rat, Liver | CS 18–24 hrs and Syn Tx |
UW solution saturated with CO gas 5% (40 µM) |
Anti-apoptosis Anti-inflammatory |
ERK1/2 inhibition | (62) |
| Rat, Liver | CS 48 hrs and ex vivo perfusion |
CORM-3 in UW solution 50 µM | Vasodilatation | NA | (71) |
| Rat, heart | CS 4–6 hrs ex vivo perfusion |
CORM-3 in St. Thomas H solution 50 µM |
Vasodilatation | NA | (70, 162) |
| Rat, lung | CS 6 hr and Syn Tx | UW solution saturated with CO gas 5% (~40 µM) |
Anti-inflammatory | NA | (63) |
| Rat, intestine |
CS 6 hr and Syn Tx | UW solution saturated with CO gas 5% (~40 µM) |
Anti-inflammatory Vasodilatation |
sGC activation | (65) |
|
RECIPIENT Rat, Kidney |
CS 24 hrs and Syn Tx |
CO inhalation recipient 250 ppm |
Anti-inflammatory Anti-apoptosis Vasodilatation |
HIF-1α activation and VEGF upregulation. |
(108, 157) |
| Pig, Kidney | WI 60 min + CS 24 hrs and Allo Tx |
CO inhalation recipient 2–3 mg/kg (COHb 4–9%) |
Anti-inflammatory Anti-apoptosis |
Enhance proliferative repair | (141) |
| Pig, Kidney | WI 10 min + CS 16– 18 hrs and ex vivo perfusion |
CORM-3 in perfusion solution 50– 400 µM |
Vasodilatation | NA | (74) (73) |
| Rat, Liver | CS 24 hrs and ex vivo perfusion |
Perfusate (Ht 15%) saturated with CO gas 300 ppm |
Vasodilatation | p38 MAPK activation | (59) |
| Rat, Liver | CS 18 hrs and Syn Tx |
CO inhalation recipient 20–250 ppm (COHb 5–25%) |
Anti-inflammatory Anti-thrombosis |
ERK1/2 MAPK inhibition | (115, 138, 163) |
| Rat, lung | CS 6 hr and Syn Tx | CO inhalation donor/recipient 250 ppm |
Anti-inflammatory | p38 MAPK activation ERK1/2 inhibition |
(80, 139) |
| Rat, intestine |
CS 6 hr and Syn Tx | CO inhalation recipient 250 ppm | Anti-inflammatory Anti-apoptosis |
sGC activation | (109, 140) |
|
WARM ISCHEMIA Mouse, kidney |
WI 40 min |
[Ru(CO)3Cl2]2 iv 10 mg/kg CORM-3 ip 40 mg/kg |
NA |
HO-1 upregulation |
(150) |
| Rat, Liver | WI 70% liver 60 min |
CORM-2 iv 8 mg/kg | Anti-inflammatory Anti-apoptosis |
NFκB inhibition | (164) |
| Mice, heart | LAD ligation 30 min | CORM-3 iv 3.54 mg/kg | NA | NA | (147) |
| Rat, heart | LAD ligation 30 min | CO inhalation 250–1000 ppm | Anti-inflammatory | p38 MAPK, Akt, eNOS, and sGC activation |
(146) |
| Rat, heart | LAD ligation 30 min | Hemospan saturated with CO gas | NA | NA | (165) |
| Rat, heart | WI 30min and ex vivo perfusion |
CORM-3 10–50 µM | NA | NA | (72, 166) |
| Pig, heart | LAD ligation 45 min | CO inhalation COHb 5% | NA | NA | (167) |
| Mouse, lung | WI left lung 60 min | CO inhalation 1000 ppm (COHb 24.8%) |
Anti-thrombosis | sGC activation PAI-1 inhibition |
(114) |
| Mouse, lung | WI left lung 30 min | CO inhalation 500 ppm | Anti-apoptosis | P38 MAPK activation | (119) |
| Pig, lung | WI left lung 90 min | CO inhalation 250 ppm (COHb 12.1%) |
Anti-inflammatory | Low HMGB-1 | (168) |
Allo Tx: allogenic transplantation
CS: cold storage
LAD: left anterior descending coronary artery
Syn Tx: syngenic transplantation
WI: warm ischemia
7. CO delivery to donor can decrease I/R injury
I/R injury in transplantation is a complex cascade of interrelated diverse injurious pathways with efficient self-amplifying loops. In essence, hypoxic condition induces depletion of ATP and results in failure to maintain intracellular homeostasis, including altered cytosolic pH, disturbances of cellular ion homeostasis, increased cytosolic Na+ and Ca2+ concentrations, activation of cytotoxic enzymes (e.g. proteases, phospholipases, endonucleases) and degradation of their substrates, and increases in the permeability of cellular membranes (77, 78). These changes take place in both warm and cold ischemia. The cold storage method decreases the rate of anoxic cell injury process. However, hypothermia itself could cause cell injury (see below), and significant alterations occur in endothelial cells during cold storage.
In the donor stage, cadaveric donors with brain death suffer from profound physiological disturbances, including severe hemodynamic dysfunction, profound metabolic alterations, and malfunction in endocrine, immune, and coagulation systems. In addition, donation after cardiac death (DCD) associates with direct warm ischemic injury. Donor factors, such as known medical conditions, including hypertension, diabetes, and metabolic disorders also contribute to graft injury and outcomes of transplantation. CO can be used in this stage as donor treatment.
Efficacy of donor CO treatment was directly examined in the rat heart and lung transplantation models with 24 hrs and 6 hrs cold storage and syngenic transplantation. Donor CO inhalation (250–1000 ppm) mitigated I/R injury and improved graft function and survival after transplantation (79–81). CO showed anti-inflammatory, anti-apoptotic, and anti-thrombosis effects after reperfusion with suppressions of ERK1/2 MAPK activation, Erg-1 expression, and Erg DNA-binding activity (81). In the rat liver transplantation model, donor CO 250 ppm inhalation also inhibited I/R injury and improved posttransplant graft function. The protective effects associated with HSP70, but not HO-1 or HSP60, upregulation in graft livers before transplantation (unpublished data).
Clinically, various organs have been obtained from donors with fatal CO poisoning and used for transplantation. As the central nervous system and myocardium are most susceptible to CO toxicity (82), most publications focus on the use of intrathoracic organs (heart and lung) and aim to validate the use of such donors as a part of donor pool expansion (Table 2). Early transplant experiences with CO-poisoned donors (COHb levels 16.4–48%) were rather disappointing due to high mortality (83–86). Although the causal relationship between graft losses and CO were not firmly confirmed, the use of organs from CO intoxicated donors was controversial. However, recent individual case reports demonstrated successful transplantation from carefully selected CO poisoned donors. Outcomes of transplantation (heart, lung, liver, kidney and pancreas) from CO intoxicated donors without CO-induced tissue damage appear to be comparable to those with nonintoxicated donors (87–91). Thus, grafts obtained from selected hemodynamically stable CO poisoning donors with minimal tissue damage could be transplanted; however, these reports do not investigate whether donors exposed to CO show reduced degree of I/R injury. Bojakowski has reported unexpected excellent outcome of kidney graft from a CO poisoned donor in spite of complicated transplantation procedure with prolonged warm ischemia to 100 minutes (89). Further studies to identify the optimized condition to use CO as donor treatment are warranted.
Table 2.
Organ transplantation with CO intoxicated donors
| Organ Tx (#) |
# donors |
Survival >2M |
Cause of failure | Comments | Year | Authors | Ref |
|---|---|---|---|---|---|---|---|
| Heart (1) | 1 | 1/1 | NA | COHb: high levels | 2008 | Martìn-Suàrez S | (87) |
| Heart (1) | 1 | 1/1 | NA | CO exposure 72h | 2008 | Sezgin A | (88) |
| Kidney (2) | 1 | 2/2 | NA | COHb: 48.7%, warm ischemia 100min | 2007 | Bojakowski K | (89) |
| Heart,(1) Kidney (2) Pancreas (1) Liver (1) |
1 | 5/5 | NA | COHb: 36% Early heart graft dysfunction |
2001 | Rodrigus IE | (90) |
| Heart (6) Lung (1) |
6 | 6/7 | Heart graft failure | Lung recipient died of lung infection (8M) | 2001 | Luckraz H | (91) |
| Heart (2) | 2 | 2/2 | NA | 2001 | Bentley MJ | (169) | |
| Heart (5) | 5 | 4/5 | Technical (24h) | COHb: 16.4–20% 2 additional death due to intestinal ischemia (3M) and pancreatic cancer (4M) |
1997 | Koerner MM | (83) |
| Liver (2) | 2 | 2/2 | NA | COHb: 25–32% | 1996 | Verran D | (170) |
| Heart (1) | 1 | 1/1 | NA | Graft loss (14M) | 1995 | Roberts JR | (171) |
| Heart (1) Kidney (2) Liver (1) Pancreas (1) |
1 | 4/5 | Heart graft failure | 1995 | Hantson P | (84) | |
| Heart (1) | 1 | 1/1 | NA | COHb: 29% | 1993 | Iberer F | (172) |
| Heart (1) Lung (1) |
1 | 1/2 | Heart graft failure | CO exposure 4h Heart was diffusely hypokinetic in the donor |
1992 | Shennib H | (85) |
| Kidney (2) | 1 | 2/2 | NA | One recipient with delayed graft function | 1992 | Hebert MJ | (173) |
| Heart (2) | 2 | 2/2 | NA | COHb: 14.7–43% | 1992 | Smith JA | (174) |
| Heart (1) | 1 | 0/1 | Heart graft failure (4d) |
COHb: 48% | 1989 | Karwande SV | (86) |
8. CO delivery during cold storage and inhibition of apoptosis
Although cold perfusion and preservation of organ grafts are the principle procedure to slow down cell metabolism and decrease oxygen and energy consumption, hypothermic/hypoxic condition during cold storage of organs initiates injury process and sets a stage for apparent damage by reperfusion. Hypothermic condition per se induces losses of ionic homeostasis, membrane integrity, and membrane associated transporter and enzyme functions, resulting in metabolic, structural and functional cell injury (92–94). In particular, hypothermia has been shown to induce apoptosis in various types of cultured cells by increasing the opening of the permeability transition pores and subsequent mitochondrial swelling, and generation of ROS and direct DNA damage, as well as by an activation of proteasomes and serine proteases (95–98). Previous experimental studies have shown that prolonged cold ischemia alone increased apoptotic cell death in organ grafts (96, 99, 100). Further, caspase inhibitors added into cold preservation solution have been shown to inhibit the apoptotic process, reduce I/R injury, and improve graft function (99–101). Moreover, clinical studies demonstrate that increased frequencies of apoptotic renal tubular epithelial cells in kidney graft biopsies before transplantation associate with the development of delayed graft function (102, 103). Thus, activation of apoptosis pathway during cold storage plays significant roles in transplant-induced cold I/R injury and posttransplant graft function.
Accordingly, application of CO to excised grafts during cold storage period becomes attractive to inhibit ischemic injury and improve graft viability owing to CO’s anti-apoptosis action (see below). Although brief CO inhalation treatment would be applicable for clinical transplantation, significant obstacles may exist in applying in vivo CO treatment, due to the CO binding to hemoglobin and possible CO's adverse effects. Ex vivo treatment of excised bloodless grafts with gaseous CO could minimize the concerns associating with in vivo CO inhalation and significantly advance the application of CO in a clinical setting (see above and Figure 2). These possible advantages of the graft CO treatment have been confirmed in experimental studies. When rat kidney, liver, lung, and intestine grafts were perfused and stored in CO-containing UW preservation solution, I/R injury was significantly mitigated with improved graft function and survival after transplantation (43, 62, 63, 65). Preservation solutions containing CORM also protected rabbit kidney, and rat liver and heart grafts from I/R injury with improved blood flow and function after reperfusion in ex vivo perfusion circuits (69–71). Using the preclinical large animal model of kidney transplantation with 48 hrs static cold storage, ex vivo treatment of excised porcine kidney grafts with CO during cold storage inhibited renal I/R injury with shorter initial oliguria periods, lower serum creatinine levels, less urinary protein secretion, and better graft histopathology, when compared to those in control UW solution. These beneficial effects of CO did not associate with any adverse event or COHb elevation in recipient animals (64).
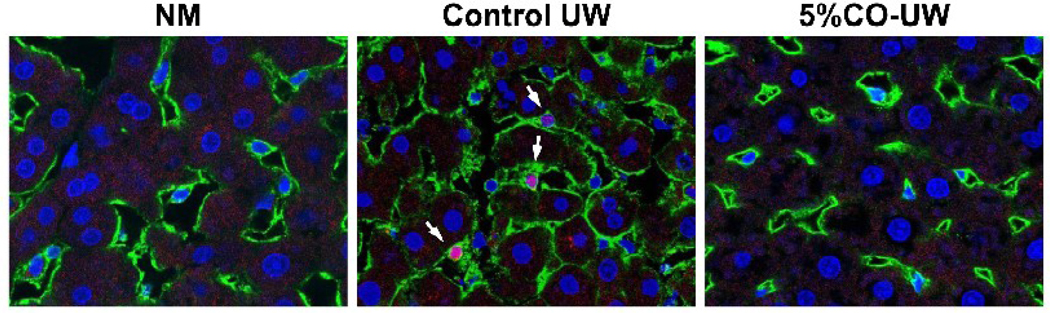
The inhibition of I/R injury in the graft treatment with CO in preservation solution associates with anti-inflammatory and vasodilative effects after reperfusion. Ikeda examined effects of CO on liver grafts at the end of cold storage in CO-containing UW solution and demonstrated significantly decreased frequencies of sinusoidal endothelial cell death, reduced intercellular cell adhesion molecule translocation, and less matrix metalloproteinase release during cold preservation (62) (Figure 3). Together with the vasodilatative effect of CO in preservation solution, graft CO treatment might be effective in protecting vascular endothelial cells against damage caused by the hypothermic/hypoxic condition. Although graft CO treatment does not cause COHb elevation after reperfusion, high CO levels in preservation solution might be unsuitable. CO solubility of 40~100 µM appeared to be beneficial using CORM or 5–10% gaseous CO for bubbling preservation solutions; however, higher soluble CO levels in preservation solution resulted in no beneficial effects in the porcine kidney transplantation study (64) and poor survival after the liver transplantation experiment (62). As graft tissue CO levels increase by the end of cold storage depending on the concentration of soluble CO levels in preservation solution, high CO concentrations in preservation solutions and excess CO bindings to grafts may introduce impaired graft hemeprotein function.
Figure 3. Inhibition of sinusoidal endothelial cell death with CO during cold storage.
Rat liver grafts were preserved for 18 hours in control UW solution or UW solution containing CO. At the end of cold preservation, grafts were perfused with a propidium iodide (PI) solution, fixed, cut into 6-µm sections, and stained with sinusoidal endothelial cell-specific SE-1 monoclonal antibody (green) and Hoechst nuclear dye (blue). Normal liver (NM) showed networks of healthy SECs with smooth SE-1 surface staining and flat nuclear staining. In liver grafts preserved in control UW solution, many SECs showed colocalization (pink, white arrows) of nuclear Hoechst (blue) and PI (red). There were very few injured PI+ SE-1+ SECs in liver grafts with CO-containing UW solution.
9. Reperfusion and recipient CO treatment to minimize I/R injury
Reperfusion of grafts with warm oxygenated blood causes an excess of oxygen and generates ROS, resulting in significantly augmented cell injury. Damage or loss of vascular endothelial cells (VECs) causes disturbance of microcirculation, and anoxic cell injury may persist after graft reperfusion. Further, damaged cells, in particular VECs upregulate adhesion molecules (e.g. ICAM, VCAM) and activate noncellular elements, such as the complement system, blood coagulation cascade, chemokines, and proinflammatory cytokines, resulting in influx of host inflammatory infiltrates. In addition, the damaged cell debris and disrupted tissue matrix activate macrophages, dendritic, and other cells via binding to Toll-like receptors. Thus, the graft reperfusion promptly initiates vigorous inflammatory events that are complicated with the microcirculation disturbance. The complexity of the cellular injury processes during early reperfusion associates with activation of various intracellular signaling pathways, and MAPK, NF-κB, and apoptotic pathways have all been reported to be activated in the reperfusion phase.
9-1. Vasodilatation effects
Endogenously produced CO plays a key role in vivo in regulating vasomotor tones mostly through the activation of sGC and subsequent generation of cGMP, which is an intracellular signaling molecule involved in the regulation of cellular events, such as smooth muscle relaxation, inhibition of platelet aggregation, and synaptic transmission (11–15). Suematsu has shown in the ex vivo liver perfusion circuit that the inhibition of endogenous CO generation with an HO-1 inhibitor increases perfusion pressure, and exogenous CO or cGMP analogues are able to reverse the effects of the HO-1 inhibitor (104). In vitro study suggests that hypoxia-induced HO-1 and CO generation in the vasculature regulate the expression of endothelin-1 (ET-1) and platelet-derived growth factor-B (PDGF-B) through sGC/cGMP pathway (105). Exogenously provided CO in human pulmonary artery smooth muscle cell culture inhibits ET-1 expression and release (105, 106). Damaged VECs release a variety of vasoconstrictive proteins such as ET-1, which is the most powerful natural mammalian vasoconstrictive agent (107). CO may induce vasodilatation via the suppression of ET-1. Since the damage in the graft vascular system and significant disturbance in graft microcirculation are key events in I/R injury associating with organ transplantation, CO’s potent vasodilatation effects would have significant impacts in minimizing graft injury.
As seen with donor and/or graft CO treatment, recipient CO treatment during perireperfusion period effectively inhibits I/R injury with significant vasodilative effects. In the kidney and intestine I/R injury models with prolonged cold storage, recipient CO inhalation significantly improves graft blood flow (108, 109). As the beneficial effect is abolished by a selective sGC inhibitor, CO‘s vasodilative effects in I/R injury might be mediated through sGC/cGMP pathway. In the hepatic I/R injury experiment using ex vivo perfusion circuit, CO reduces portal venous resistance via the activation of p38 MAPK signaling pathway (59). Recent studies in the pig using an isolated organ-perfusion system with autologous pig blood show that the addition of CORM in perfusion solution increases renal blood flow, creatinine clearance, and urine output in an experimental model of 60 min warm ischemia with 18 hrs cold storage (73, 74). In these studies, the doses of 50–100 µM CORM-3 show benefits, while higher doses of 200–400 µM CORM resulted in renal dysfunction.
9-2. Anti-coagulation effects
Vascular injury during I/R injury leads to platelet adhesion, activation, and aggregation, resulting in the formation of microvascular thrombosis. I/R injury also activates tissue factor and coagulation system. When activated platelets adhere to sites of vascular injury, the platelet activating factors, such as ADP, thromboxane A2 (TXA2), serotonin, collagen and thrombin, are produced to recruit additional circulating platelets for aggregation and to directly activate platelets to express proinflammatory molecules (e.g. P-selectin, soluble CD40 ligand and others). HO-1 and CO are known to play pivotal roles in regulating vascular integrity, as the patient with HO-1 deficiency and HO-1 knockout mice have been reported to develop advanced systemic vascular endothelial cell injury (110, 111). As early as 1982, CO is shown to inhibit the arachidonic acid induced platelet aggregation (112). Subsequently, it is shown that CO inhibits human platelet aggregation triggered with arachidonate, ADP, collagen, thrombin, or the prostaglandin endoperoxide analogue via sGC/cGMP activation (113).
These anti-coagulation effects greatly benefit in inhibiting graft injury due to I/R. In the lung warm I/R injury, CO suppresses fibrin accumulation and tissue injury by inhibiting PAI-1, the major inhibitor of the fibrinolytic axis (114). The protective effects of CO and PAI-1 inhibition are abolished by the inhibition of sGC activity, confirming that CO potentiates fibrinolysis by activating sGC/cGMP pathway. In the rat lung transplant-induced I/R model, donor CO inhalation protects lung grafts by inhibiting proinflammatory and prothrombotic mediators through cGMP-dependent ERK inhibition and Erg-1 expression (81). In rat liver transplant-induced I/R injury, CO significantly inhibited platelet accumulation as early as 1 hr after reperfusion (115).
9-3. Anti-apoptotic function
Tissue necrosis is a common pathophysiological finding of I/R injury; however, apoptosis of graft cells, particularly of VECs, is evident before and after reperfusion with an activation of initiator and effector caspases and of Bcl-2 family members via the intrinsic and extrinsic pathways of apoptosis. As mentioned above, mitigation of I/R injury by caspase inhibitors indicates an important role of apoptosis in developing graft injury (99–101). Indeed, apoptotic cell death has been shown to be a crucial event in initiating inflammation and subsequent necrotic tissue injury (116).
In vitro studies have clearly shown CO’s anti-apoptotic effects during various injurious stimulations. When VECs are exposed to exogenous CO (10,000 ppm), TNF-α-mediated apoptosis is suppressed through the activation of the p38 MAPK pathway and NFκB (117, 118). Low level exogenous CO (15 ppm) attenuates anoxia-reoxygenation-induced lung VEC apoptosis through p38 MAPK activation (119). Further study in anoxia-reoxygenation injury shows that the anti-apoptotic effect of CO in VECs is also depend on the activation of STAT3 via PI3K/Akt and p38 MAPK pathways with a subsequent attenuation of Fas expression and caspase 3 activity (120). Apoptosis of vascular smooth muscle cells (SMCs) induced by cytokines is inhibited with CO (50–200 ppm) in a concentration-dependent manner through the activation of sGC with inhibitions of mitochondrial cytochrome c release and p53 expression (121). Thus, a direct inhibition of apoptosis afforded by CO may be mediated by several different mechanisms, probably depending on the variety of stimuli to induce apoptosis and the types of responding cells.
Inhibition of VEC apoptosis with CO also is seen after reperfusion in the experimental transplantation models; although it is unclear whether the inhibition of apoptosis in in vivo experiments is caused by CO’s direct anti-apoptotic function or is the results of other mechanisms of CO. I/R injury induced by prolonged cold storage of renal and intestinal grafts results in significant morphological alteration of graft VECs, and recipient treatment with inhaled CO 250 ppm protects VECs and preserves vascular morphology and integrity (108, 109). Apoptosis of graft parenchymal cells after transplantation is also inhibited with CO. Recipient CO inhalation (250 ppm) inhibits the expression of cleaved caspase 3 on renal tubular epithelial cells in rat kidney grafts and intestinal crypt epithelial cells in small bowel grafts after prolonged cold preservation and transplantation with a downregulation of early Bax mRNA and upregulation of antiapoptotic Bcl-2 (108, 109). Myocardial TUNEL positive apoptotic cells in rat heart grafts also decreased after 24 hrs cold storage and transplantation with donor, graft and recipient CO treatment (79).
9-4. Anti-inflammatory effect
Potent anti-inflammatory actions of CO have been well documented in various experimental models, and are the most extensively studied CO’s function. Inhaled CO or CORM inhibits endotoxin-induced inflammatory tissue injury (122–126), sepsis-induced lethality in polymicrobial sepsis (127), hyperoxia-induced acute lung injury (128), ventilator-induced lung injury (129–131), autoimmune neuroinflammation (132), postoperative ileus (133, 134), inflammatory liver damage (135), and graft damage due to rejection (17, 18, 21, 22, 136). These anti-inflammatory effects of CO associated with potent downregulation of proinflammatory cytokines, chemokines, and inflammatory infiltrates. In vitro experiments show that CO inhibits RAW 264.7 macrophages to produce inflammatory cytokine production (e.g. TNF-α, IL1β, MIP-1) while increased anti-inflammatory IL-10 in response to LPS via p38 MAPK activation (122) or inhibition of JNK/AP-1 signaling pathways (123). CO also inhibits LPS-induced growth factor (GM-CSF) induction of RAW 264.7 macrophages via NFκB inhibition (137). These in vitro studies suggest that CO might function mainly on macrophage populations in mediating anti-inflammatory effects.
In rodent transplant experiments, recipient CO inhalation significantly reduces inflammatory responses in I/R injury and resulted in improved graft function of kidney, liver, heart, lung and small intestine grafts with extended cold preservation and syngenic transplantation (108, 138–140). Anti-inflammatory effects of CO are observed as decreased mRNA and protein levels for proinflammatory mediators (e.g. IL-6, TNF-α, IL-1β, iNOS, cyclooxygenase-2) and decreased cellular infiltration. Using the porcine kidney transplantation model, peritransplant recipient CO inhalation reduces I/R injury of kidney grafts with 60 min warm ischemia and 24 hrs cold storage (141). I/R injury induce prompt activations of several intracellular signaling pathways (62, 138), and protective effects of CO, particularly anti-inflammatory functions, appear to correlate with its regulation of MAPK pathways. MAPK signaling pathways integrate cellular responses to the environmental stresses and inflammation (142). The pathways include parallel and interacting cascades that phosphorylate the three main MAPK families, p38 MAPK, ERK, and JNK. Each of pathways is preferentially recruited by distinct sets of stimuli through diverse receptor families to allow the cells to respond coordinately to multiple divergent inputs. Early in vitro study demonstrating CO’s anti-inflammatory properties in TNF-α-induced rat pulmonary artery VEC injury shows an inhibition of ERK1/2 pathway and activation of p38 MAPK (143). Although the mechanisms of action in in vivo experiments are complex, anti-inflammatory actions of CO in organ transplantation also associate with regulation of MAPK signaling pathways. Recipient CO inhalation inhibits hepatic I/R injury with 18 hrs cold storage with a significant inhibition of inflammatory responses via ERK1/2 inhibition (138). Lung I/R injury and inflammatory cytokine upregulation induced by 6 hrs cold preservation in rats are ameliorated by CO inhalation and associated with significant inhibition of ERK1/2 and marginal p38 MAPK activation (139). In ex vivo rat liver perfusion circuit model, CO’s anti-inflammatory effects, such as a decrease of cell infiltration, are abrogated by a p38 MAPK inhibitor (59). In addition, CO might also have direct antimicrobial effects in controlling inflammation, as CORM-3 increases survival of immunoincompetent mice following P. aeruginosa bacteremia via inhibition of pathogen’s respiratory chain (144). Taken together, large numbers of preclinical studies emphasize a potential mechanism of CO modulating the inflammatory response by differentially regulating the MAPKs.
10. Warm I/R injury and CO
Although significant differences in injury sites and mechanisms exist between warm and cold ischemic injury, they also share common injurious pathways (145). CO is able to alleviate warm ischemic injury induced by brief interruption of blood supply and subsequent reperfusion in various organs. In the rat heart warm I/R injury model, inhalation of CO at 250–1000 ppm reduces infarct size and improved cardiac function (146). Likewise, in the mouse heart warm I/R injury model with 30 min left anterior descending coronary artery ligation, IV injection of CORM-3 reduces infarct size and improves function (147–149). Renal function after 40 min warm I/R injury also is improved with CORMs (150). The beneficial effects of gaseous CO or injection of CORM are seen in various warm I/R models of kidney, liver, and lung using rodents and pigs with similar ranges of CO doses through anti-inflammatory and anti-apoptotic actions (Table 1).
11. Tissue repair and anti-fibrotic effects
In vitro experiments, CO treatment results in a prompt burst of mitochondrial ROS, presumably due to CO’s binding to the heme group of cytochrome c oxidase (41, 151). This ROS burst is proposed to activate the p38 MAPK and other signal transduction pathways, including the hypoxia-inducible factor 1 alpha (HIF-1α) (152). HIF-1α is a stress response transcription factor, and regulates numerous genes involved in angiogenesis, metabolism, and cell survival, including TGF-β and vascular endothelial growth factor (VEGF). VEGF is a multifunctional growth factor that induces VEC growth, differentiation, and regeneration through its receptors VEGFR-1 and VEGFR-2 (153, 154). HIF-1α/VEGF pathway has been shown to be involved in some of the cytoprotective effects of CO. VEC culture with CORM leads to VEGF upregulation and angiogenesis (155). Astrocytes stimulated with CORM promote angiogenesis by increasing VEGF expression and secretion via increasing HIF-1α protein level (156). In renal I/R injury model, inhaled CO promotes VEGF upregulation through its upstream signal, HIF-1 activation (157). CO has been shown in the cardiac warm I/R model to significantly increase c-kit+ progenitor cell migration into the infarct area and subsequent vascular and myocardial regeneration via HIF-1α, stromal cell derived factor-1α and VEGF upregulation (158). These data suggest that CO also potentially has an important action promoting proliferative repair after I/R injury.
Interestingly, CO alternatively suppresses in vitro proliferation of cultured human airway SMC with increased cellular levels of p21Cip1 and decreased levels of cyclins A and D (159, 18). The same effects are seen in in vivo mice bleomycin-induced lung fibrosis model, and CO suppresses collagen-1 production and matrix deposition by fibroblast through the transcriptional regulator Id1 (160). CO also inhibits renal fibrosis in the mouse unilateral ureteral obstruction model via the MKK3 pathway (161). Severe I/R injury could result in the failure in proper repairing, and subsequent replacement of the graft parenchyma by fibrotic tissue could lead to loss of organ function. Thus, CO’s actions to promote proliferative repair and inhibit fibrosis would have a great potential to combat long-term complications of I/R injury.
12. Conclusion
Gaseous modulators, such as CO, NO, and hydrogen sulphide, are important physiological mediators in the body. Although these gases were initially viewed as toxic substances, they play important roles in the body as signaling molecules. In particular, endogenous CO plays crucial roles in regulating vascular tones, platelet and endothelial cell function. Significant amounts of preclinical data indicate that exogenously provided CO is able to ameliorate I/R injury associating with organ transplantation. Currently, a prospective, multicenter, single-blind, placebo-controlled, phase II trial is being conducted to examine safety and tolerability of inhaled CO in kidney transplant patients (ClinicalTrials.gov Identifier: NCT00531856). In early history, CO was viewed as a poison due to the environmental generation of high concentrations; however, CO is an efficient endogenous gaseous regulator with potent cytoprotective effects. If used properly, this simple molecule would have enormous potential.
ABBREVIATIONS
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- CORM
CO-releasing molecules
- ERG-1
early growth response protein-1
- ERK
extracellular signal-regulated kinase
- ET1
endothelin-1
- GC
guanylyl cyclase
- HIF
hypoxia-induced factor
- HO
heme oxygenase
- I/R
ischemia/reperfusion injury
- JNK
c-Jun NH2 terminal protein kinase
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- ROS
reactive oxygen species
- SMC
smooth muscle cells
- VECS
vascular endothelial cells
- VEGF
vascular endothelial growth factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard C. Paris: Bailliere; 1857. [Google Scholar]
- 2.Haldane JB. Carbon Monoxide as a Tissue Poison. Biochem J. 1927;21:1068–1075. doi: 10.1042/bj0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas CG, Haldane JS, Haldane JB. The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol. 1912;44:275–304. doi: 10.1113/jphysiol.1912.sp001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjostrand T. Endogenous formation of carbon monoxide; the CO concentration in the inspired and expired air of hospital patients. Acta Physiol Scand. 1951;22:137–141. doi: 10.1111/j.1748-1716.1951.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 5.Siosteen SM, Sjostrand T. A method for determination of low concentrations of carbon monoxide in the blood and the relation between the CO-concentration in the blood and that in the alveolar air. Acta Physiol Scand. 1951;22:129–136. doi: 10.1111/j.1748-1716.1951.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 6.Coburn RF, Blakemore WS, Forster RE. Endogenous carbon monoxide production in man. J Clin Invest. 1963;42:1172–1178. doi: 10.1172/JCI104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostrand T. The in vitro formation of carbon monoxide in blood. Acta Physiol Scand. 1952;24:314–332. doi: 10.1111/j.1748-1716.1952.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrand T. The formation of carbon monoxide by the decomposition of haemoglobin in vivo. Acta Physiol Scand. 1952;26:338–344. doi: 10.1111/j.1748-1716.1952.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 11.Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- 12.Watkins CC, Boehning D, Kaplin AI, Rao M, Ferris CD, Snyder SH. Carbon monoxide mediates vasoactive intestinal polypeptide-associated nonadrenergic/noncholinergic neurotransmission. Proc Natl Acad Sci U S A. 2004;101:2631–2635. doi: 10.1073/pnas.0308695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvester JT, McGowan C. The effects of agents that bind to cytochrome P-450 on hypoxic pulmonary vasoconstriction. Circ Res. 1978;43:429–437. doi: 10.1161/01.res.43.3.429. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 17.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, et al. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 19.Chauveau C, Bouchet D, Roussel JC, Mathieu P, Braudeau C, Renaudin K, Tesson L, et al. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant. 2002;2:581–592. doi: 10.1034/j.1600-6143.2002.20702.x. [DOI] [PubMed] [Google Scholar]
- 20.Neto JS, Nakao A, Toyokawa H, Nalesnik MA, Romanosky AJ, Kimizuka K, Kaizu T, et al. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am J Physiol Renal Physiol. 2006;290:F324–F334. doi: 10.1152/ajprenal.00026.2005. [DOI] [PubMed] [Google Scholar]
- 21.Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, et al. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 23.Maines MD, Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974;71:4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Takahashi S, Kikuchi G. Partial purification and reconstitution of the heme oxygenase system from pig spleen microsomes. J Biochem. 1974;75:1187–1191. doi: 10.1093/oxfordjournals.jbchem.a130494. [DOI] [PubMed] [Google Scholar]
- 25.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 26.Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem. 1986;261:11131–11137. [PubMed] [Google Scholar]
- 27.McCoubrey WK, Jr., Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 28.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 29.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 31.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 32.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, et al. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 33.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–1726. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 34.Scharte M, Bone HG, Van Aken H, Meyer J. Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun. 2000;267:423–426. doi: 10.1006/bbrc.1999.1936. [DOI] [PubMed] [Google Scholar]
- 35.Zegdi R, Caid R, Van De Louw A, Perrin D, Burdin M, Boiteau R, Tenaillon A. Exhaled carbon monoxide in mechanically ventilated critically ill patients: influence of inspired oxygen fraction. Intensive Care Med. 2000;26:1228–1231. doi: 10.1007/s001340000590. [DOI] [PubMed] [Google Scholar]
- 36.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 37.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 38.Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D. Basis of guanylate cyclase activation by carbon monoxide. Proc Natl Acad Sci U S A. 1995;92:2568–2571. doi: 10.1073/pnas.92.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohlrab H, Ogunmola GB. Carbon monoxide binding studies of cytochrome a3 hemes in intact rat liver mitochondria. Biochemistry. 1971;10:1103–1106. doi: 10.1021/bi00783a001. [DOI] [PubMed] [Google Scholar]
- 40.Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol. 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- 41.Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 42.Leemann T, Bonnabry P, Dayer P. Selective inhibition of major drug metabolizing cytochrome P450 isozymes in human liver microsomes by carbon monoxide. Life Sci. 1994;54:951–956. doi: 10.1016/0024-3205(94)00496-x. [DOI] [PubMed] [Google Scholar]
- 43.Nakao A, Faleo G, Shimizu H, Nakahira K, Kohmoto J, Sugimoto R, Choi AM, et al. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–1016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 44.Obolenskaya M, Vanin AF, Mordvintcev PI, Mulsch A, Decker K. Epr evidence of nitric oxide production by the regenerating rat liver. Biochem Biophys Res Commun. 1994;202:571–576. doi: 10.1006/bbrc.1994.1966. [DOI] [PubMed] [Google Scholar]
- 45.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirota M, Inoue M, Ando Y, Hirayama K, Morino Y, Sakamoto K, Mori K, et al. Inhibition of stress-induced gastric injury in the rat by glutathione. Gastroenterology. 1989;97:853–859. doi: 10.1016/0016-5085(89)91488-1. [DOI] [PubMed] [Google Scholar]
- 47.Kosaka H, Sawai Y, Sakaguchi H, Kumura E, Harada N, Watanabe M, Shiga T. ESR spectral transition by arteriovenous cycle in nitric oxide hemoglobin of cytokine-treated rats. Am J Physiol. 1994;266:C1400–C1405. doi: 10.1152/ajpcell.1994.266.5.C1400. [DOI] [PubMed] [Google Scholar]
- 48.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 49.Von Burg R. Carbon monoxide. J Appl Toxicol. 1999;19:379–386. doi: 10.1002/(sici)1099-1263(199909/10)19:5<379::aid-jat563>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Gorman D, Drewry A, Huang YL, Sames C. The clinical toxicology of carbon monoxide. Toxicology. 2003;187:25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 51.Stewart RD. The effect of carbon monoxide on humans. Annu Rev Pharmacol. 1975;15:409–423. doi: 10.1146/annurev.pa.15.040175.002205. [DOI] [PubMed] [Google Scholar]
- 52.Gorman DF, Runciman WB. Carbon monoxide poisoning. Anaesth Intensive Care. 1991;19:506–511. doi: 10.1177/0310057X9101900403. [DOI] [PubMed] [Google Scholar]
- 53.Hausberg M, Somers VK. Neural circulatory responses to carbon monoxide in healthy humans. Hypertension. 1997;29:1114–1118. doi: 10.1161/01.hyp.29.5.1114. [DOI] [PubMed] [Google Scholar]
- 54.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 55.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, et al. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989;321:1426–1432. doi: 10.1056/NEJM198911233212102. [DOI] [PubMed] [Google Scholar]
- 56.Adams KF, Koch G, Chatterjee B, Goldstein GM, O'Neil JJ, Bromberg PA, Sheps DS. Acute elevation of blood carboxyhemoglobin to 6% impairs exercise performance and aggravates symptoms in patients with ischemic heart disease. J Am Coll Cardiol. 1988;12:900–909. doi: 10.1016/0735-1097(88)90452-4. [DOI] [PubMed] [Google Scholar]
- 57.Sheps DS, Herbst MC, Hinderliter AL, Adams KF, Ekelund LG, O'Neil JJ, Goldstein GM, et al. Production of arrhythmias by elevated carboxyhemoglobin in patients with coronary artery disease. Ann Intern Med. 1990;113:343–351. doi: 10.7326/0003-4819-113-5-343. [DOI] [PubMed] [Google Scholar]
- 58.Naik JS, Walker BR. Homogeneous segmental profile of carbon monoxide-mediated pulmonary vasodilation in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1436–L1443. doi: 10.1152/ajplung.2001.281.6.L1436. [DOI] [PubMed] [Google Scholar]
- 59.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, et al. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 60.Gunther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, Bach FH, et al. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Lee SS, Gao W, Czismadia E, McDaid J, Ollinger R, Soares MP, et al. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54:1400–1406. doi: 10.2337/diabetes.54.5.1400. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda A, Ueki S, Nakao A, Tomiyama K, Ross MA, Stolz DB, Geller DA, et al. Liver graft exposure to carbon monoxide during cold storage protects sinusoidal endothelial cells and ameliorates reperfusion injury in rats. Liver Transpl. 2009;15:1458–1468. doi: 10.1002/lt.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohmoto J, Nakao A, Sugimoto R, Wang Y, Zhan J, Ueda H, McCurry KR. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2008;136:1067–1075. doi: 10.1016/j.jtcvs.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida J, Ozaki KS, Nalesnik MA, Ueki S, Castillo-Rama M, Faleo G, Ezzelarab M, et al. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am J Transplant. 2010;10:763–772. doi: 10.1111/j.1600-6143.2010.03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 66.Vreman HJ, Wong RJ, Kadotani T, Stevenson DK. Determination of carbon monoxide (CO) in rodent tissue: effect of heme administration and environmental CO exposure. Anal Biochem. 2005;341:280–289. doi: 10.1016/j.ab.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 68.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 69.Sandouka A, Fuller BJ, Mann BE, Green CJ, Foresti R, Motterlini R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006;69:239–247. doi: 10.1038/sj.ki.5000016. [DOI] [PubMed] [Google Scholar]
- 70.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Pizarro MD, Rodriguez JV, Mamprin ME, Fuller BJ, Mann BE, Motterlini R, Guibert EE. Protective effects of a carbon monoxide-releasing molecule (CORM-3) during hepatic cold preservation. Cryobiology. 2009;58:248–255. doi: 10.1016/j.cryobiol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 73.Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85:576–581. doi: 10.1097/TP.0b013e318160516a. [DOI] [PubMed] [Google Scholar]
- 74.Hosgood SA, Bagul A, Kaushik M, Rimoldi J, Gadepalli RS, Nicholson ML. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg. 2008;95:1060–1067. doi: 10.1002/bjs.6174. [DOI] [PubMed] [Google Scholar]
- 75.Pratschke J, Weiss S, Neuhaus P, Pascher A. Review of nonimmunological causes for deteriorated graft function and graft loss after transplantation. Transpl Int. 2008;21:512–522. doi: 10.1111/j.1432-2277.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 76.Szwarc I, Garrigue V, Delmas S, Deleuze S, Chong G, Mourad G. [Delayed graft function: a frequent but still unsolved problem in renal transplantation] Nephrol Ther. 2005;1:325–334. doi: 10.1016/j.nephro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 77.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279–3288. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 80.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, et al. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140:179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, et al. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci U S A. 2006;103:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turino GM. Effect of carbon monoxide on the cardiorespiratory system. Carbon monoxide toxicity: physiology and biochemistry. Circulation. 1981;63:253A–259A. [PubMed] [Google Scholar]
- 83.Koerner MM, Tenderich G, Minami K, Morshuis M, Mirow N, Arusoglu L, Gromzik H, et al. Extended donor criteria: use of cardiac allografts after carbon monoxide poisoning. Transplantation. 1997;63:1358–1360. doi: 10.1097/00007890-199705150-00027. [DOI] [PubMed] [Google Scholar]
- 84.Hantson P, Vekemans MC, Squifflet JP, Mahieu P. Outcome following organ removal from poisoned donors: experience with 12 cases and a review of the literature. Transpl Int. 1995;8:185–189. doi: 10.1007/BF00336535. [DOI] [PubMed] [Google Scholar]
- 85.Shennib H, Adoumie R, Fraser R. Successful transplantation of a lung allograft from a carbon monoxide-poisoning victim. J Heart Lung Transplant. 1992;11:68–71. [PubMed] [Google Scholar]
- 86.Karwande SV, Hopfenbeck JA, Renlund DG, Burton NA, Gay WA., Jr. An avoidable pitfall in donor selection for heart transplantation. Utah Heart Transplant Program. J Heart Transplant. 1989;8:422–424. [PubMed] [Google Scholar]
- 87.Martin-Suarez S, Mikus E, Pilato E, Bacchini M, Savini C, Grigioni F, Coccolo F, et al. Cardiac transplantation from a carbon monoxide intoxicated donor. Transplant Proc. 2008;40:1563–1565. doi: 10.1016/j.transproceed.2008.03.155. [DOI] [PubMed] [Google Scholar]
- 88.Sezgin A, Akay TH, Ozkan S, Gultekin B. Successful cardiac transplantation from donor with carbon monoxide intoxication: a case report. Transplant Proc. 2008;40:324–325. doi: 10.1016/j.transproceed.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 89.Bojakowski K, Gaciong Z, Grochowiecki T, Szmidt J. Carbon monoxide may reduce ischemia reperfusion injury: a case report of complicated kidney transplantation from a carbon monoxide poisoned donor. Transplant Proc. 2007;39:2928–2929. doi: 10.1016/j.transproceed.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 90.Rodrigus IE, Conraads V, Amsel BJ, Moulijn AC. Primary cardiac allograft failure after donor carbon monoxide poisoning treated with biventricular assist device. J Heart Lung Transplant. 2001;20:1345–1348. doi: 10.1016/s1053-2498(01)00331-x. [DOI] [PubMed] [Google Scholar]
- 91.Luckraz H, Tsui SS, Parameshwar J, Wallwork J, Large SR. Improved outcome with organs from carbon monoxide poisoned donors for intrathoracic transplantation. Ann Thorac Surg. 2001;72:709–713. doi: 10.1016/s0003-4975(01)02808-9. [DOI] [PubMed] [Google Scholar]
- 92.Kruuv J, Glofcheski D, Cheng KH, Campbell SD, Al-Qysi HM, Nolan WT, Lepock JR. Factors influencing survival and growth of mammalian cells exposed to hypothermia. I. Effects of temperature and membrane lipid perturbers. J Cell Physiol. 1983;115:179–185. doi: 10.1002/jcp.1041150212. [DOI] [PubMed] [Google Scholar]
- 93.Rauen U, Reuters I, Fuchs A, de Groot H. Oxygen-free radical-mediated injury to cultured rat hepatocytes during cold incubation in preservation solutions. Hepatology. 1997;26:351–357. doi: 10.1002/hep.510260215. [DOI] [PubMed] [Google Scholar]
- 94.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–H1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 95.Salahudeen AK, Huang H, Joshi M, Moore NA, Jenkins JK. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am J Transplant. 2003;3:273–280. doi: 10.1034/j.1600-6143.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 96.Kerkweg U, Li T, de Groot H, Rauen U. Cold-induced apoptosis of rat liver cells in University of Wisconsin solution: the central role of chelatable iron. Hepatology. 2002;35:560–567. doi: 10.1053/jhep.2002.31869. [DOI] [PubMed] [Google Scholar]
- 97.Doeppner TR, Grune T, de Groot H, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: involvement of the proteasome. Transplantation. 2003;75:1946–1953. doi: 10.1097/01.TP.0000065291.02855.6A. [DOI] [PubMed] [Google Scholar]
- 98.Tian T, Lindell SL, Henderson SC, Mangino MJ. Protective effects of ezrin on cold storage preservation injury in the pig kidney proximal tubular epithelial cell line (LLC-PK1) Transplantation. 2009;87:1488–1496. doi: 10.1097/TP.0b013e3181a43f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant. 2004;4:1246–1254. doi: 10.1111/j.1600-6143.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 100.Quadri SM, Segall L, de Perrot M, Han B, Edwards V, Jones N, Waddell TK, et al. Caspase inhibition improves ischemia-reperfusion injury after lung transplantation. Am J Transplant. 2005;5:292–299. doi: 10.1111/j.1600-6143.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 101.Mueller TH, Kienle K, Beham A, Geissler EK, Jauch KW, Rentsch M. Caspase 3 inhibition improves survival and reduces early graft injury after ischemia and reperfusion in rat liver transplantation. Transplantation. 2004;78:1267–1273. doi: 10.1097/01.tp.0000141095.06273.10. [DOI] [PubMed] [Google Scholar]
- 102.Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol. 1999;10:2006–2013. doi: 10.1681/ASN.V1092006. [DOI] [PubMed] [Google Scholar]
- 103.Schwarz C, Hauser P, Steininger R, Regele H, Heinze G, Mayer G, Oberbauer R. Failure of BCL-2 up-regulation in proximal tubular epithelial cells of donor kidney biopsy specimens is associated with apoptosis and delayed graft function. Lab Invest. 2002;82:941–948. doi: 10.1097/01.lab.0000021174.66841.4c. [DOI] [PubMed] [Google Scholar]
- 104.Suematsu M, Wakabayashi Y, Ishimura Y. Gaseous monoxides: a new class of microvascular regulator in the liver. Cardiovasc Res. 1996;32:679–686. [PubMed] [Google Scholar]
- 105.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stanford SJ, Walters MJ, Mitchell JA. Carbon monoxide inhibits endothelin-1 release by human pulmonary artery smooth muscle cells. Eur J Pharmacol. 2004;486:349–352. doi: 10.1016/j.ejphar.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 107.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 109.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, et al. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163:1587–1598. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, et al. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 112.Mansouri A, Perry CA. Alteration of platelet aggregation by cigarette smoke and carbon monoxide. Thromb Haemost. 1982;48:286–288. [PubMed] [Google Scholar]
- 113.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 114.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 115.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- 116.Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 119.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, et al. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 121.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 122.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 123.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 124.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, et al. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- 125.Cepinskas G, Katada K, Bihari A, Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G184–G191. doi: 10.1152/ajpgi.00348.2007. [DOI] [PubMed] [Google Scholar]
- 126.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, et al. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19:2045–2047. doi: 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- 127.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 129.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 131.Goebel U, Siepe M, Mecklenburg A, Stein P, Roesslein M, Schwer CI, Schmidt R, et al. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology. 2008;108:1025–1036. doi: 10.1097/ALN.0b013e3181733115. [DOI] [PubMed] [Google Scholar]
- 132.Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, et al. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 134.De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347–356. doi: 10.1136/gut.2008.155481. [DOI] [PubMed] [Google Scholar]
- 135.Sass G, Seyfried S, Parreira Soares M, Yamashita K, Kaczmarek E, Neuhuber WL, Tiegs G. Cooperative effect of biliverdin and carbon monoxide on survival of mice in immune-mediated liver injury. Hepatology. 2004;40:1128–1135. doi: 10.1002/hep.20450. [DOI] [PubMed] [Google Scholar]
- 136.Minamoto K, Harada H, Lama VN, Fedarau MA, Pinsky DJ. Reciprocal regulation of airway rejection by the inducible gas-forming enzymes heme oxygenase and nitric oxide synthase. J Exp Med. 2005;202:283–294. doi: 10.1084/jem.20050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002;27:739–745. doi: 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- 138.Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, Geller DA, et al. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236–G244. doi: 10.1152/ajpgi.00144.2007. [DOI] [PubMed] [Google Scholar]
- 139.Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, Shimizu H, et al. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279–2290. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 140.Nakao A, Moore BA, Murase N, Liu F, Zuckerbraun BS, Bach FH, Choi AM, et al. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278–1285. doi: 10.1136/gut.52.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10:2421–2430. doi: 10.1111/j.1600-6143.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- 142.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 143.Sethi JM, Otterbein LE, Choi AM. Differential modulation by exogenous carbon monoxide of TNF-alpha stimulated mitogen-activated protein kinases in rat pulmonary artery endothelial cells. Antioxid Redox Signal. 2002;4:241–248. doi: 10.1089/152308602753666299. [DOI] [PubMed] [Google Scholar]
- 144.Desmard M, Davidge KS, Bouvet O, Morin D, Roux D, Foresti R, Ricard JD, et al. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 2009;23:1023–1031. doi: 10.1096/fj.08-122804. [DOI] [PubMed] [Google Scholar]
- 145.Ikeda T, Yanaga K, Kishikawa K, Kakizoe S, Shimada M, Sugimachi K. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992;16:454–461. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- 146.Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, et al. Carbon monoxide protects against cardiac ischemia--reperfusion injury in vivo via MAPK and Akt--eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 147.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005;38:127–134. doi: 10.1016/j.yjmcc.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clark JE, Kottam A, Motterlini R, Marber MS. Measuring left ventricular function in the normal, infarcted and CORM-3-preconditioned mouse heart using complex admittance-derived pressure volume loops. J Pharmacol Toxicol Methods. 2009;59:94–99. doi: 10.1016/j.vascn.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 150.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 151.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d'Avila JC, Esterbauer H, Chin BY, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 152.Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 154.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Volti G, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, et al. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal. 2005;7:704–710. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 156.Choi YK, Kim CK, Lee H, Jeoung D, Ha KS, Kwon YG, Kim KW, et al. Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein. J Biol Chem. 2010;285:32116–32125. doi: 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 158.Lakkisto P, Kyto V, Forsten H, Siren JM, Segersvard H, Voipio-Pulkki LM, Laine M, et al. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 159.Song R, Mahidhara RS, Liu F, Ning W, Otterbein LE, Choi AM. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2002;27:603–610. doi: 10.1165/rcmb.4851. [DOI] [PubMed] [Google Scholar]
- 160.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, et al. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol. 2005;166:27–37. doi: 10.1016/S0002-9440(10)62229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang L, Lee JY, Kwak JH, He Y, Kim SI, Choi ME. Protective effects of low-dose carbon monoxide against renal fibrosis induced by unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2008;294:F508–F517. doi: 10.1152/ajprenal.00306.2007. [DOI] [PubMed] [Google Scholar]
- 162.Musameh MD, Fuller BJ, Mann BE, Green CJ, Motterlini R. Positive inotropic effects of carbon monoxide-releasing molecules (CO-RMs) in the isolated perfused rat heart. Br J Pharmacol. 2006;149:1104–1112. doi: 10.1038/sj.bjp.0706939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229–235. doi: 10.1016/j.surg.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 164.Wei Y, Chen P, de Bruyn M, Zhang W, Bremer E, Helfrich W. Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats. BMC Gastroenterol. 2010;10:42. doi: 10.1186/1471-230X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, Winslow RM. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol. 2008;154:1649–1661. doi: 10.1038/bjp.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Varadi J, Lekli I, Juhasz B, Bacskay I, Szabo G, Gesztelyi R, Szendrei L, et al. Beneficial effects of carbon monoxide-releasing molecules on post-ischemic myocardial recovery. Life Sci. 2007;80:1619–1626. doi: 10.1016/j.lfs.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 167.Ahlstrom K, Biber B, Aberg AM, Abrahamsson P, Johansson G, Ronquist G, Waldenstrom A, et al. Exogenous carbon monoxide does not affect cell membrane energy availability assessed by sarcolemmal calcium fluxes during myocardial ischaemia-reperfusion in the pig. Eur J Anaesthesiol. 2010 doi: 10.1097/EJA.0b013e32833eab96. [DOI] [PubMed] [Google Scholar]
- 168.Sahara H, Shimizu A, Setoyama K, Okumi M, Oku M, Samelson-Jones E, Yamada K. Carbon monoxide reduces pulmonary ischemia-reperfusion injury in miniature swine. J Thorac Cardiovasc Surg. 2010;139:1594–1601. doi: 10.1016/j.jtcvs.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 169.Bentley MJ, Mullen JC, Lopushinsky SR, Modry DL. Successful cardiac transplantation with methanol or carbon monoxide-poisoned donors. Ann Thorac Surg. 2001;71:1194–1197. doi: 10.1016/s0003-4975(01)02402-x. [DOI] [PubMed] [Google Scholar]
- 170.Verran D, Chui A, Painter D, Shun A, Dorney S, McCaughan G, Sheil R. Use of liver allografts from carbon monoxide poisoned cadaveric donors. Transplantation. 1996;62:1514–1515. doi: 10.1097/00007890-199611270-00024. [DOI] [PubMed] [Google Scholar]
- 171.Roberts JR, Bain M, Klachko MN, Seigel EG, Wason S. Successful heart transplantation from a victim of carbon monoxide poisoning. Ann Emerg Med. 1995;26:652–655. doi: 10.1016/s0196-0644(95)70021-8. [DOI] [PubMed] [Google Scholar]
- 172.Iberer F, Konigsrainer A, Wasler A, Petutschnigg B, Auer T, Tscheliessnigg K. Cardiac allograft harvesting after carbon monoxide poisoning. Report of a successful orthotopic heart transplantation. J Heart Lung Transplant. 1993;12:499–500. [PubMed] [Google Scholar]
- 173.Hebert MJ, Boucher A, Beaucage G, Girard R, Dandavino R. Transplantation of kidneys from a donor with carbon monoxide poisoning. N Engl J Med. 1992;326:1571. doi: 10.1056/nejm199206043262315. [DOI] [PubMed] [Google Scholar]
- 174.Smith JA, Bergin PJ, Williams TJ, Esmore DS. Successful heart transplantation with cardiac allografts exposed to carbon monoxide poisoning. J Heart Lung Transplant. 1992;11:698–700. [PubMed] [Google Scholar]