Abstract
Cochlear implants provide hearing by electrically stimulating the auditory nerve. Implant function can be hindered by device design variables, including electrode size and electrode-to-nerve distance, and cochlear environment variables, including the degeneration of the auditory nerve following hair cell loss. We have developed a dual component cochlear implant coating to improve both the electrical function of the implant and the biological stability of the inner ear, thereby facilitating the long-term perception of sound through a cochlear implant. This coating is a combination of an arginine-glycine-aspartic acid (RGD)-functionalized alginate hydrogel and the conducting polymer poly(3, 4-ethylenedioxythiophene) (PEDOT). Both in vitro and in vivo assays on the effects of these electrode coatings demonstrated improvements in device performance. We found that the coating reduced electrode impedance, improved charge delivery, and locally released significant levels of a trophic factor into cochlear fluids. This coating is non-cytotoxic, clinically relevant, and has the potential to significantly improve the cochlear implant user’s experience.
Keywords: alginate, drug delivery, electroactive polymer, growth factor, hydrogel, neural prosthesis
1. Introduction
In the undamaged ear, sound waves are transduced by cochlear hair cells, which initiate neural impulses in the auditory nerve. In the damaged cochlea, where hair cells are missing or dysfunctional, acoustic hearing is severely compromised. The cochlear implant (CI) provides electric hearing to patients with certain types of severe hearing loss induced by hair cell loss. CIs bypass the function of lost hair cells by stimulating the auditory nerve and initiate neural impulses [1]. The nerve then sends signal-related information to higher auditory areas where it is perceived as sound. The CI has provided or restored hearing to over 200,000 patients worldwide; however, the sound perceived by CI users is not yet as complete as acoustic sound. This altered sound perception is caused by limitations in both the construction of the stimulating electrode array and the stability of the surviving cochlear tissue, which often degenerates following hair cell loss [1–8].
The local environment of the CI presents a challenge for biomedical technologies. Many implantable prostheses, including cardiac pacemakers and deep brain stimulators, are placed in direct contact with the tissue or neural structures that are stimulated. CIs, however, are placed into the scala tympani, a fluid-filled space in the cochlea, and can be located up to 2 mm from the target neurons [9]. The stimuli from the implant must pass through fluid, bone, and soft tissue before reaching the cells of the auditory nerve (spiral ganglion neurons, SGNs). These obstacles increase the electrode-neuron impedance, degrade signal fidelity, and increase the voltage required to excite the neural structures to threshold and initiate action potentials [10]. These obstacles can lead to undesirable adverse effects that include hydrolysis, tissue damage, and non-specific neural stimulation. A more focused current delivery system could provide a significant benefit to cochlear implant stimulation in terms of both tissue survival and implant performance.
One way to focus the current and reduce non-specific stimulation is to increase the number of electrodes on the implant [4]. However, an increased density of electrodes is useful only if these electrodes can be used to stimulate discrete regions of the nerve, i.e., function as independent channels. Discrete neural stimulation could be achieved by either decreasing the electrode-to-target tissue impedance or maintaining neural survival; a combination of these aspects could be optimal. We have therefore designed a long-term in vivo CI coating designed to address these factors simultaneously. This coating is composed of the conducting polymer poly(3, 4-ethylenedioxythiophene) (PEDOT), to improve the electrode-fluid charge transfer characteristics, and an arginine-glycine-aspartic acid (RGD)-functionalized alginate hydrogel, to deliver trophic drugs and attenuate the degeneration of the cochlea and auditory nerve.
Inherently conducting polymers, including polyaniline, polypyrrole, and PEDOT, have been studied in neural probe and prosthetic research as a means of improving the electrode-tissue interface [11, 12]. These organic materials rely on a conjugated polymer backbone to provide both electrical and ionic conductivity. The application of conducting polymers to both stimulating and recording electrodes has been shown to improve the signal-to-noise ratio, decrease impedances, reduce scar tissue formation, and improve charge transport [13, 14]. For cochlear implants, conducting polymers offer the ability to manipulate the environment immediately surrounding the implant in ways that could improve electric hearing [15].
Hydrogels have been used in both clinical and basic research to aid in tissue and organ regeneration and engineering [16, 17]. Hydrogels are water-swollen networks of lightly cross-linked polymer chains, which allow the hydrogel to reversibly dehydrate and re-swell depending on the environment, thus enabling drug uptake and release. In the cochlea, hydrogels have been effectively used to deliver neurotrophic factors when placed on the round window [18, 19]. Maximal effect on cochlear tissues, however, could be achieved if a drug-loaded hydrogel was placed not near but within the scala tympani. In addition, an RGD-functionalized hydrogel can act as an artificial extracellular matrix by providing scaffolds to support neuronal and tissue growth [20, 21]; this property could aid neural regeneration in a degenerated cochlea. The CI provides a convenient method to introduce a drug-loaded hydrogel directly into the cochlea.
We analyzed the effects of the individual and combined elements of a PEDOT/hydrogel coating on cochlear implant stimulation under several paradigms. We tested the functional effects of the PEDOT using in vitro electrochemical impedance spectroscopy. The drug-delivery capability of the hydrogel was assayed using 2 methods of incorporating brain-derived neurotrophic factor (BDNF) into the hydrogel followed by in vivo BDNF release measurements. BDNF is important for both the development and maintenance of the cochlea and has been shown in numerous studies to promote auditory nerve survival following hair cell loss [22–27]. Finally, a chronic implantation tested the long-term stability and biocompatibility of this specialized dual-component coating using in vivo electrochemical impedance spectroscopy and histological analysis of the cochlea.
2. Materials and Methods
2.1. Implant fabrication and coating
Cochlear implants were constructed in-house using Teflon-coated 75 μm diameter platinum/iridium alloy (Pt/Ir, 90 %/10 %) wire (A-M Systems, Sequim, WA, USA). The wire was melted using a natural gas/oxygen flame to produce a ball electrode with a diameter of 300–450 μm (Figure 1). For only the chronic implantations, a 60 mm lead wire from the electrode was threaded through silicone tubing and connected to a base pedestal that was attached to the animal’s skull. An additional electrode was added to the chronic implant, as previously described [28]; briefly, a 2 mm piece of polyimide tubing was placed on the lead wire of the ball electrode, and another wire was wrapped around the polyimide to create a helix-shaped electrode that was 300 μm long. Silastic BioMedical Grade Elastomer (Dow Corning, Midland, MI, USA) was used to seal exposed wire and junctions in all in vitro and in vivo implants.
Figure 1. Custom-built cochlear implants.
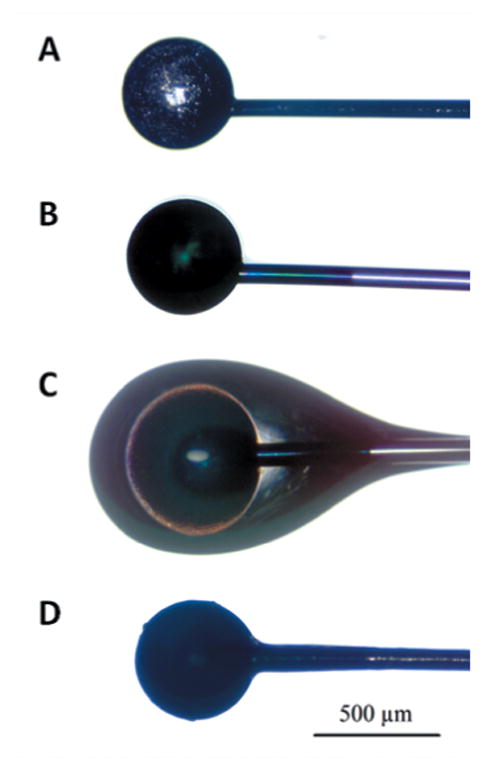
Implants were made from Teflon-coated platinum-iridium (Pt/Ir) wire. (A) Bare Pt/Ir cochlear electrode. (B) PEDOT-coated cochlear electrode. (C) RGD-alginate hydrogel and PEDOT-coated cochlear electrode. (D) Dehydrated RGD-alginate hydrogel and PEDOT-coated cochlear electrode.
Electrodes were electrochemically coated with poly(3, 4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT-PSS), as previously described [13] (Figure 1B). PEDOT-PSS was deposited from a solution containing 0.1 % (w/v) ethylenedioxythiophene (EDOT; H.C. Starck, Newton, MA, USA) and 0.2 % (w/v) PSS (Acros Organics, Waltham, MA, USA) in deionized water. The cochlear electrode was immersed in the monomer solution and served as the working electrode, on which the monomer oxidized to form the conducting polymer. A 6 × 6 mm platinum foil served as the counter electrode. Galvanostatic currents of 1–5 μA were applied using an AutoLab PGStat12 potentiostat/galvanostat (Metrohm Autolab, Utrecht, The Netherlands). To equalize the amount of electrical stimulation that each implant received, non-coated implants were activated using equal levels of charge while in deionized water.
For hydrogel application, the implant, up to approximately 3 mm beyond the ball electrode, was coated with an alginate hydrogel that was covalently modified with the RGD adhesion ligand (Figure 1C). This hydrogel was generously provided by Dr. David Mooney at Harvard University [29–31]. The implant was dip-coated in a solution of 1 % (w/v) RGD-alginate in phosphate buffered saline (PBS) and then dipped into a solution of 2 % (w/v) CaCl2 in PBS [32]. Implants were dipped in the solutions repeatedly to build up a coating on the ball electrode that was approximately 100 μm thick and then allowed to air dry. Once the gel was dehydrated, the coating was very thin (Figure 1D) and did not significantly impact the implantation procedure.
For the dual-component coating, hydrogel application followed the PEDOT application. The coated implants were sterilized with 70 % ethanol for in vitro testing and in vivo BDNF release measurement and by ethylene oxide gas for chronic implantation.
2.2. In vitro electrochemical impedance spectroscopy and cyclic voltammetry
Electrochemical impedance spectroscopy (EIS) was performed on both PEDOT/hydrogel-coated and bare cochlear electrodes in saline after coating and prior to implantation. A 3-electrode setup was used in which the cochlear electrode was the working electrode, a 6 × 6 mm platinum foil was the counter electrode and a saturated calomel electrode was the reference electrode. The electrolyte used was room temperature PBS (HyClone Media, pH = 7.4). The impedance was measured at frequencies from 1 to 100,000 Hz upon application of a 5 mV root mean square sinusoid wave between the working and counter electrodes using a potentiostat with a frequency response analysis module (AutoLab PGStat 12, Metrohm Autolab, Utrecht, The Netherlands). Cyclic voltammetry determined the charge storage capacity at a scan rate of 100 mV/sec over a voltage range of -0.6 to +0.8 V, which was within the window of safe voltages that avoid hydrolysis or potentially harmful bubble formation.
2.3. Scanning electron microscopy
Scanning electron microscopy was performed using a FEI Nova 200 Nanolab Dualbeam SEM/FIB (University of Michigan Electron Microbeam Analysis Laboratory). Samples were sputtered with approximately 1 nm of gold prior to scanning electron imaging.
2.4. Subjects
Male pigmented guinea pigs (Elm Hill, Chelmsford, MA, USA) were used in this study. Animals were placed in 1 of 2 groups. The first group (n = 17) received electrically non-functional hydrogel-coated implants and had cochlear fluids sampled 1 or 2 weeks post-implantation to measure BDNF release from the coating. The second group (n = 4) received ototoxic treatments to destroy hair cells, received electrically functional dual-component coated implants, and underwent regular impedance spectroscopy during a chronic implantation. Acoustically-evoked pure tone auditory brainstem responses were recorded in this group both prior to the deafening procedure, to ensure normal hearing levels, and after the deafening procedure, to verify hearing loss. Animal weights ranged from 290–690 g at the time of implantation. This study was performed in accordance with National Institutes of Health Guidelines and the University Committee on the Use and Care of Animals at the University of Michigan approved the experimental protocols. Veterinary care and animal husbandry were provided by the Unit for Laboratory Animal Medicine, in facilities certified by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
2.5. Surgery
For cochlear implantation in the first group of animals that received electrically non-functional implants, guinea pigs were anesthetized with a ketamine (40 mg/kg) and xylazine (10 mg/kg) mix (IP) and given buprenorphine (0.05 mg/kg, SQ) as an analgesic and chloramphenicol (30 mg/kg, SQ) as an antibiotic. Lidocaine with epinephrine was used as a local anesthetic. A post-auricular incision was made and the underlying tissue was cleared to expose the bulla. Using the tip of a scalpel blade, a hole was made in the bulla to expose the cochlea; a small cochleostomy was then drilled adjacent to the lip of the round window of the cochlea to provide access to the scala tympani. The cochlear implant was inserted approximately 3–4 mm into the scala tympani and the cochleostomy was plugged with fascia tissue around the implant. The implant was secured by holding it to the edge of the hole in the bulla with fascia tissue and cyanoacrylate. Carboxylate cement (Durelon) was applied to close this bulla hole and the implant wire was cut where it exited this cement. The skin flap was sutured in 2 layers, and the animal was allowed to recover.
In the second group of animals that received electrically functional implants, animals were systemically deafened using a combination of kanamycin (400 mg/kg, SQ) and ethacrynic acid (40 mg/kg, IV, 2 hr following kanamycin) 1 week prior to implantation. Animals were given a ketamine (40 mg/kg) and xylazine (10 mg/kg) mix (IM) for general anesthesia and lidocaine was used as a local anesthetic for skin incisions. For cochlear implantation with electrically functional implants, an incision was made down the midline of the head, and muscle and connective tissue were gently pushed apart to reveal the skull. Three screws were placed in the skull and formed a triangle around bregma. These screws held the head of an inverted restraining bolt, which was used as a ground electrode during EIS. The cochlea was exposed as described above. Implants were inserted approximately 2–3 mm into the basal turn of the cochlea. The insulated lead wire from the implant was tunneled under the skin and secured to a base pedestal on the animal’s skull. Durelon was applied to the bulla hole to seal the middle ear space and secure the position of the implant. A ground electrode (500 μm ball electrode, Pt/Ir) was placed in the post-auricular muscle. The skin incision was sutured in 2 layers, the exposed skull and hardware was sealed using dental acrylic, and the animal was given warm subcutaneous fluids and allowed to recover.
2.6. Growth factor release measurement
We tested 2 methods of incorporating BDNF into the hydrogel to assess drug loading and release. In the first method, BDNF was loaded into poly(lactic-co-glycolic acid) (PLGA) particles, a degradable polymer that is FDA-approved for several clinical uses [33]. PLGA particles with BDNF were prepared by the single oil-in-water emulsion/solvent evaporation method. A solution of 800 mg PLGA in 15 ml of dichloromethane was mixed with a solution of 10 μg human recombinant BDNF (Millipore, Billerica, MA, USA) in 15 ml ethanol. This mixture was then added drop-by-drop to a solution of 5 % (w/v) polyvinyl alcohol in deionized water. This mixture was sonicated for 10 min to produce a homogenous emulsion. The solvent was evaporated over 12 hr while the emulsion was slowly stirred. The particles were separated from unreacted reagents through ultracentrifugation (35,000 rpm) then collected and loaded into the alginate hydrogel at 10 % (w/v). PLGA-BDNF hydrogel-coated implants were then sterilized using 70 % ethanol.
In the second incorporation method, human recombinant BDNF, as above, was loaded directly into the hydrogel by soaking the sterilized hydrogel-coated implant in a BDNF solution. The electrode end of the implant was immersed in 1–5 μl of 500 ng/ml BDNF in PBS and then allowed to dry prior to implantation.
At 1 or 2 weeks post-implantation, animals were anesthetized, as above, and decapitated. Both temporal bones were removed and opened to expose the cochlea. An opening was made in the scala tympani of the basal turn, and microcapillary tubes (1 μl Microcap, Drummond Scientific Co, Broomall, PA, USA) were used to extract 2 μl of fluid from the cochlea (in 1 case, only 1 μl of fluid was collected). This fluid was transferred to a gasket-sealed, screw-cap microcentrifuge tube for storage. Due to the potential extension of the basal turn cochleostomy into the scala media and possible breeching of the bony roof of scala tympani by the microcapillary tubes, the fluid collected was most likely composed of both perilymph and endolymph. The fluid samples were stored at −80 °C until the time of assay. The BDNF concentration in the cochlear fluids was quantified using a BDNF-specific enzyme-linked immuno-sorbent assay (ELISA) kit (Millipore, Billerica, MA, USA) following kit instructions, and results were read with a plate reader (SpectraMax 340, Molecular Devices, Inc., Sunnyvale, CA, USA). For the ELISA, the samples were diluted with 100 μl of the standard/sample diluent, except for the 1 μl sample, which was diluted with 101 μl of the diluent.
2.7. In vivo electrochemical impedance spectroscopy
The group of 4 chronically implanted animals received PEDOT/hydrogel/BDNF-coated electrically functional implants. BDNF was incorporated via the direct soaking method immediately prior to implantation. EIS was performed in a similar manner as described above for in vitro EIS, using the same potentiostat/galvanostat with a frequency response analysis module. Once implanted, EIS was performed using a 2-electrode setup. The cochlear implant electrode was connected to the working electrode, and the stainless steel bolt mounted on the animal's skull at bregma was connected to the counter and reference electrodes. Impedances were measured at 35 frequencies from 10 to 10,000 Hz upon application of a 2.5 μA root mean square sinusoid wave. In preliminary in vivo studies, the impedance of the cochlear electrodes was the same with either 2.5 μA or 5 mV sinusoid waves.
In addition to the values obtained during EIS testing, the impedance values were also tested between EIS sessions to track changes over time and verify implant integrity. Values were obtained several times a month and were measured using a 1 μA root mean square, 1 kHz sinusoid wave from an in-house constructed impedance meter. The obtained values were statistically compared up to 80 days post-implantation. At this point, 1 animal from the bare implant group had impedance values that exceeded the limits of the meter and were deemed non-measurable, thus precluding further group analysis. All other animals were implanted for 6 months.
2.8. Spiral ganglion cell assessment
The 4 chronically implanted animals were deeply anesthetized and decapitated, and the temporal bones were harvested. Each cochlea was locally perfused with 4 % paraformaldehyde (PFA) and immersed in 4 % PFA overnight. Cochleae were rinsed in PBS and placed in a 3 % ethylenediaminetetraacetic acid (EDTA) solution to decalcify until sufficiently soft for sectioning. Once decalcified, the implant was removed from the cochlea, and each cochlea was embedded in JB-4 resin and sectioned in the mid-modiolar plane, which provided 6 measurable profiles of Rosenthal’s canal [34]. Sections were 3 μm thick, and every 3rd section was kept and stained with 1 % (w/v) toluidine blue in 1 % (w/v) sodium borate. Three slides from each cochlea were chosen by a random number generator for evaluation. The Rosenthal’s canal region of the basal turn in each of these 3 slides was evaluated using a SPOT Camera (Diagnostic Instruments, Inc., Sterling Heights, MI) for data acquisition and Image J software (National Institutes of Health) for data analysis. The outer edge of each region of Rosenthal’s canal was traced in Image J and the 2-dimensional area was calculated by the software. The number of SGNs within each Rosenthal’s canal region was counted, and a density value for each canal region was determined by dividing the number of cells per region by the area of that region.
3. Results
3.1. Electrode surface morphology
Electron microscopic analysis of PEDOT-coated electrodes (Figure 2) revealed that the surface of the coated electrodes had a rough, nodular texture with features on the order of 10–100 nm. Larger grains with dimensions of 10–50 μm were present on both the bare Pt/Ir electrodes and PEDOT-coated electrodes and are thought to correspond to the grain structure of the Pt/Ir formed during the heating and cooling associated with electrode manufacture.
Figure 2. Scanning electron micrograph of a PEDOT-coated cochlear electrode.
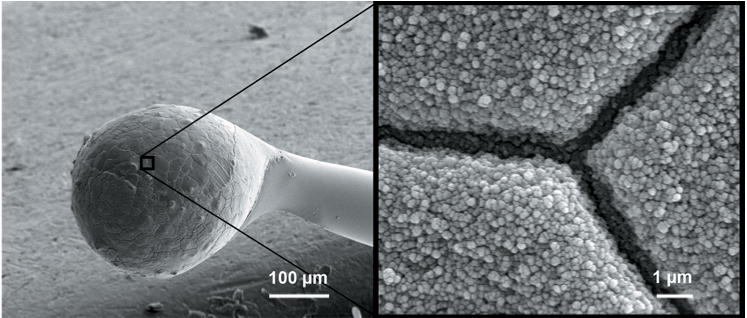
The image on the right shows a magnified view of the textured surface of PEDOT on the Pt/Ir cochlear electrode.
3.2. In vitro impedance, phase angle, and charge storage measurements
The PEDOT coating reduced electrode impedance and shifted the phase angle during in vitro EIS (Figure 3). When the thinnest layer of PEDOT (approximately 100 nm) was applied to the electrodes with 30 μC of charge, there was a decrease in electrode impedance (Figure 3A) at all frequencies measured (1–100,000 Hz) and a decrease in the phase angle (Figure 3B) above 100 Hz, when compared to the bare implant (0 μC deposition charge). These effects were a function of PEDOT coating thickness; increased deposition charge, with a range of 30 to 930 μC, lead to decreased impedances at frequencies below 1 kHz and decreased phase angle at frequencies above 10 Hz.
Figure 3. In vitro electrochemical impedance spectroscopy.
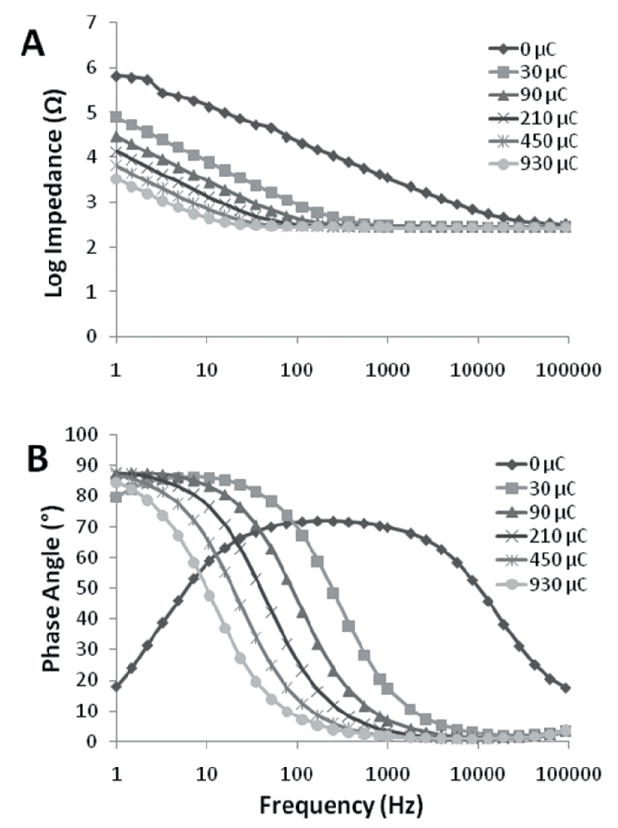
(A) Impedance values with PEDOT coatings of increasing thickness (given as deposition charge) on Pt/Ir cochlear electrodes. (B) Phase angle responses to impedance spectroscopy.
The phase angle of bare Pt/Ir cochlear electrodes was closer to 90 ° and predominantly non-faradaic, or capacitive, at frequencies of 10–10,000 Hz, but showed mixed faradaic and non-faradaic charge transfer at frequencies below 10 Hz and above 10,000 Hz (Figure 3B). The charge transfer became more faradaic with PEDOT application, which corresponds to electron transfer from the electrode to electrolyte and vice-versa. For safe stimulation with implanted electrodes, it is desirable to avoid irreversible faradaic reactions, which can include hydrolysis, large pH changes, and salt precipitation. During electrochemical testing and stimulation of cochlear electrodes, we avoided hydrolysis by restricting the voltages to the water window range and did not detect any deposition of salts on the electrodes. Thus, we conclude that the charge transfer reactions occurring with PEDOT-coated electrodes are predominantly of the reversible faradaic type.
The PEDOT coating also increased the charge storage capacity (Figure 4). With increasing PEDOT film deposition, the charge storage capacity rose from 20.4 μC on bare Pt/Ir (0 μC deposition charge) electrodes to 93.0 μC with 930 μC of deposition charge. This corresponds to a 356 % increase in the amount of charge that could be safely delivered from a PEDOT-coated implant while avoiding hydrolysis on the electrode.
Figure 4. In vitro cyclic voltammetry.
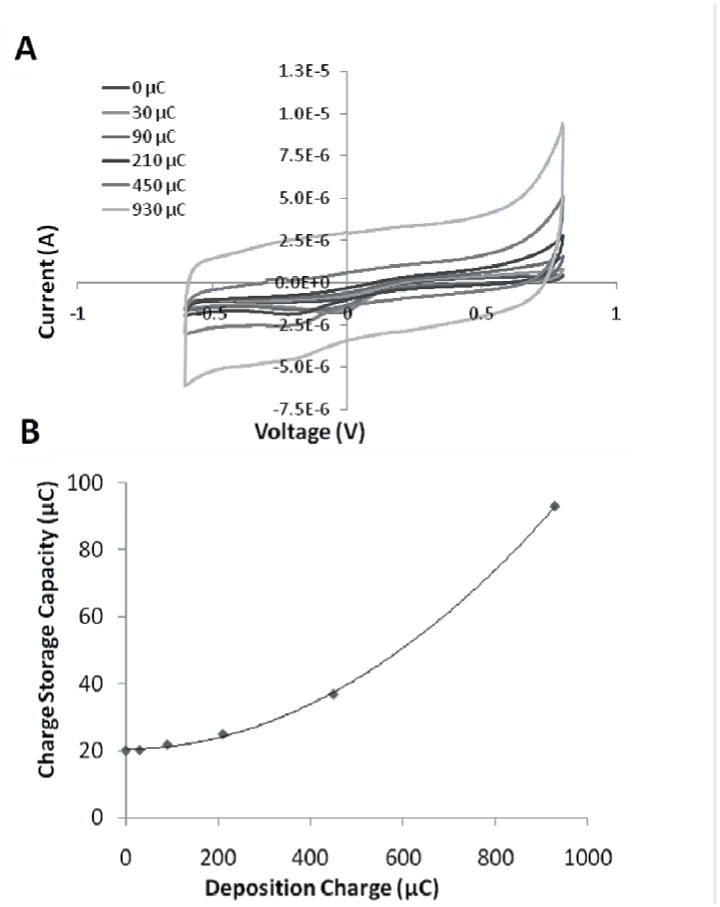
(A) Cyclic voltammetry with PEDOT coatings of increasing thickness (given as deposition charge) on Pt/Ir cochlear electrodes. (B) Charge storage capacity with PEDOT coatings of increasing thickness on Pt/Ir cochlear electrodes. Polynomial fit: CSC(x) = 8.34*10-5 x2 + 2.31*10-4x + 20.4. R2 = 0.99.
3.3. BDNF release into cochlear fluids
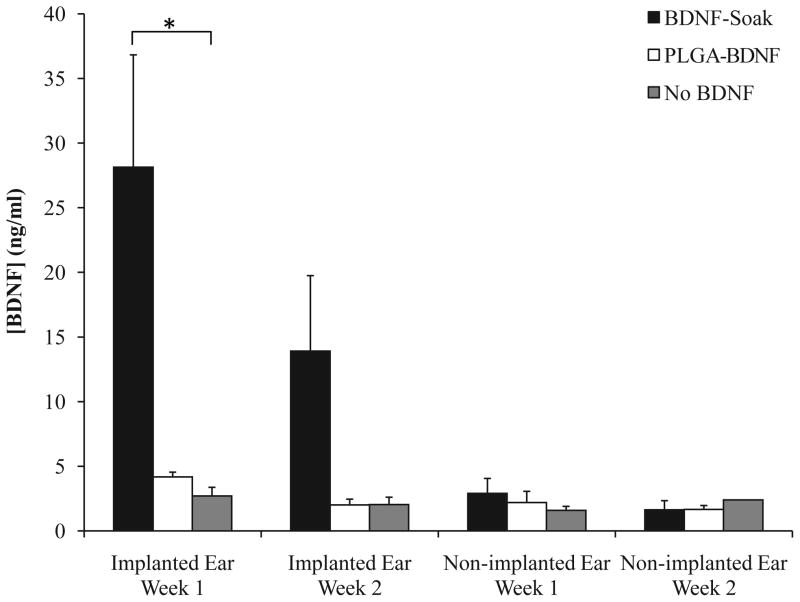
BDNF was released from the hydrogel coating into the cochlea at biologically significant levels, but the amount of release varied with the incorporation method (Figure 5). When BDNF was directly incorporated into the hydrogel via soaking (BDNF-Soak), the BDNF concentration in the cochlear fluids of the implanted ear was 28.14 ng/ml at 1 week post-implantation and 13.91 ng/ml at 2 weeks post-implantation. This was a statistically significant difference from the non-BDNF-loaded hydrogel, which had 2.7 ng/ml at 1 week and 2.04 ng/ml at 2 weeks (ANOVA, p < 0.05 at 1 week). When the BDNF was incorporated into the hydrogel using PLGA particles (PLGA-BDNF), a modest but not significant increase in BDNF concentration above control was seen at 1 week post-implantation (4.18 ng/ml). BDNF levels, however, dropped down to near baseline (2.01 ng/ml) at 2 weeks following PLGA-BDNF hydrogel implantation. There were no significant differences in the BDNF levels of the contralateral, non-implanted ears between groups at either time point, indicating a spatial restriction of the BDNF that was released from the hydrogel and a minimal transfer of BDNF into the contralateral ears via cerebrospinal fluid.
Figure 5. BDNF release into cochlear fluids following implantation with coated implants.
ELISA analysis of the BDNF concentrations (ng/ml) in the fluids from implanted and non-implanted cochleae at 1 and 2 weeks post-implantation. There was a statistically higher BDNF concentration 1 week following implantation with a BDNF-soaked hydrogel implant coating (black bars) compared to both the non-BDNF-treated controls (gray bars) and the PLGA-BDNF implants (white bars) (ANOVA, * indicates p < 0.05). Error bars represent the standard error of the mean.
3.4. In vivo impedances
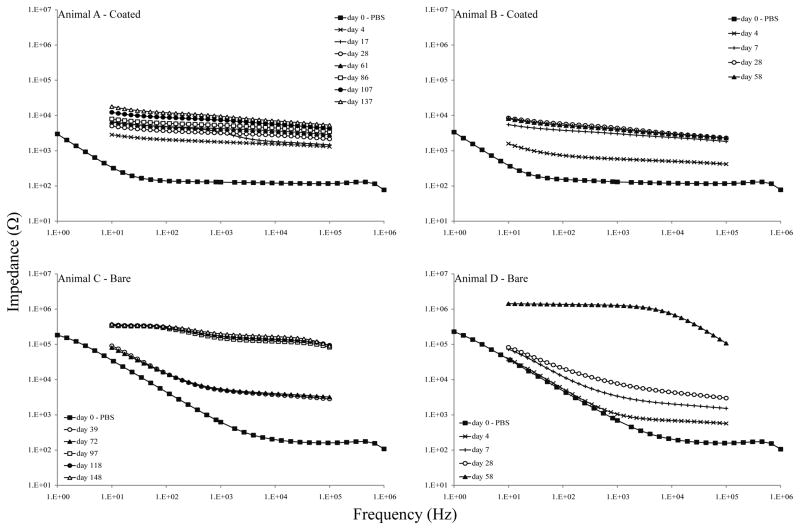
In vivo EIS data showed lower impedances with PEDOT/hydrogel/BDNF-coated implants (Figure 6, Animals A & B) than with bare implants (Figure 6, Animals C & D) throughout the experiment and over a range of frequencies; these data were similar to the in vitro data. Prior to implantation (day 0, tested in PBS), coated implants had lower impedances than bare implants across all frequencies. Following implantation, all implants exhibited stereotypical impedance increases [35, 36]. PEDOT/hydrogel-coated implants maintained consistently low impedance values throughout the chronic implantation, while bare implant impedances increased with time post-implantation.
Figure 6. In vivo electrochemical characterization of the PEDOT/hydrogel/BDNF coating.
Each graph represents a single animal; Animals A & B (top) received PEDOT/hydrogel/BDNF-soaked implants and Animals C & D (bottom) received bare implants. EIS data was collected prior to implantation (day 0) and at regular intervals over a 6-month period. At 81 days post-implantation, the impedances for Animal D exceeded the limitations of our system, becoming non-measurable, and no further data could be collected for this animal.
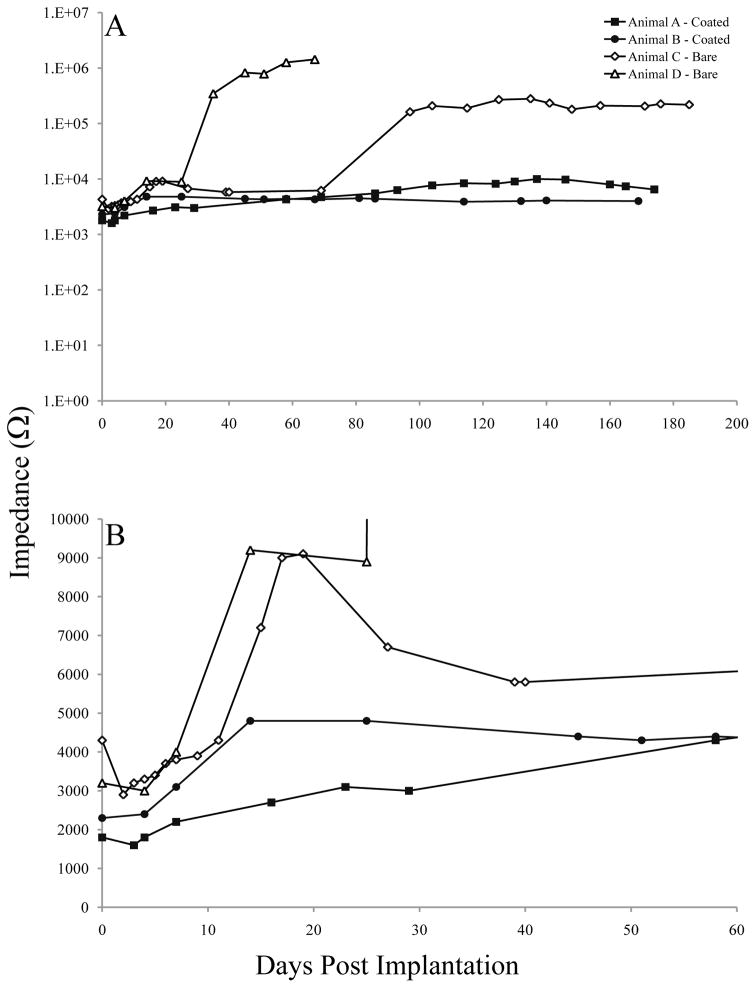
Impedance values at 1 kHz were collected at more frequent intervals than EIS testing and also demonstrate the stabilizing effects of the PEDOT/hydrogel coating over time (Figure 7). Prior to implantation, the average impedances at 1 kHz were 130 Ω for the coated group and 660 Ω for the bare group. Immediately after implantation, the average impedance at 1 kHz was 2,100 Ω for the coated implant group and 3,800 Ω for the bare group. After 1 month of implantation, average impedances were 3,805 Ω for the coated implant group and 6,390 Ω for the bare group. The differences between the PEDOT/hydrogel group and the bare implant group were statistically significant throughout the testing period (repeated measures ANOVA, p < 0.05). Changes in the impedance values over time were analyzed by linear regression of the impedance vs. time function. Slopes of this function (Ω/day) were 0.04 (Animal A, coated), 0.005 (Animal B, coated), 1.5 (Animal C, bare) and 22.1 (Animal D, bare). The slope of impedances changes over time for Animal B, with a PEDOT/hydrogel-coated implant, was not significantly different from zero (p = 0.23), while the slopes for the other animals were statistically different from zero. These data indicate that the impedance values for Animal B, with a PEDOT/hydrogel-coated implant, were stable over time and did not significantly increase or decrease during the 6 months following implantation.
Figure 7. In vivo impedances at 1 kHz over time.
Impedances for PEDOT/hydrogel/BDNF-soaked implants (Animals A & B, closed symbols) and bare implants (Animals C & D, open symbols) were measured at 1 kHz at more frequent intervals than the full EIS assessment. At 81 days post-implantation, the impedances for Animal D exceeded the limitations of our system, becoming non-measurable, and no further data could be collected for this animal. (A) Impedances at 1 kHz over the entire 6-month testing period. (B) Impedances for the first 60 days post-implantation.
3.5. Long-term auditory nerve survival
Cross-sectional ganglion cell density was calculated and compared between cochleae in the chronic implantation group. We did not find a difference in SGN survival between the coated and uncoated groups, indicating no detrimental effect of the PEDOT/hydrogel/BDNF coating on the auditory nerve. The neuronal density (expressed as the mean ± standard deviation) was 4.2 ± 2.8 neurons/10,000 μm2 for the PEDOT/hydrogel-coated group and 4.8 ± 1.5 neurons/10,000 μm2 for the uncoated group (Student’s t-test, p = 0.63).
4. Discussion
4.1. Improved cochlear implant function
In both the in vitro and in vivo EIS data, we found significant differences between PEDOT/hydrogel-coated implants and uncoated Pt/Ir implants. The application of PEDOT to the Pt/Ir electrodes increased the effective surface area of the electrodes without altering the device footprint or insertion procedure (Figure 2). This increase in the effective surface area led to lower electrode impedances and greater charge storage capacity at all tested frequencies from 1 to 100,000 Hz. This pattern was correlated to the amount of PEDOT deposited in the in vitro testing, where, in a stepwise fashion, larger deposition charge, and the subsequently higher levels of PEDOT coating, led to lower impedance values (Figure 3). At frequencies higher than 1,000 Hz, however, there was no change in impedances with increased levels of PEDOT deposition.
With chronic in vivo implantation, animals with PEDOT/hydrogel-coated cochlear implants showed significantly lower impedances throughout the 6-month testing period. EIS data demonstrated minimal increases over time over the full range of tested frequencies; impedances for the coated implants began lower, stayed lower, and had less variability over time than those for the bare implants. These are important considerations for the function of neural prostheses, as stability over time could improve CI user experience and reduce the need for replacement surgeries [37]. Impedance fluctuations in chronic implantations can lead to increased voltage requirements, which necessitate higher battery consumption, a major concern for the battery-operated CI. High battery consumption used for stimulation can take power from other components of the prosthesis, including the sound processing hardware, which in turn limits the software that can be used. Thus, the potential to reduce battery consumption and improve signal fidelity using this novel coating could allow for more complex processing paradigms and more naturalistic stimulation. The PEDOT/hydrogel coating may be of significance not only for cochlear implants but also for other chronically implanted devices, such as cardiac rhythm management and neuromodulation devices, whose electrical stimulation efficacy is often degraded with extended implantations.
4.2. Direct cochlear drug delivery
We found a significant increase in the concentration of BDNF within cochlear fluids at 1 week following implantation with a BDNF-soaked PEDOT/hydrogel-coated cochlear implant compared to coated implants without BDNF incorporation. The level of BDNF in the cochlear fluids was also higher in the BDNF-Soak group than in the non-BDNF group at 2 weeks post-implantation, although there was a decline from week 1. The values following the BDNF soak were 10 times higher than the baseline BDNF levels at 1 week post-implantation and 6 times higher than the baseline at 2 weeks post-implantation. This increase in available BDNF was most pronounced when the hydrogel itself was soaked in a BDNF solution and not when the BDNF was loaded into the hydrogel via PLGA particles. Although we did see an increase in the concentration of BDNF within the cochlea following implantation with PLGA-BDNF-loaded CIs, the effect was minimal and more transient than with the direct-soaking method. These data suggest that a larger amount of BDNF was immediately available from the BDNF-soaked hydrogel than could be contained in and/or released from the PLGA particles. Although these particles have been shown in other studies [38, 39] to be an effective drug-delivery vehicle, in the current application they were not as effective a method as the direct loading of BDNF into the hydrogel. This difference in efficacy between loading methods could be due to lower overall loading related to the additional polymer components of the PLGA or to BDNF entrapment within the slowly-degrading PLGA particles. These PLGA particles could be modified for use in future research assessing the time-controlled release of low levels of BDNF into the cochlea.
The decrease in the concentration of BDNF from week 1 to week 2 in the BDNF-Soak group suggests that the majority of the growth factor release from the hydrogel occurred soon after implantation. Drug release from hydrogel compounds typically occurs quickly, due to the porosity and high-water content of the hydrogel matrix [18]; this action may explain why we did not see an effect of BDNF release on SGN survival at the termination of the chronic implantation. BDNF and other growth factors have been shown to promote SGN survival following hair cell loss, even in cases where the delivery method is more indirect than the method used here [26, 27, 40, 41]. However, the time between the maximal BDNF release from the hydrogel and the SGN assessment, while necessary in this study to characterize the long-term electrochemical effects of the implant coating, may have been too long to see the effects typically associated with growth factor release in a damaged cochlea. Conversely, SGN survival in the implanted ears was comparable to that of the non-implanted ears, providing evidence of the non-cytotoxic nature of the coating.
Current methods of drug delivery to the cochlea leave room for improvement [15]. Bolus injections of drugs into the cochlea, perhaps administered at the time of implantation, are clinically relevant but only effective for hours or days, as the exogenous factor degrades quickly once placed in the cochlear fluids. The relatively slower release of drugs from a hydrogel could extend the effective time of this type of treatment. Implantable osmotic pumps are often used in animal models of cochlear damage because of the potential for long-term drug delivery, but are not clinically viable due to a high infection risk [42, 43]. The clinical applications of hydrogel-based cochlear implant coatings are immediate. Several hydrogels are already approved for clinical use in a number of areas, including wound healing and tissue regeneration [44]. The hydrogel-mediated release of BDNF in this study was robust over the first 2 weeks following implantation, indicating that this CI coating is an effective methodology that could be optimized for extended and controlled cochlear drug delivery.
5. Conclusions
The combination of the conducting polymer PEDOT with an RGD-modified alginate hydrogel loaded with BDNF created an effective, non-cytotoxic, and clinically relevant cochlear implant coating. Applying this coating to cochlear implants led to a decrease in electrode impedances and an increase in the safe charge delivery capabilities. These results were seen in both in vitro and in vivo experiments. These improvements in impedance and charge delivery density could impact chronic stimulating prostheses, where, over many years, signal fidelity can degrade and decrease peak performance. Stable electrical properties could provide consistent cochlear implant function over long periods of time and reduce the need for visits to adjust cochlear implant parameters. In addition, there was a substantial release of bioactive BDNF into the cochlear fluids within 1 week of implantation with a BDNF-soaked hydrogel-coated implant; this effect was sustained 2 weeks after implantation. The ability of this coating to release BDNF into cochlear fluids offers the potential of enticing SGN processes into the hydrogel matrix, where they might interact more intimately with cochlear implant electrodes. The combination of the PEDOT and BDNF-delivering hydrogel did not hamper the effects of either component, giving this dual-component cochlear implant coating potential for future clinical applications to improve the safety, function, and efficacy of cochlear implants.
Acknowledgments
The authors are grateful to Lisa Beyer, Deborah Colesa, Mark Crumling, Diane Prieskorn, Donald Swiderski, and Gina Su for their technical assistance in the experiments. This project was funded by the R. Jamison and Betty Williams Professorship, the A. Alfred Taubman Medical Research Institute, NIH Grants R01-DC01634, R01-DC05401, F31-DC009134, and P30-DC05188, NSF Grants DMR-0320740 and DMR-0518079, the University of Michigan COE GAP Program, the National Academies Keck Futures Initiative on Smart Prosthetics, ARO MURI Grant W911NF-06-1-0218, and Biotectix, LLC. JLH, SMR and DCM are founders and owners of Biotectix LLC, a company specializing in the commercialization of conducting polymer materials.
Footnotes
The University of Michigan manages this conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson BS, Dorman MF. Cochlear implants: current designs and future possibilities. J Rehabil Res Dev. 2008;45(5):695–730. doi: 10.1682/jrrd.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- 2.Webster M, Webster DB. Spiral ganglion neuron loss following Organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108(1–2):112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 4.Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008;242(1–2):3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo J, Parkins CW. A model of electrical excitation of the mammalian auditory-nerve neuron. Hear Res. 1987;31(3):287–311. doi: 10.1016/0378-5955(87)90197-3. [DOI] [PubMed] [Google Scholar]
- 6.Pfingst BE, Sutton D. Relation of cochlear implant function to histopathology in monkeys. Ann N Y Acad Sci. 1983;405:224–239. doi: 10.1111/j.1749-6632.1983.tb31635.x. [DOI] [PubMed] [Google Scholar]
- 7.Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33(1):11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 8.Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79(3–4):266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- 9.Rebscher SJ, Hetherington A, Bonham B, Wardrop P, Whinney D, Leake PA. Considerations for design of future cochlear implant electrode arrays: electrode array stiffness, size, and depth of insertion. J Rehabil Res Dev. 2008;45(5):731–747. doi: 10.1682/jrrd.2007.08.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan YY, Clark GM, Cowan RS. A study of intra-cochlear electrodes and tissue interface by electrochemical impedance methods in vivo. Biomaterials. 2004;25(17):3813–3828. doi: 10.1016/j.biomaterials.2003.09.107. [DOI] [PubMed] [Google Scholar]
- 11.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29(24–25):3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 12.Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30(13):2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, et al. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56(2):261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3(1):59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 15.Hendricks JL, Chikar JA, Crumling MA, Raphael Y, Martin DC. Localized cell and drug delivery for auditory prostheses. Hear Res. 2008;242(1–2):117–131. doi: 10.1016/j.heares.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4(17):999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coviello T, Matricardi P, Alhaique F. Drug delivery strategies using polysaccharidic gels. Expert Opin Drug Deliv. 2006;3(3):395–404. doi: 10.1517/17425247.3.3.395. [DOI] [PubMed] [Google Scholar]
- 18.Noushi F, Richardson RT, Hardman J, Clark G, O'Leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26(3):528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- 19.Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, et al. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115(11):2016–2020. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- 20.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 21.Katz JS, Burdick JA. Hydrogel mediated delivery of trophic factors for neural repair. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):128–139. doi: 10.1002/wnan.10. [DOI] [PubMed] [Google Scholar]
- 22.Raphael Y, Colesa DJ, Lee C-C, Minoda R, Nakaizumi T, Pfingst BE. Postdeafening treatment of the mature cochlea by upregulation of BDNF: Histological and functional effects. Association for Research in Otolaryngology MidWinter Meeting; 2004; Daytona Beach, FL. 2004. [Google Scholar]
- 23.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 24.Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21(16):6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295(3):369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- 26.Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(4–5):631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 27.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 28.Miller CA, Woodruff KE, Pfingst BE. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear Res. 1995;92(1–2):85–99. doi: 10.1016/0378-5955(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 29.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80(11):2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 30.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28(30):4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60(2):217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Abidian M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res A. 2004;71(4):577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 33.Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, et al. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454(3):350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 35.Su GL, Colesa DJ, Pfingst BE. Effects of deafening and cochlear implantation procedures on postimplantation psychophysical electrical detection thresholds. Hear Res. 2008;241(1–2):64–72. doi: 10.1016/j.heares.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105(1–2):1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- 37.Spelman FA. Cochlear electrode arrays: past, present and future. Audiol Neurootol. 2006;11(2):77–85. doi: 10.1159/000090680. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 40.Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann N Y Acad Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- 41.Chikar JA, Colesa DJ, Swiderski DL, Polo AD, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245(1–2):24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson RT, Noushi F, O'Leary S. Inner ear therapy for neural preservation. Audiol Neurootol. 2006;11(6):343–356. doi: 10.1159/000095896. [DOI] [PubMed] [Google Scholar]
- 43.Pettingill LN, Richardson RT, Wise AK, O'Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54(6 Pt 1):1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann U, Mimietz S, Zimmermann H, Hillgartner M, Schneider H, Ludwig J, et al. Hydrogel-based non-autologous cell and tissue therapy. Biotechniques. 2000;29(3):564–572. 574, 576. doi: 10.2144/00293rv01. passim. [DOI] [PubMed] [Google Scholar]