Abstract
Health-related quality of life (HRQOL) is associated with seizure recency amongst adults with epilepsy. In a prospective, community-based study of long-term outcomes of childhood-onset epilepsy, we evaluated whether worse HRQOL is associated with more recent seizures amongst children and adolescents with epilepsy. We used the Child Health Questionnaire (CHQ), a generic measure with child and parent-proxy versions, to measure HRQOL. Amongst 277 children with epilepsy (CWE) assessed 9 years after diagnosis, parent-proxy reported but not child self-reported HRQOL was significantly worse for those having seizures in the prior year compared to those who were seizure-free ≥1 year across the majority of scales. There were no differences between CWE in remission for 1–5 years compared to ≥ 5 years for child and parent-proxy reported HRQOL with the exception of the parent emotional impact scale, thereby suggesting that HRQOL differences related to seizure recency level off after the initial year of remission.
Keywords: Epilepsy, Children and adolescents, Health-related quality of life, Seizure recency, Seizure remission, Parent-proxy reports
I. Introduction
Given its broad physical and psychosocial impacts, assessment of health-related quality of life (HRQOL) is a critical patient-reported outcome measure for persons with childhood-onset epilepsy [1–2]. In a community-based study of children with newly diagnosed epilepsy followed prospectively for 9-years after diagnosis, we previously found that, based on parent-proxy report, children with epilepsy (CWE) had worse HRQOL than sibling controls. By contrast, based on self-report, CWE had HRQOL comparable to sibling controls [3]. Prior research has supported the notion that there is a gradient of HRQOL related to the level of “active” epilepsy amongst CWE. In particular, Austin et al. and Cramer et al. demonstrated that CWE with “more severe” or “active” epilepsy have worse HRQOL compared to those with “less severe” or “inactive” epilepsy [4–5]. In theses studies, “active” epilepsy was defined by a composite score established by metrics of both seizure frequency and type and, in the case of Austin et al., number of anti-epileptic drugs (AEDs) and associated side-effects; “active” epilepsy was not defined by remission status. Although seizure remission status has been shown to be an important predictor of improved HRQOL in pediatric epilepsy surgical cohort studies, such studies did not examine whether variable remission times differentially impact HRQOL [6–8].
To our knowledge, no studies to date have examined the impact of seizure remission duration on HRQOL in CWE. We aim to determine if there is a gradient between seizure recency, or duration of seizure remission, and HRQOL at a 9-year follow-up. Given evidence from adult epilepsy surgery cohorts that has demonstrated improvements in HRQOL that are noted most significantly in the first 6 months to 2 years after surgery and then subsequently level off, we hypothesized that there is an analogous gradient among CWE with worse HRQOL associated with more recent seizures (seizures within the past year) compared to better HRQOL amongst CWE seizure-free for 1–5 year or ≥ 5 years amongst CWE at 9 year follow-up [9–12]. Additionally, because CWE and their parents can have different perspectives about the overall impact of epilepsy with HRQOL, a secondary aim was to determine if parents and children report associations between seizure recency and HROQL differently; we hypothesized that parent-child differences will exist [3, 13–15].
II. Methods
a. Sample
The Connecticut Study of Epilepsy is a prospective, community-based cohort study that recruited 613 children with newly diagnosed epilepsy (January 1993 to December 1997). Details of methods, recruitment, and follow-up procedures have been previously published [3, 16–18]. The characteristics of the CWE in this Connecticut cohort are highly comparable to those of a Canadian study that is generally accepted as population-based with respect to age at onset, gender, proportion of participants with certain well-recognized forms of epilepsy, intellectual disability and mortality [16, 19–23].
Eight to nine years after enrollment (March 2002 to February 2006) CWE and family members from the original cohort participated in a reassessment protocol. Retention in this 9-year assessment was high with 83% (n=502) of the original cohort participating. The 111 children from the original cohort that did not participate in this 9-year follow-up included 13 children who died, 70 children who were lost to follow-up and 28 children who declined participation. This 9-year assessment protocol included evaluation of HRQOL of the child with epilepsy by both the child with epilepsy and the child's parent. HRQOL was assessed in CWE who were less than 18 years old at the time of the 9-year assessment using the Child Health Questionnaire (CHQ). Of the 502 children who participated in the 9-year assessment protocol at any level, 374 were less than 18 years old, of whom 278 parent-child dyads completed the CHQ. One child-parent dyad was subsequently excluded from this analysis for confidential reasons making the final sample 277 parent-child dyads.
Institutional Review Board approval was obtained at all sites. At the time of initial enrollment and at the 9-year assessment, written informed consent was obtained from the parent and written assent from the child.
b. Data collection
Clinical and demographic data of children and parents were obtained via structured in-person interview with the parent by trained research associates during initial enrollment and at the 9-year assessment, in conjunction with ongoing review of neurological medical records and interim telephone interviews. Details of this data collection protocol have been previously published [3, 17–18]. At the 9-year assessment, remission status was defined as being 5-years seizure-free or not, a definition consistent with prior literature that has examined outcomes amongst persons with childhood-onset epilepsy and is in accordance with the definition of remission set forth by the 1993 epidemiologic guidelines of the International League Against Epilepsy (ILAE) [24–28]. CWE were classified as either currently taking or not taking AEDs. To more closely examine the association of seizure remission duration, or time since last seizure, children were further categorized into the following seizure recency groups: (a) those seizure-free ≥ 5 years, (b) those seizure free 1 to 5 years, and (c) those who were not seizure-free and had experienced a seizure within the past year, or seizure-free < 1 year. These categories were based in part on the frequency distribution of the time since last seizure for the cohort at the time of the 9-year assessment, agreement amongst authors, in addition to prior literature from adult epilepsy surgical cohorts [9, 11–12, 29–30].
c. Measures
As previously noted, HRQOL of CWE was assessed using the CHQ, a self-administered, generic HRQOL measure with child (CHQ-CF87) and parent-proxy reported (CHQ-PF50) versions [31]. The child version includes 87 items in 11 scales and 2 global items. The CHQPF50 includes 50 items in 12 scales and 2 summary scores. Raw scores for each scale are transformed to a 0 to 100 scale, with higher scores indicating better health (100 = best health). Physical and psychosocial summary scores (CHQ-PF50 version only) are transformed to T-scored (mean = 50; SD = 10) calculated against a general reference U.S. population.
d. Analysis
Sociodemographic and clinical characteristics of CWE and parents were calculated using univariate statistics. Internal consistency reliability for each scale of the CHQ-CF87 and the CHQ-PF50 were estimated using Cronbach's alpha. We examined the relationship between seizure recency and HRQOL at 9-year assessment by comparing child and parent-proxy reported HRQOL of children who were seizure-free: (a) ≥ 5 years, (b) 1–5 years or (c) < 1 year using age- and gender-adjusted analysis of covariance (ANCOVA) with Type III sums of squares using each respective CHQ-CF87 or CHQ-PF50 scale, global item or summary score as the dependent variable. ANCOVAs were not adjusted for current AED status because it was highly correlated with seizure recency groups (r ≥ 0.70). We used the Tukey-Kramer multiple range test to assess differences in adjusted means between the 3 different seizure recency groups. All analyses were performed using SAS (9.2), setting an a priori p-value of p ≤ 0.05 for statistical significance.
III. Results
a. Sociodemographic and clinical characteristics of CWE and their parents
As previously noted, 277 child-parent dyads completed the CHQ at the time of the 9-year assessment; sociodemographic and clinical characteristics for these CWE and their parents are shown in Table 1. The mean age of epilepsy onset was 4.4 (SD=2.6) years; 46.6% were female. The average age of parents was 42.5 years (SD=5.4), over 90% were female and nearly half of parents had at least a college education. At the time of the 9-year assessment CWE were, on average, 13.0 years old (SD=2.6 years); nearly one-third were taking AEDs. Nearly two-thirds (n=177; 63.9%) were seizure-free ≥ 5 years; 18.4% had been seizure-free for 1–5 years, while 17.7% had experienced a seizure within the past year and were thus categorized as seizure-free < 1 year. Internal consistency reliability was high (Cronbach's α ≥ 0.75) for all child and parent-proxy scales.
Table 1.
Demographic and clinical characteristics for children with epilepsy and parents (n=277 dyads)
| Child demographics and clinical characteristics | N (%) |
|---|---|
|
| |
| Female | 129 (46.6) |
| Age of epilepsy onset, yrs, mean (SD) | 4.4 (2.6) |
| Age of epilepsy diagnosis, yrs, mean (SD) | 5.1 (2.5) |
| Age at CHQ administration, yrs, mean (SD) | 13.0 (2.6) |
| Education level at CHQ administration¶ | |
| Preschool – 5th grade | 67 (24.5) |
| 6th grade – 8th grade | 107 (39.2) |
| 9th grade – 12th grade | 99 (36.3) |
| Seizure remission status at 9-year follow-up | |
| Seizure-free ≥ 5 years | 177 (63.9) |
| Seizure free 1 to 5 years | 51 (18.4) |
| Seizure free for < 1 year | 49 (17.7) |
| Currently taking anti-epileptic drugs (AEDs) | 87 (31.4) |
| Full Scale IQ ≥ 80† | 230 (83.0) |
| Normal neurological exam¥ | 232 (83.8) |
| Parent-respondent demographics | N (%) |
|---|---|
|
| |
| Female | 252 (91.0) |
| Age, yrs, mean (SD) | 42.5 (5.4) |
| Race¶ | |
| Caucasian | 222 (80.4) |
| African American | 33 (12.0) |
| Other§ | 21 (7.6) |
| College education or higher¶ | 119 (43.1) |
| Employed full or part time¶ | 216 (78.3) |
| Married¶ | 198 (71.7) |
| Biological Parent | 265 (95.7) |
Missing data from child education (2 children graduated high school and 2 children were in vocational school), 1 missing from parent race, parent education, parent employment, and parent marital status
At 9-year follow-up
At baseline or 9-year follow-up
Includes Hispanic and Asian and Other
b. The association of seizure recency with HRQOL in CWE
In age- and gender-adjusted ANCOVAs of HRQOL and seizure recency, there were significant seizure recency between-group differences (Type III sum of squares) for child-reported HRQOL on 4 of 11 scales and for parent-proxy reported HRQOL on 10 of 12 scales, 2 of 2 items and both summary scores (all p's ≤ 0.03; Table 2). Comparison of seizure recency group adjusted means revealed no significant differences between CWE who were ≥ 5 years seizure-free compared to those who were 1–5 years seizure-free on any child-reported or parent-proxy reported scale or item with the exception of the parent-reported parental emotional impact scale (p < 0.0001; Table 2; Figure 1b). There were, however, significant differences in HRQOL amongst CWE that were seizure free <1 year compared to those were seizure free ≥ 5 years or 1–5 years for both child-reported and parent-proxy reported HRQOL (Table 2; Figure 1a, 1b). Specifically, compared those that were seizure free ≥ 1, CWE who were seizure free < 1 year had lower (worse) mean HRQOL scores on 2 of 11 child-reported scales including role/social limitations – physical and family activities scales (p≤0.05; Table 2; Figure 1a). Furthermore, CWE who were seizure-free < 1 year had lower HRQOL compared to those seizure free ≥ 5 years on 2 of 11 child-reported scales including physical function and behavior scales (p≤0.05; Table 2; Figure 1a). Findings from parent-proxy reported HROQL were similar, although much more pervasive than child-reported HRQOL. Notably, CWE who were seizure free < 1 year had lower mean parent-proxy reported HRQOL on 9 of 12 scales, global general health items and physical and psychosocial summary scores compared to those were seizure-free ≥ 1 year (p≤0.05; Table 2; Figure 1b). Furthermore, CWE who were seizure-free < 1 year had lower HRQOL compared to those seizure free ≥ 5 years on the mental health scale and the global behavior item (p≤0.05; Table 2; Figure 1b). Significant differences between seizure recency groups were not seen for the bodily pain/discomfort and family cohesion parent-proxy reported scales (Table 2; Figure 1b).
Table 2.
Association of seizure recency with HRQOL in children with epilepsy at 9-year follow-up (n = 277)
| Seizure recency | F-value† | p-value† | |||
|---|---|---|---|---|---|
| Seizure-free ≥ 5 years (N=177) | Seizure-free 1 year to 5 years (N=51) | Seizure-free < 1 year (N=49) | |||
| Child Report (CHQ-CF87) | |||||
| Physical function | 94.5 a | 93.9 a,b | 88.9 b | 4.08 | 0.02 |
| Role/social limitations – physical | 96.8 a | 95.9 a | 86.3 b | 10.78 | <0.0001 |
| Role/social limitations – behavioral | 94.6 a | 93.2 a | 89.5 a | 2.02 | 0.13 |
| Role/social limitations – emotional | 91.3 a | 91.5 a | 85.9 a | 1.96 | 0.14 |
| Mental health | 76.7 a | 77.3 a | 73.5 a | 1.01 | 0.37 |
| Self-esteem | 81.3 a | 82.5 a | 79.0 a | 0.85 | 0.43 |
| Behavior | 79.2 a | 77.9 a,b | 72.6 b | 4.52 | 0.01 |
| Bodily pain/ discomfort | 82.9 a | 80.3 a | 80.3 a | 0.60 | 0.55 |
| General health perceptions | 73.8 a | 72.6 a | 68.2 a | 2.71 | 0.07 |
| Family activities | 80.6 a | 80.5 a | 70.6 b | 4.13 | 0.02 |
| Family cohesion | 75.0 a | 71.3 a | 69.1 a | 1.32 | 0.27 |
| Global behavior | 83.0 a | 82.0 a | 76.3 a | 2.28 | 0.10 |
| Global general health | 81.6 a | 78.9 a | 76.9 a | 1.46 | 0.23 |
| Parent Report (CHQ-PF50) | |||||
| Physical function | 97.0 a | 95.2 a | 87.5 b | 9.15 | 0.0001 |
| Role/Social limitations – physical | 95.2 a | 96.6 a | 86.2 b | 5.87 | 0.003 |
| Role/Social limitations – emotional/ behavioral | 89.5 a | 90.3 a | 70.8 b | 10.41 | <0.0001 |
| Mental health | 79.2 a | 78.7 a,b | 72.4 b | 3.59 | 0.03 |
| Self-esteem | 80.2 a | 78.4 a | 68.7 b | 6.98 | 0.001 |
| Behavior | 77.8 a | 75.8 a | 66.9 b | 6.20 | 0.002 |
| Bodily pain/ discomfort | 83.1 a | 83.3 a | 79.1 a | 0.82 | 0.44 |
| General health perceptions | 74.6 a | 71.3 a | 61.7 b | 12.28 | <0.0001 |
| Family activities | 83.6 a | 84.2 a | 71.5 b | 5.99 | 0.003 |
| Family cohesion | 73.2 a | 74.2 a | 67.9 a | 1.31 | 0.27 |
| Global behavior | 79.3 a | 77.3 a, b | 66.8 b | 4.86 | 0.009 |
| Global general health | 88.0 a | 85.3 a | 74.6 b | 12.57 | <0.0001 |
| Parent impact on time | 90.1a | 88.0 a | 72.8 b | 12.09 | <0.0001 |
| Parent emotional impact | 78.5 a | 68.0b | 50.1c | 27.56 | <0.0001 |
| Summary scores (T-scores) | |||||
| Physical summary score | 53.7 a | 52.9 a | 47.8 b | 11.67 | <0.0001 |
| Psychosocial summary score | 51.4 a | 49.8 a | 42.0 b | 12.50 | <0.0001 |
ANCOVAs of CHQ (scale, item or summary score) and seizure recency adjusting for child's age and gender.
Bold indicates ANCOVA (Type III sum of squares) significance of p ≤ 0.05
Means within a row with different letters differ significantly (p ≤ 0.05; Tukey-Kramer multiple range test)
Figure 1.
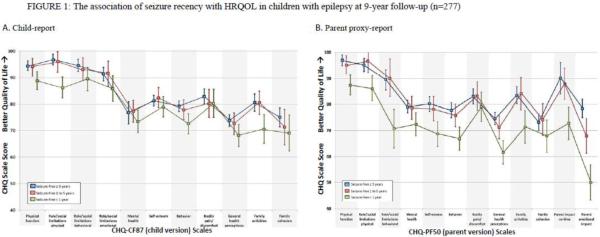
Adjusted predicted HRQOL mean scale scores (± CI's) calculated from ANCOVAs for seizure recency.
IV. Discussion
In this 9-year follow-up of adolescents with newly diagnosed epilepsy in childhood followed prospectively, we found that HRQOL is worse for those having seizures in the prior year compared to those who were seizure-free for one year or more. Parent-proxy reports were pervasive across the majority of scales, items and summary scores, while child-reported differences were noted on a smaller proportion of scales. There were no differences, however, between CWE in remission for 1–5 years compared to those in remission for ≥ 5 years for both child and parent-proxy reported HRQOL, with the exception of the parent emotional impact scales. While such findings suggest that HRQOL differences related to seizure recency are not linear and level off after the initial year of remission, they also highlight the hidden cost that childhood-onset epilepsy has on parents and families, particularly if children have experienced seizures recently.
To our knowledge, this is the first study to examine the relationship between HRQOL and seizure recency, or duration of seizure remission, in CWE. Several studies of adults with epilepsy, however, have demonstrated that HRQOL is associated with seizure-free duration. Notably, in a study of 136 adults with epilepsy on AEDs from three outpatient clinics in the UK, Wagner et al. found that HRQOL decrements were related to the time since last seizure; patients who were seizure-free for > 6 months had better HROQL scores compared to those with more recent seizures [32]. Similarly, Jacoby found that improved feelings about epilepsy and its impact on quality of life was associated with a longer duration of seizure freedom amongst adults seizure-free ≥ 2 years, while Pulsipher et al. found that days since last seizure was the strongest predictor of epilepsy-related effects in the QOLIE-89 in a multivariate analysis of 93 adult with temporal lobe epilepsy [30, 33]. Interestingly, Cramer et al. found that amongst 201 adult patients with partial epilepsy on two or more AEDs, decrements in perceived (hypothetical) health, if experiencing a seizure today, were greatest for those with more recent seizures [34]. In contrast, however, in a non-surgical sample of 139 adults with epilepsy from three US centers, time since last seizure was not uniquely related to HRQOL as measured by the SF-36 in a multivariate analysis [35].
Our finding that HRQOL is worse for those having seizures in the prior year compared to those who were seizure-free for ≥1 year, with minimal differences between seizure free 1–5 and ≥ 5 year groups, suggests that the relationship between HROQL and seizure recency is not linear, a finding that is consistent with prior literature of HRQOL in adult epilepsy surgical cohorts [9–10, 12]. In adult epilepsy surgical cohort studies by Elsharkawy et al., time since last seizure was a significant predictor of improved HRQOL as measured by the QOLIE-31, with most subscales demonstrating a steep, non-linear increase in scores within the first 2–3 years of seizure freedom that then remained stable [9–10]. In the Multicenter Study of Epilepsy Surgery, Spencer et al. demonstrated improvements in QOLIE-89 scores within the first 6 months after epilepsy surgery, regardless of seizure outcome, with subsequent improvements, however, that paralleled the length of time seizure free and then stabilized at 2 years; McLachlan et al. similarly found that changes in HRQOL were not evident until the second post-operative year amongst those seizure-free and those with at least a 90% reduction in seizure frequency [11–12].
While seizure recency was strongly associated across multiple domains of HROQL in CWE for parent-proxy reports, such findings were less robust across child self-reported HRQOL. Differences in seizure recency groups in child-reported HRQOL were primarily seen across scales related to physical function, role/social limitations physical, behavior and family activities while differences in parent-rated HRQOL of CWE were pervasive across the majority of scales, items and summary scores. Such child-parent differences are consistent with our prior findings of parent-child differences in report of HRQOL of CWE; parent proxies rated children with epilepsy's HRQOL as worse than sibling controls, while children with epilepsy (CWE) self-reported HRQOL comparable to sibling controls that parents [3]. Furthermore, this parent-child reporting discrepancy may, indeed, reflect differences in the perception of HRQOL in CWE as has been found in several studies to date. In a study by Hamiwka et al., parents rated HRQOL of children who had experienced a first time seizure as lower than normative controls on one-half of parent-proxy CHQ scales, including the two parent impact scales, although children did not, while Verhey et al. found that parents rated children's HRQOL lower compared with child's report as measured by an epilepsy-specific measure, the CHEQOL-25 [13, 15]. These parent-child differences may, in part, reflect the large impact that childhood-onset epilepsy has on parents and families with regard to day to day functioning, family activities and even parental mood, all of which may impact a parent's rating of their child's HROQL [36–38]. In fact, compared to parents without epilepsy, parents of CWE often have higher levels of psychological distress and depression and report poorer QOL [39–40]. Alternatively, the parent-child reporting differences of the association of seizure recency with HRQOL in CWE may reflect that the CHQ-PF50 (parent-proxy version) has better construct validity for epilepsy disease severity compared to the CHQ-CF87 (child self-report version).
There are several limitations to our study. The observed differences in parent-proxy versus child reports of the association of seizure recency with HRQOL in CWE may be due in part to the fact that the CHQ is a generic instrument that is not sensitive to the nuances of epilepsy severity, particularly for child-report. In this regard, the CHQ-CF87 may have less construct validity for epilepsy severity compared to the CHQ-PF50. Secondly, differences in child and parent-proxy reports represent general trends only because the CHQ-CF87 and CHQ-PF50 have varied wording of similar items, some distinct items altogether, a few scales with non-overlapping content, and content that maps from one scale of one version to multiple scales of the other version. For example, the parent “physical function” scale has 6 questions while the child “physical function” scale is comprised of 9 questions. On the parent version “role limitations emotional/behavioral” is one scale, while on the child version it is two scales, “role limitations – behavioral” and “role limitations – emotional”. Two scales on the parent version, “parent impact on time” and “parent emotional impact” do not exist on the child version. Similarly, physical and psychosocial summary scores only exist for the parent version. As such, direct child and parent-proxy comparisons are limited and we have previously shown that parent-child agreement is low [3]. Lastly, because the subjects in our sample were recruited from one US state (Connecticut), were predominantly Caucasian (>80%) and had parents that were highly educated (nearly half had college education or more) and employed (78%), the generalizability of our findings may be limited.
At 9-year follow-up of a cohort of CWE, HRQOL is worse for those having seizures in the prior year compared to those who were seizure-free for one year or more; HRQOL differences related to seizure recency amongst CWE are not linear and level off after the initial year of remission. Associations of seizure recency with HRQOL in CWE were more pronounced for parent-proxy reported HRQOL than for child-reported HRQOL, the findings of which may reflect differences in perspectives of children and their parents that are indicative of the large impact that childhood-onset epilepsy has on parents and families, particularly if children have experienced seizures recently. In contrast, these reporting differences may suggest that the parent-version of the CHQ has better construct validity for epilepsy disease severity compared to the child self-report version.
Acknowledgments
Financial disclosure: Supported by grant from National Institutes of Health, NINDS R37-NS31146 (PI-Berg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None of the authors have any conflicts of interest
VI. References
- [1].Lach LM, Ronen GM, Rosenbaum PL, Cunningham C, Boyle MH, Bowman S, et al. Health-related quality of life in youth with epilepsy: theoretical model for clinicians and researchers. Part I: the role of epilepsy and co-morbidity. Qual Life Res. 2006;15:1161–71. doi: 10.1007/s11136-006-0051-7. [DOI] [PubMed] [Google Scholar]
- [2].Ronen GM, Streiner DL, Rosenbaum P. Health-related quality of life in childhood epilepsy: moving beyond 'seizure control with minimal adverse effects'. Health Qual Life Outcomes. 2003;1:36–45. doi: 10.1186/1477-7525-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baca CB, Vickrey BG, Hays RD, Vassar SD, Berg AT. Differences in child versus parent reports of the child's health-related quality of life in children with epilepsy and healthy siblings. Value Health. 2010;13:778–86. doi: 10.1111/j.1524-4733.2010.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Austin JK, Huster GA, Dunn DW, Risinger MW. Adolescents with active or inactive epilepsy or asthma: a comparison of quality of life. Epilepsia. 1996;37:1228–38. doi: 10.1111/j.1528-1157.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- [5].Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the Quality of Life in Epilepsy Inventory for Adolescents: the QOLIE-AD-48. Epilepsia. 1999;40:1114–21. doi: 10.1111/j.1528-1157.1999.tb00828.x. [DOI] [PubMed] [Google Scholar]
- [6].Sabaz M, Lawson JA, Cairns DR, Duchowny MS, Resnick TJ, Dean PM, et al. The impact of epilepsy surgery on quality of life in children. Neurology. 2006;28(66):557–61. doi: 10.1212/01.wnl.0000197788.38783.09. [DOI] [PubMed] [Google Scholar]
- [7].Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76:1330–7. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zupanc ML, Rubio EJ, Werner RR, Schwabe MJ, Mueller WM, Lew SM, et al. Epilepsy surgery outcomes: quality of life and seizure control. Pediatr Neurol. 2010;42:12–20. doi: 10.1016/j.pediatrneurol.2009.07.018. [DOI] [PubMed] [Google Scholar]
- [9].Elsharkawy AE, May T, Thorbecke R, Ebner A. Predictors of quality of life after resective extratemporal epilepsy surgery in adults in long-term follow-up. Seizure. 2009;18:498–503. doi: 10.1016/j.seizure.2009.04.010. [DOI] [PubMed] [Google Scholar]
- [10].Elsharkawy AE, May T, Thorbecke R, Koch-Stoecker S, Villagran A, Urak L, et al. Long-term outcome and determinants of quality of life after temporal lobe epilepsy surgery in adults. Epilepsy Res. 2009;86:191–9. doi: 10.1016/j.eplepsyres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- [11].McLachlan RS, Rose KJ, Derry PA, Bonnar C, Blume WT, Girvin JP. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997;41:482–9. doi: 10.1002/ana.410410411. [DOI] [PubMed] [Google Scholar]
- [12].Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Haut S, et al. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007;62:327–34. doi: 10.1002/ana.21131. [DOI] [PubMed] [Google Scholar]
- [13].Hamiwka L, Singh N, Niosi J, Wirrell E. Perceived health in children presenting with a “first seizure”. Epilepsy Behav. 2008;13:485–8. doi: 10.1016/j.yebeh.2008.05.019. [DOI] [PubMed] [Google Scholar]
- [14].Stevanovic D, Jancic J, Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia. 2011;52:e75–8. doi: 10.1111/j.1528-1167.2011.03133.x. [DOI] [PubMed] [Google Scholar]
- [15].Verhey LH, Kulik DM, Ronen GM, Rosenbaum P, Lach L, Streiner DL. Quality of life in childhood epilepsy: what is the level of agreement between youth and their parents? Epilepsy Behav. 2009;14:407–10. doi: 10.1016/j.yebeh.2008.12.008. [DOI] [PubMed] [Google Scholar]
- [16].Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–52. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- [17].Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, DiMario F. Behavior and social competency in idiopathic and cryptogenic childhood epilepsy. Dev Med Child Neurol. 2007;49:487–92. doi: 10.1111/j.1469-8749.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- [18].Baca C, Vickrey BG, Caplan R, Vassar S, Berg A. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics. doi: 10.1542/peds.2011-0245. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–14. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- [20].Berg AT, Shinnar S, Testa FM, Levy SR, Smith SN, Beckerman B. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. 2004;158:1147–52. doi: 10.1001/archpedi.158.12.1147. [DOI] [PubMed] [Google Scholar]
- [21].Camfield CS, Camfield PR. Long-term social outcomes for children with epilepsy. Epilepsia. 2007;48(Suppl 9):3–5. doi: 10.1111/j.1528-1167.2007.01390.x. [DOI] [PubMed] [Google Scholar]
- [22].Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- [23].Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet. 2002;359:1891–5. doi: 10.1016/S0140-6736(02)08779-2. [DOI] [PubMed] [Google Scholar]
- [24].Shackleton DP, Kasteleijn-Nolst Trenite DG, de Craen AJ, Vandenbroucke JP, Westendorp RG. Living with epilepsy: long-term prognosis and psychosocial outcomes. Neurology. 2003;61:64–70. doi: 10.1212/01.wnl.0000073543.63457.0a. [DOI] [PubMed] [Google Scholar]
- [25].Sillanpaa M, Haataja L, Shinnar S. Perceived impact of childhood-onset epilepsy on quality of life as an adult. Epilepsia. 2004;45:971–7. doi: 10.1111/j.0013-9580.2004.44203.x. [DOI] [PubMed] [Google Scholar]
- [26].Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–22. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- [27].Wakamoto H, Nagao H, Hayashi M, Morimoto T. Long-term medical, educational, and social prognoses of childhood-onset epilepsy: a population-based study in a rural district of Japan. Brain Dev. 2000;22:246–55. doi: 10.1016/s0387-7604(00)00121-2. [DOI] [PubMed] [Google Scholar]
- [28].Guidelines for epidemiologic studies on epilepsy Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34:592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [29].Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110:1135–46. doi: 10.3171/2008.6.JNS17613. [DOI] [PubMed] [Google Scholar]
- [30].Pulsipher DT, Seidenberg M, Jones J, Hermann B. Quality of life and comorbid medical and psychiatric conditions in temporal lobe epilepsy. Epilepsy Behav. 2006;9:510–4. doi: 10.1016/j.yebeh.2006.07.014. [DOI] [PubMed] [Google Scholar]
- [31].Landgraf JMAL, Ware JA. The CHQ user's manual. 1st edition ed. The Health Institute, New England Medical Centre; Boston: 1996. [Google Scholar]
- [32].Wagner AK, Keller SD, Kosinski M, Baker GA, Jacoby A, Hsu MA, et al. Advances in methods for assessing the impact of epilepsy and antiepileptic drug therapy on patients' health-related quality of life. Qual Life Res. 1995;4:115–34. doi: 10.1007/BF01833606. [DOI] [PubMed] [Google Scholar]
- [33].Jacoby A. Epilepsy and the quality of everyday life. Findings from a study of people with well-controlled epilepsy. Soc Sci Med. 1992;34:657–66. doi: 10.1016/0277-9536(92)90193-t. [DOI] [PubMed] [Google Scholar]
- [34].Cramer JA, Brandenburg NA, Xu X, Vera-Llonch M, Oster G. The impact of seizures and adverse effects on global health ratings. Epilepsy Behav. 2007;11:179–84. doi: 10.1016/j.yebeh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- [35].Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK. Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology. 1999;13;53:162–6. doi: 10.1212/wnl.53.1.162. [DOI] [PubMed] [Google Scholar]
- [36].Aytch LS, Hammond R, White C. Seizures in infants and young children: an exploratory study of family experiences and needs for information and support. J Neurosci Nurs. 2001;33:278–85. doi: 10.1097/01376517-200110000-00008. [DOI] [PubMed] [Google Scholar]
- [37].Ferro MA, Avison WR, Campbell MK, Speechley KN. The impact of maternal depressive symptoms on health-related quality of life in children with epilepsy: a prospective study of family environment as mediators and moderators. Epilepsia. 2011;52:316–25. doi: 10.1111/j.1528-1167.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- [38].Williams J, Steel C, Sharp GB, DelosReyes E, Phillips T, Bates S, et al. Parental anxiety and quality of life in children with epilepsy. Epilepsy Behav. 2003;4:483–6. doi: 10.1016/s1525-5050(03)00159-8. [DOI] [PubMed] [Google Scholar]
- [39].Lv R, Wu L, Jin L, Lu Q, Wang M, Qu Y, et al. Depression, anxiety and quality of life in parents of children with epilepsy. Acta Neurol Scand. 2009;120:335–41. doi: 10.1111/j.1600-0404.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- [40].Shore CP, Austin JK, Huster GA, Dunn DW. Identifying risk factors for maternal depression in families of adolescents with epilepsy. J Spec Pediatr Nurs. 2002;7:71–80. doi: 10.1111/j.1744-6155.2002.tb00153.x. [DOI] [PubMed] [Google Scholar]


