Abstract
Objective
To assess the validity of parental report for seasonal and monovalent H1N1 influenza vaccinations among children 6 months-<18 years who were recommended to receive both vaccines in 2009–2010.
Methods
Children with fever or respiratory symptoms were prospectively enrolled in both emergency departments in Forsyth County, North Carolina and the only pediatric hospital in the region. Enrollment occurred from September 1, 2009 through April 12, 2010, during the H1N1 influenza pandemic. A parental questionnaire was administered by trained interviewers to ascertain the status of seasonal and monovalent H1N1 influenza vaccines. Parental report was compared to that documented in the medical record and/or the North Carolina immunization registry.
Results
Among 297 enrolled children 6 months -<18 years of age, 174 (59%) were 6 months-4 years, 67 (23%) were 5–8 years and 56 (19%) were 9-<18 years. Parents reported that 140 (47%) children had received ≥1 dose of 2009–2010 influenza vaccine-- 128 (43%) for seasonal vaccine and 63 (21%) for H1N1 vaccine. Confirmed vaccination data indicated that 156 (53%) children had received ≥1 dose of any 2009–2010 vaccine—120 (40%) for seasonal vaccine and 53 (18%) for H1N1 vaccine.
Parental report of any seasonal influenza vaccination was 92% sensitive and 86% specific and had a kappa of 0.76. Parental report for any H1N1 influenza vaccination was 88% sensitive and 92% specific with a kappa of 0.71.
Conclusion
Parental report of 2009–2010 seasonal and monovalent H1N1 influenza vaccinations was sensitive and specific and had reasonable agreement with the medical record and/or immunization registry.
What’s New
During the 2009–2010 H1N1 pandemic when seasonal and monovalent H1N1 vaccines were recommended for all children, we found that parental report for both influenza vaccines among children aged 6 months-<18 years had reasonable sensitivity, specificity and validity as compared to the medical record and/or immunization registry.
Keywords: influenza vaccine, parental report, child, validity, accuracy, seasonal influenza vaccine, monovalent H1N1 influenza vaccine
For decades, influenza vaccine was recommended for children with high-risk medical conditions.1 Since 2002, the Advisory Committee on Immunization Practices has significantly expanded its influenza vaccine recommendations. During the 2009–2010 influenza season, all children 6 months-18 years of age were recommended to receive both seasonal and monovalent H1N1 influenza vaccines.2,3
Many logistical issues make administering influenza vaccine to all children ≥6 months of age complicated. The upper age limit for children needing two doses of influenza vaccine in 2009–2010 differed by one year for seasonal and monovalent H1N1 vaccines. In 2009, all children <10 years of age were recommended to receive two doses of monovalent H1N1 influenza vaccine one month apart, whereas those ≥10 years were recommended to receive one dose.4 All children <9 years who had not previously received two doses of seasonal influenza vaccine in one influenza season were recommended to receive two doses of 2009–2010 seasonal vaccine one month apart; children ≥9 years and those <9 years who had been previously fully vaccinated against seasonal influenza were recommended to receive one dose.2 Many children receive one or more doses of influenza vaccine outside their primary medical home.5 This occurrence results in fragmented medical records documenting the administration of influenza vaccines. Accurate assessment of influenza vaccination status is important for both effective clinical care and pandemic control, as well as for research and influenza surveillance programs. Parental report of childhood influenza vaccination is often used for these assessments. Although a few studies have previously assessed the accuracy of parental report, no studies have been performed during a year when two distinct influenza vaccines were recommended. Hence, we sought to describe the influenza vaccination status for both 2009–2010 vaccines and to determine the validity of parental reports for assessing seasonal and monovalent H1N1 influenza vaccine status among children seen in the inpatient and emergency department setting.
Methods
Study population
Children with fever and/or respiratory symptoms were prospectively enrolled in either of two hospital emergency departments in Forsyth County, North Carolina and in the only pediatric hospital in the region. More than 95% of Forsyth County children who are seen in either the emergency department setting or are admitted to the hospital are cared for at these institutions. Clinical symptoms conferring study eligibility included parental report of fever, cough, nasal congestion, difficulty breathing, earache, sore throat, or wheezing. Children were eligible for study enrollment if they resided in Forsyth County, NC or one of 7 contiguous NC Counties, and if their parents spoke English or Spanish. Eligible children were systematically approached for study enrollment during 8 hours of surveillance each day shift for 4–5 days per week. Systematic enrollment entailed consecutively approaching families of eligible children in the order in which they checked into the emergency department. Enrollment occurred from September 1, 2009 through April 12, 2010, during the H1N1 influenza pandemic.
Approval
This study was reviewed and approved by the Wake Forest School of Medicine institutional review board. The Wake Forest School of Medicine institutional review board had an authorization agreement with the Forsyth Medical Center institutional review board.
Parental report
After written informed consent from the parent/guardian and child assent when appropriate, trained interviewers administered a parental questionnaire to ascertain the status of the 2009–2010 seasonal and monovalent H1N1 influenza vaccinations, demographic information, and past medical history. High-risk medical conditions included all medical conditions with a specific 2009–2010 influenza vaccine indication (e.g. congenital heart disease).2
Vaccination confirmation
Parents signed a release of medical records to allow confirmation of influenza vaccinations. We faxed a request to the medical home practice of enrolled children to confirm the 2009–2010 influenza vaccination status. We also confirmed the influenza vaccination status in the North Carolina Immunization Registry. Schools and other locations were contacted if the vaccination was reported to be given at that location and the vaccination could not be otherwise confirmed by the medical home practice and/or the North Carolina Immunization Registry. The North Carolina Immunization Registry is an adaptation of the Wisconsin Immunization Registry used by all of North Carolina’s local health departments and >600 providers of pediatric vaccines as of 2010.6 All health departments and most pediatric and family practice offices in this region of North Carolina report their vaccinations in the North Carolina immunization registry. Classification of Confirmed Influenza Vaccination Status
Parental report of influenza vaccination status was considered to be “confirmed” or “valid” if review of medical records from medical home practice, health department, North Carolina immunization registry or other site providing vaccination (e.g. school) reported a 2009–2010 influenza vaccination. The current and past influenza vaccination status was used to classify each child as unvaccinated, partially vaccinated, or fully vaccinated, and this was done using vaccination status data that had been confirmed by medical record review.
For both seasonal and monovalent H1N1 pandemic influenza vaccine, children were considered unvaccinated if they did not have any documented doses of influenza vaccine in 2009–2010.
For seasonal influenza vaccine, children <9 years were considered partially vaccinated if they received one dose of this 2009–2010 vaccine and had not received seasonal vaccine in the past. Children were considered fully vaccinated if they met any of the following conditions. They were <9 years of age and received two doses in 2009–2010 at least 24 days apart; they were <9 years of age, received one dose in 2009–2010 and received at least one dose of vaccine in a previous season; or they were 9–17 years of age and received one dose in 2009–2010.
For monovalent H1N1 vaccine, children were considered partially vaccinated if they were <10 years of age and received one of two recommended doses. Children were considered fully vaccinated if they were <10 years of age and received two doses of monovalent H1N1 vaccine at least 24 days apart or if they were 10–17 years and received one dose of monovalent H1N1 vaccine.
Additional details of the 2009–2010 season
During the 2009–2010 influenza season in North Carolina, influenza vaccine was provided for free to physician offices and the health department.7,8 Physician offices could charge administration fees, and the health department did not. Pharmacies in North Carolina were given temporary authority to administer seasonal and monovalent H1N1 influenza vaccine to children ≥14 years of age from October 9, 2009 through July 31, 2010.9
Analysis
Tests of association between vaccination status (none/partial/full) and demographic variables were performed using Fisher’s exact tests. The primary outcome was the comparison of the parental report of seasonal and monovalent H1N1 influenza vaccines to that reported in the medical record and/or the North Carolina immunization registry. For each vaccine, we computed the sensitivity, specificity, positive predictive value, and negative predictive value of parental report as well as the kappa statistic as compared to the confirmed vaccination status. McNemar’s test was used to compare the proportion of children who received at least one dose of the monovalent H1N1 vaccine to the proportion who received at least one dose of the seasonal influenza vaccine at the time of enrollment, and at the end of the influenza season using a second test. In secondary analyses, we sought to determine if any demographic variables predicted agreement between the parental report and the confirmed influenza vaccination status using Fisher’s exact tests. Statistical analysis was performed using Stata, version 8.1, and/or SAS statistical software, version 9.2.
Results
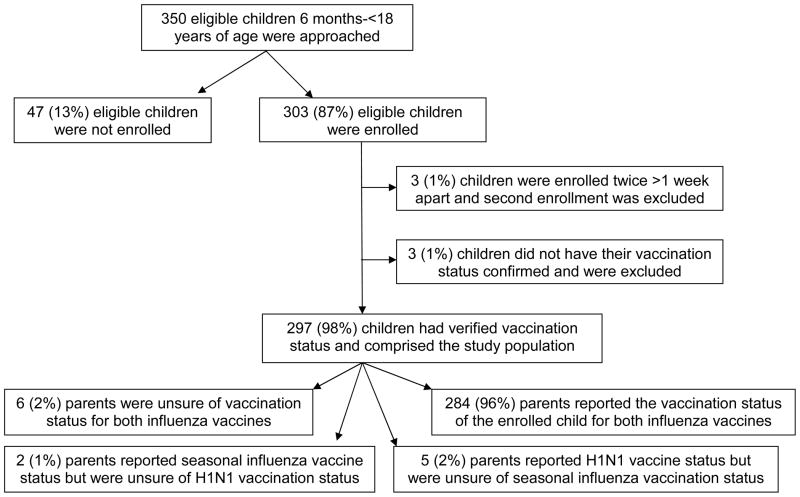
A total of 350 children 6 months-<18 years of age were invited to participate in this study, and 303 (87%) were enrolled (Figure 1). Three children were enrolled twice, but only the first enrollment was included in this analysis. We also excluded three children for whom influenza vaccination status could not be confirmed: two parents reported that their children had not received any influenza vaccines and one parent reported that the child had received both influenza vaccines. The resulting study population of 297 children consists of study subjects for whom influenza vaccinations could be confirmed by medical record review.
Figure 1.
Study Population
The majority of study children (59%) were 6 months-4 years of age, 23% were 5–8, 3% were 9 years of age, and 16% were 10-<18 years of age (Tables 1 and 2). Almost half the children were female, and 54% were black, 26% were white and 20% were Hispanic. Most children (69%) were enrolled between November and February and had public insurance (82%).
Table 1.
Demographic characteristics of the study population by the confirmed 2009–2010 seasonal influenza vaccine status at enrollment and at the end of the season in April 2010.
| Demographic characteristic | Enrolled | Seasonal Influenza Vaccination Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At enrollment, N (%) | At end of season, N (%) | |||||||||
| Frequency Count | None | Partial | Full | p-value | None | Partial | Full | p-value | ||
| All | 297 | 177 (60%) | 60 (20%) | 60 (20%) | 161 (54%) | 57 (19%) | 79 (27%) | |||
| Age group | 6 mos to 4 yrs | 174 | 90 (52%) | 38 (22%) | 46 (26%) | 0.002† | 80 (46%) | 33 (19%) | 61 (35%) | 0.002† |
| 5 to 8 yrs | 67 | 44 (66%) | 22 (33%) | 1 (1%) | 42 (63%) | 24 (36%) | 1 (1%) | |||
| 9 to 17 yrs | 56 | 43 (77%) | N/A | 13 (23%) | 39 (70%) | N/A | 17 (30%) | |||
| Gender | Female | 142 | 79 (56%) | 30 (21%) | 33 (23%) | 0.363 | 74 (52%) | 24 (17%) | 44 (31%) | 0.228 |
| Male | 155 | 98 (63%) | 30 (19%) | 27 (17%) | 87 (56%) | 33 (21%) | 35 (23%) | |||
| Race/Ethnicity | Hispanic | 60 | 30 (50%) | 20 (33%) | 10 (17%) | 0.059 | 25 (42%) | 19 (32%) | 16 (27%) | 0.071 |
| Black | 159 | 100 (63%) | 29 (18%) | 30 (19%) | 94 (59%) | 24 (15%) | 41 (26%) | |||
| White | 77 | 46 (60%) | 11 (14%) | 20 (26%) | 42 (55%) | 13 (17%) | 22 (29%) | |||
| Admitted to the hospital | No | 226 | 151 (67%) | 39 (17%) | 36 (16%) | <.001 | 140 (62%) | 36 (16%) | 50 (22%) | <.001 |
| Yes | 70 | 26 (37%) | 20 (29%) | 24 (34%) | 21 (30%) | 21 (30%) | 28 (40%) | |||
| Month of enrollment | Sept–Oct 2009 | 41 | 33 (80%) | 5 (12%) | 3 (7%) | 0.059 | 23 (56%) | 3 (7%) | 15 (37%) | 0.230 |
| Nov–Dec 2009 | 91 | 55 (60%) | 21 (23%) | 15 (16%) | 51 (56%) | 22 (24%) | 18 (20%) | |||
| Jan–Feb 2010 | 115 | 62 (54%) | 23 (20%) | 30 (26%) | 60 (52%) | 22 (19%) | 33 (29%) | |||
| Mar–Apr 2010 | 50 | 27 (54%) | 11 (22%) | 12 (24%) | 27 (54%) | 10 (20%) | 13 (26%) | |||
| Hish-risk medical condition* | No | 181 | 115 (64%) | 34 (19%) | 32 (18%) | 0.199 | 105 (58%) | 30 (17%) | 46 (25%) | 0.203 |
| Yes | 116 | 62 (53%) | 26 (22%) | 28 (24%) | 56 (48%) | 27 (23%) | 33 (28%) | |||
| Insurance | Private | 44 | 28 (64%) | 6 (14%) | 10 (23%) | 0.082 | 25 (57%) | 7 (16%) | 12 (27%) | 0.263 |
| Public | 243 | 139 (57%) | 54 (22%) | 50 (21%) | 127 (52%) | 50 (21%) | 66 (27%) | |||
| None | 10 | 10 (100%) | 0 (0%) | 0 (0%) | 9 (90%) | 0 (0%) | 1 (10%) | |||
High-risk medical conditions include cancer, diabetes mellitus, heart disease, immune deficiency, kidney disease, liver disease, sickle cell disease, seizure disorder, cognitive impairment or developmental delay, asthma, cystic fibrosis, and chronic lung disease.
Note: Statistically significant results as determined by Fisher’s exact tests are highlighted with gray background.
Because partial vaccination is not possible for the oldest age group, we compared no vaccination to partial or full vaccination for age group only using the Fisher’s exact tests.
Table 2.
Demographic characteristics of the study population by the confirmed 2009–2010 monovalent H1N1 influenza vaccine status at enrollment and at the end of the season in April 2010.
| Demographic characteristic | Enrolled | H1N1 Influenza Vaccination Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At enrollment, N (%) | At end of season, N (%) | |||||||||
| Frequency Count | None | Partial | Full | p-value | None | Partial | Full | p-value | ||
| All | 297 | 244 (82%) | 35 (12%) | 18 (6%) | 214 (72%) | 42 (14%) | 41 (14%) | |||
| Age group | 6 mos to 4 yrs | 174 | 136 (78%) | 29 (17%) | 9 (5%) | 0.012† | 115 (66%) | 33 (19%) | 26 (15%) | 0.016† |
| 5 to 9 yrs | 76 | 63 (83%) | 6 (8%) | 7 (9%) | 59 (78%) | 9 (12%) | 8 (11%) | |||
| 10 to 17 yrs | 47 | 45 (96%) | N/A | 2 (4%) | 40 (85%) | N/A | 7 (15%) | |||
| Gender | Female | 142 | 115 (81%) | 18 (13%) | 9 (6%) | 0.871 | 98 (69%) | 23 (16%) | 21 (15%) | 0.515 |
| Male | 155 | 129 (83%) | 17 (11%) | 9 (6%) | 116 (75%) | 19 (12%) | 20 (13%) | |||
| Race/Ethnicity | Hispanic | 60 | 37 (62%) | 14 (23%) | 9 (15%) | <.001 | 25 (42%) | 18 (30%) | 17 (28%) | <.001 |
| Black | 159 | 139 (87%) | 16 (10%) | 4 (3%) | 129 (81%) | 17 (11%) | 13 (8%) | |||
| White | 77 | 67 (87%) | 5 (6%) | 5 (6%) | 60 (78%) | 6 (8%) | 11 (14%) | |||
| Admitted to the hospital | No | 226 | 188 (83%) | 24 (11%) | 14 (6%) | 0.503 | 170 (75%) | 29 (13%) | 27 (12%) | 0.075 |
| Yes | 70 | 55 (79%) | 11 (16%) | 4 (6%) | 43 (61%) | 13 (19%) | 14 (20%) | |||
| Month of enrollment | Sept–Oct 2009 | 41 | 41 (100%) | 0 (0%) | 0 (0%) | 0.002 | 26 (63%) | 4 (10%) | 11 (27%) | 0.316 |
| Nov–Dec 2009 | 91 | 76 (84%) | 13 (14%) | 2 (2%) | 69 (76%) | 13 (14%) | 9 (10%) | |||
| Jan–Feb 2010 | 115 | 90 (78%) | 13 (11%) | 12 (10%) | 82 (71%) | 17 (15%) | 16 (14%) | |||
| Mar–Apr 2010 | 50 | 37 (74%) | 9 (18%) | 4 (8%) | 37 (74%) | 8 (16%) | 5 (10%) | |||
| Hish-risk medical condition* | No | 181 | 151 (83%) | 20 (11%) | 10 (6%) | 0.749 | 135 (75%) | 25 (14%) | 21 (12%) | 0.348 |
| Yes | 116 | 93 (80%) | 15 (13%) | 8 (7%) | 79 (68%) | 17 (15%) | 20 (17%) | |||
| Insurance | Private | 44 | 38 (86%) | 4 (9%) | 2 (5%) | 0.784 | 31 (70%) | 5 (11%) | 8 (18%) | 0.646 |
| Public | 243 | 196 (81%) | 31 (13%) | 16 (7%) | 174 (72%) | 37 (15%) | 32 (13%) | |||
| None | 10 | 10 (100%) | 0 (0%) | 0 (0%) | 9 (90%) | 0 (0%) | 1 (10%) | |||
High-risk medical conditions include cancer, diabetes mellitus, heart disease, immune deficiency, kidney disease, liver disease, sickle cell disease, seizure disorder, cognitive impairment or developmental delay, asthma, cystic fibrosis, and chronic lung disease.
Note: Statistically significant results as determined by Fisher’s exact tests are highlighted with gray background.
Because partial vaccination is not possible for the oldest age group, we compared no vaccination to partial or full vaccination for age group only using the Fisher’s exact tests.
For the 297 study children, we received influenza vaccination status information from medical home practices for 268 (90%) children, obtained registry data for 290 (98%) children, and obtained information from both sources for 261 (88%) children. The majority of children were followed in pediatric practices (76%), 18% in family practices, and 6% at a health department.
Of 297 families interviewed, 140 (47%) reported that their child was vaccinated--128 (43%) for seasonal vaccine and 63 (21%) for monovalent H1N1 vaccine. A total of 8 and 11 parents respectively reported that the status of the monovalent H1N1 vaccine and seasonal influenza vaccine for their child was uncertain at the time of interview. One parent of a teenager reported that their child received seasonal influenza vaccine at a pharmacy, which we were unable to confirm. Five parents reported that their child received one or both influenza vaccines at school, all of which were confirmed by the practice and/or the registry.
According to the medical record and the North Carolina Immunization Registry, 156 children (53%) had received at least one dose of either influenza vaccine by April 2010--120 (40%) for seasonal vaccine and 53 (18%) for monovalent H1N1 vaccine. As shown in Tables 1 and 2, more children had received seasonal influenza vaccine than H1N1 influenza vaccine both at the time of enrollment (40% versus 18%, p<0.001) and by the end of April 2010 (46% versus 28%, p<0.001). Younger children were more likely to be vaccinated with either vaccine than older children. More Hispanic children received at least one dose of either vaccine than black or white children, but this difference was statistically significant only for monovalent H1N1 vaccine. The proportion of children who had received either vaccine at enrollment steadily increased from September–October 2009 through March–April 2010, but this difference was statistically significant only for monovalent H1N1 vaccine. More children enrolled in the inpatient setting had received seasonal influenza vaccine than those enrolled in the emergency department.
Many children 6 months-<9 years of age for seasonal vaccine and 6 months -<10 for monovalent H1N1 vaccine who received at least one dose of either vaccine did not meet criteria for complete vaccination. The overall proportion of children <9 years of age with confirmed seasonal influenza vaccinations who were fully vaccinated was 44% (47/107) at enrollment and 52% (62/119) by April 2010. Of previously unvaccinated children <9 years who received 2009–2010 seasonal influenza vaccine, the proportion of children who received both doses of vaccine were 25% (18/72) at enrollment and 33% (26/79) by April. Similarly, the proportions of fully vaccinated children <10 years of age among those who received any monovalent H1N1 vaccine and were recommended to receive two doses were 31% (16/51) at enrollment and 45% (34/76) by April.
For seasonal and monovalent H1N1 influenza vaccines, there were 286 and 289 study children, respectively, for whom immunization data was available from both parental report and medical record confirmation. Parents of 128 children reported that their child had received the 2009–2010 seasonal influenza vaccine, and 104 (81%) of these children had that vaccination confirmed by medical record review. For an additional 9 (3%) of 286 study children, parents reported that the child had not received the seasonal influenza vaccine, but that was contradicted by medical record review. Parents of 63 children reported that their child had received monovalent H1N1 vaccine, and 43 (68%) of these children had that vaccination confirmed by medical record review. For an additional 6 (2%) of these 289 study children, parents reported that the child had not received the monovalent H1N1 vaccine, but that was contradicted by medical record review. The sensitivity, specificity, positive predictive value, negative predictive value, and kappa statistic were similar for parental report of any seasonal influenza vaccination and of any monovalent H1N1 influenza vaccine (Table 3).
Table 3.
Comparison of the 2009–2010 seasonal influenza vaccine by parental report and by confirmation from provider or North Carolina immunization registry at time of enrollment.*
| Parental Report | Confirmed Vaccination Status | Sensitivity | Specificity | PPV† | NPV†† | Kappa | |
|---|---|---|---|---|---|---|---|
| None | At least 1 dose | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | K (95% CI) | |
| 2009–2010 Seasonal Influenza Vaccine | |||||||
| None | 149 | 9 | 92% (85%–96%) | 86% (80%–91%) | 75% (67%–82%) | 94% (89%–97%) | 0.76 (0.69–0.84) |
| At least 1 dose | 24 | 104 | |||||
| 2009–2010 Monovalent H1N1 Vaccine | |||||||
| None | 220 | 6 | 88% (75%–95%) | 92% (87%–95%) | 68% (55%–79%) | 97% (94%–99%) | 0.71 (0.61–0.82) |
| At least 1 dose | 20 | 43 | |||||
All vaccinations reported at school were confirmed by the provider or the registry.
PPV is the positive predictive value.
NPV is the negative predictive value.
Secondary analysis revealed that for both vaccines, the results were similar across age groups and for children with and without high-risk medical conditions with overlapping 95% confidence intervals (data not shown). Evaluation of demographic variables as predictors of agreement between parental report and confirmed influenza vaccination status yielded no predictors (all p>0.05).
Discussion
This study demonstrates that parental report for any seasonal influenza vaccine and for any monovalent H1N1 vaccine was sensitive and specific and demonstrated agreement with medical records maintained at a child’s medical home and the North Carolina Immunization Registry. Confirming vaccination status by medical record review is more accurate. However, this study suggests that parental report is quite accurate even in a year with two recommended influenza vaccines.
Our results are comparable to two pediatric studies performed since universal influenza vaccination has been recommended for young children.10,11 A study of a predominantly rural Midwestern population of children 6–59 months from 2006 through 2008 reported overlapping 95% CI for the sensitivity and specificity of parental report and had a computed Kappa of 0.84.11 Our previous cross-sectional study of children 6–59 months of age during the 2004–2005 influenza season reported 95% confidence intervals for the sensitivity, specificity and kappa statistic of parental report in 2004–2005. We found that the statistical results for both 2009–2010 influenza vaccines overlap with these earlier findings.10
Two pediatric studies report overlapping estimates for the sensitivity of parental report for influenza vaccine, but lower estimates of the specificity and kappa statistic of parental vaccination report. These studies were performed in urban, low-income children before universal influenza vaccine was recommended for all children 6–23 months of age. Their final data analyses was based on approximately one-quarter of the targeted study population because of a low response rate and limited availability of medical records.12,13
Overall, we found that 47% of children 6 months-<18 years had received at least one dose of either influenza vaccine in 2009–2010, and 43% of children had received at least one dose of seasonal influenza vaccine. This result is slightly lower than the national estimate that reported 55% of children 6 months-<18 years had received at least one dose of either influenza vaccine and 44% of children had received at least one dose of seasonal influenza vaccine.14
We also found that more children in each age group received seasonal than monovalent H1N1 influenza vaccine in 2009–2010, and the proportion vaccinated increased from September–October through March–April. Given that the 2009–2010 influenza season in the United States was almost exclusively H1N1 influenza, these results highlighted the need to enhance vaccine coverage for all recommended influenza vaccines each year. For children who had received at least one dose of either seasonal or monovalent H1N1 influenza vaccine and were recommended to receive two doses, partial vaccination was common. This observation is clinically important because partial vaccination with seasonal and monovalent H1N1 influenza provides inadequate protection against influenza disease.15,16 Providing complete influenza vaccination coverage for all children within a practice is logistically challenging.17 Strategies that have been reported to be effective in children include recall-reminders, year-round scheduling for influenza vaccine visits, scheduling practice visits for October through January and influenza vaccination clinics in the evening or over the weekend.18–22
This study has several limitations. There could be systematic differences between enrolled and non-enrolled children. Children were systematically enrolled during day shifts in the emergency departments and the children’s hospital in one urban county in North Carolina in 2009–2010. Even though the demographic characteristics of children seen in the emergency department are similar across shifts, the vaccination coverage could have varied. Hence, the generalizability of the results to children in other settings, years and other regions is not known. Nonetheless these results are internally consistent with our previous study performed in an outpatient clinic five years ago, and overall vaccine coverage is consistent with a national estimate.10,14 It is possible that we failed to confirm influenza vaccines for children that were vaccinated at a location such as school, hospital or urgent care clinic if they did not report that location to us and that vaccination was not reported to their physician or the North Carolina Immunization Registry. If we failed to confirm vaccines that were reported and received, then we would have underestimated the accuracy of parental report.
In conclusion, parental report of both pediatric seasonal and monovalent H1N1 influenza vaccines in 2009–2010 was sensitive and specific and a reasonably accurate information source when medical record review of influenza vaccination status is impractical. Only 47% of children received any influenza vaccine by April 2010 and many vaccinated children who were recommended to receive two doses of that vaccine were only partially vaccinated. This finding highlights the need to improve influenza vaccine coverage in children.
Acknowledgments
We thank all the patients and their families who graciously participated in this study as well as the physicians and staff in the emergency department and inpatient settings at Forsyth Medical Center and Brenner Children’s Hospital at Wake Forest Baptist Health who made this study possible. We thank Kristin Simms as well as the members of the Novant Clinical Research Institute who facilitated the process or enrolled patients during the 2009–2010 influenza season: Brian Coleman, James Hobbs, Wendy Hobbs, Eugenia Hutchinson, Debra Norwood, Keishia Rodriguez, Robert Romanchuk and Yvonne Whitley. We also thank the anonymous reviewers whose suggestions significantly improved this manuscript.
Footnotes
Disclosure: This study was supported in part by the National Institute of Allergy and Infectious Diseases (K23 AI065805 and R01 AI79226) and by the Wachovia Research Fund. The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the U.S. government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Influenza: Recommendations for Immunization. Recommendation of the U.S. Public Health Service Advisory. Committee on Immunization Practices. Influenza Vaccine--Civilian Use--1966–67. Ann Intern Med. 1966;65:797–799. [PubMed] [Google Scholar]
- 2.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
- 3.Update on influenza A (H1N1) 2009 monovalent vaccines. MMWR Morb Mortal Wkly Rep. 2009;58:1100–1101. [PubMed] [Google Scholar]
- 4.Vaccine against 2009 H1N1 Influenza Vaccine. Available at: http://www.cdc.gov/h1n1flu/vaccination/public/vaccination_qa_pub.htm.
- 5.Santoli JM, Szilagyi PG, Rodewald LE. Barriers to immunization and missed opportunities. Pediatr Ann. 1998;27:366–374. doi: 10.3928/0090-4481-19980601-11. [DOI] [PubMed] [Google Scholar]
- 6.NCIR: North Carolina Immunization Registry. Up-to-date progress of statewide rollout! Available at: http://www.immunizenc.com/NewImmRegistry.htm.
- 7.Influenza A (H1N1) 2009 Monovalent Vaccine and Reimbursement Guidelines for 2009/2010. Available at: www.ncha.org/public/docs/MedicaidbillingH1N1vaccine.pdf.
- 8.Seasonal Influenza Vaccine and Reimbursement Guidelines for 2009/2010. Available at: http://www.ncdhhs.gov/dma/bulletin/1009bulletin.htm#flu.
- 9.Effective October 9, 2009, pharmacists will have temporary authority to administer seasonal and H1N1 flu vaccine to patients age 14 and older. Posted by North Carolina Board of Pharmacy at: http://www.ncbop.org/H1N1info2009.htm.
- 10.Shinall MC, Jr, Plosa EJ, Poehling KA. Validity of parental report of influenza vaccination in children 6 to 59 months of age. Pediatrics. 2007;120:e783–e787. doi: 10.1542/peds.2007-0052. [DOI] [PubMed] [Google Scholar]
- 11.Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine. 2009;27:6546–6549. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Lin CJ, Zimmerman RK, Nowalk MP, et al. Parental perspectives on influenza vaccination of children with chronic medical conditions. J Natl Med Assoc. 2006;98:148–153. [PMC free article] [PubMed] [Google Scholar]
- 13.Nowalk MP, Zimmerman RK, Lin CJ, et al. Parental perspectives on influenza immunization of children aged 6 to 23 months. Am J Prev Med. 2005;29:210–214. doi: 10.1016/j.amepre.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Setse RW, Euler GL, Gonzalez-Feliciano AG, et al. Influenza Vaccination Coverage--United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2011;60:38–41. [PubMed] [Google Scholar]
- 15.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg KW, Szilagyi PG, Fairbrother G, et al. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008;122:911–919. doi: 10.1542/peds.2007-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szilagyi PG, Iwane MK, Humiston SE, et al. Time spent by primary care practices on pediatric influenza vaccination visits: implications for universal influenza vaccination. Arch Pediatr Adolesc Med. 2003;157:191–195. doi: 10.1001/archpedi.157.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Kempe A, Daley MF, Barrow J, et al. Implementation of universal influenza immunization recommendations for healthy young children: results of a randomized, controlled trial with registry-based recall. Pediatrics. 2005;115:146–154. doi: 10.1542/peds.2004-1804. [DOI] [PubMed] [Google Scholar]
- 19.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: A review. JAMA. 2000;284:1820–1827. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- 20.Gaglani M, Riggs M, Kamenicky C, Glezen WP. A computerized reminder strategy is effective for annual influenza immunization of children with asthma or reactive airway disease. Pediatr Infect Dis J. 2001;20:1155–1160. doi: 10.1097/00006454-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Paul IM, Eleoff SB, Shaffer ML, Bucher RM, Moyer KM, Gusic ME. Improving influenza vaccination rates for children through year-round scheduling. Ambul Pediatr. 2006;6:230–234. doi: 10.1016/j.ambp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Poehling KA, Fairbrother G, Zhu Y, et al. Practice and child characteristics associated with influenza vaccine uptake in young children. Pediatrics. 2010;126:665–673. doi: 10.1542/peds.2009-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]