Abstract
During conversation, interactants draw on their shared communicative context and history (“common ground”) to help decide what to say next, tailoring utterances based on their knowledge of what the listener knows. The use of common ground draws on an understanding of the thoughts and feelings of others to create and update a model of what is known by the other person, employing cognitive processes such as theory of mind. We tested the hypothesis that the ventromedial prefrontal cortex (vmPFC), a neural region involved in processing and interpreting social and emotional information, would be critical for the development and use of common ground. We studied seven patients with bilateral vmPFC damage and seven age-, sex-, and education-matched healthy comparison participants, each interacting with a familiar partner. Across 24 trials, participants verbally directed their partners how to arrange a set of 12 abstract tangram cards. Our hypothesis was not supported: the vmPFC and healthy comparison groups showed similar development and use of common ground, evident in reduction in time and words used to describe the cards, similar increases in the use of definite references (e.g., the horse), and comparable use of verbal play (playful language) in their interactions. These results argue against the idea that the vmPFC is critical for the development and use of common ground in social interaction. We propose that a cognitive and neuroanatomical bifurcation in theory of mind processes may explain this outcome. The vmPFC may be important for affective theory of mind (the ability to understand another’s feelings); however, the development and use of common ground in social interaction may place higher demands on the ability to understand another’s knowledge, or cognitive theory of mind, which may not require the vmPFC.
Keywords: ventromedial prefrontal cortex, common ground, discourse, collaborative referencing
Introduction
Conversation, the cornerstone of social interaction, requires the orchestration of numerous cognitive abilities. For example, as an interaction unfolds, individuals must update and integrate new information and place utterances into the larger communicative context and the shared histories of the interactants (Body, 2007; Clark, 1992). Speakers draw on this shared communicative context and history to decide what to say next, tailoring utterances based on their knowledge of what the listener knows (Clark & Murphy, 1982; Horton, 2007; although see Keysar, 2007). For example, the successful use of a definite reference (e.g., the car) depends on speaker and listeners jointly believing that the referent is part of their shared communicative history or is identifiable from the shared context/environment (Clark & Marshall, 1978). This knowledge of shared information is referred to as common ground (Clark, 1992; Clark & Marshall, 1978).
Decades of research have been devoted towards documenting and understanding the role of common ground in conversation. To study how conversational partners develop and use common ground, many investigators have used an experimental setup that involves a collaborative referencing paradigm, where two participants work together across numerous trials to establish names for a set of novel objects, (e.g., Clark & Wilkes-Gibbs, 1986, Krauss & Glucksberg, 1969). A consistent finding is that the development of common ground is displayed as participants create and use unique labels for the objects which simplify and shorten across trials. Participants require less time to complete the task and begin to use definite references (e.g., the camel) to signal their confidence that the referent is part of shared knowledge (Clark & Wilkes-Gibbs, 1986; Wilkes-Gibbs & Clark, 1992; Yule, 1997). The findings from these early studies led to proposals about the cognitive mechanisms and neural substrates that support common ground. The role of declarative memory has been emphasized, as the dominant view has been that participants create, update, and draw on an explicit record of shared events, and on this basis, simplify and shorten labels across trials (Clark, 1992; Clark & Marshall, 1981). Social and emotional cognitive processes, such as theory of mind, have also been implicated in common ground, as interlocutors must be able to understand the thoughts and feelings of one another in order to create and update a “model” of what is known by the other person, and use this information to construct appropriate and effective references (Clark, 1992; Krauss & Fussell, 1996).
An ongoing program of research in our laboratory has been exploring the cognitive mechanisms and neural substrates of common ground, using a collaborative referencing paradigm adapted from Clark and colleagues (Clark, 1992; Clark & Wilkes-Gibbs, 1986) and Hengst (2003). The paradigm requires participants to verbally direct a familiar communication partner how to arrange a set of 12 abstract tangrams across 24 trials while separated by a low barrier, which hides their workspaces and tangrams but allows them to see each other. One line of work has explored the role of declarative memory in building and using common ground. Contrary to predictions from the early theorists, we found that some aspects of common ground do not appear to rely on declarative memory, as patients with severe hippocampal amnesia were able to successfully develop and use unique references that became increasingly concise and simplified across the task (Duff, Hengst, Tranel, & Cohen, 2006). However, the amnesic patients did not consistently mark those referents with a definite reference (e.g., the Viking ship) to signal to their partner that they believed the referent was part of their shared knowledge (Duff, Gupta, Hengst, Tranel, & Cohen, 2011a), suggesting that this aspect of common ground is dependent on declarative memory (Duff et al., 2011a; also see Horton & Gerrig, 2005). Another line of work has begun to explore the contributions of social and emotional systems to common ground. For example, we tested a patient (known as “SM”) with bilateral amygdala damage and found that the patient was impaired at developing common ground with a communication partner (Gupta, Duff & Tranel, 2011a). SM displayed an impaired rate of reduction in the amount of time required to complete each trial, and across trials, did not seem to use the common ground shared with her partner to create efficient and effective descriptions, as she did not show the typical decrease in the number of words used in the initial description of the cards across trials. These findings suggested that the amygdala is a critical component of the neural network involved in common ground, which fits with the more general hypothesis that social and emotional cognitive processes, such as theory of mind, may be important for the development of common ground—e.g., in order to understand and utilize what is known by the other person to create efficient and effective references.
In the current study, we extend our line of work in the social/emotional realm to test the hypothesis that the ventromedial prefrontal cortex (vmPFC), a neural region that is known to be involved in processing and interpreting social and emotional information, would be critical for learning to develop and take advantage of common ground in social interaction. Before describing the study, we briefly review the relevant background for our hypothesis.
The vmPFC has been well established as being critically involved in a variety of social and emotional processes (e.g., Damasio, 1994). Damage to this area impairs social emotions such as empathy and embarrassment, and impairs social decision-making (Beadle & Tranel, in press; Bechara, Damasio, Damasio, & Anderson, 1994; Beer, Heerey, Keltner, Scabini, & Knight, 2003; Eslinger, 1998; Stone, Baron-Cohen, & Knight, 1998; Stuss & Benson, 1984). Research has suggested that the vmPFC is a critical part of a network of structures important for theory of mind, or the ability to understand the mental states of other people, especially when related to another’s emotions and feelings (e.g., Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003; Stone et al., 1998; Stuss, Gallup, & Alexander, 2001; Xi et al., 2010; but see Bird, Castelli, Malik, Frith, & Husain, 2004). Patients with damage to the vmPFC have been reported to have impairments in interpreting and using nonverbal social cues to make judgments about the relationships of others (e.g., intimacy, social status) and to understand emotional expressions (Mah, Arnold, & Grafman, 2004, 2005). Impairments in aspects of communication such as understanding humor, sarcasm and faux pas have also been reported in patients with vmPFC damage (Shamay-Tsoory, Tomer, & Aharon-Peretz, 2005; Shammi & Stuss, 1999; Stone et al., 1998). Functional imaging studies have corroborated many of these findings (e.g., Berthoz, Armony, Blair, & Dolan, 2002; Decety, Jackson, Sommerville, Chaminade, & Meltzoff, 2004; Hynes, Baird, & Grafton, 2006).
Based on this background, we predicted that patients with damage to the vmPFC would have deficits in developing and using common ground with a partner. To test this prediction, we used three standard variables for assessing the development of common ground in collaborative referencing paradigms: 1) the rate of reduction in time to complete each trial; 2) the rate of reduction in words in the initial description of the cards across trials; and 3) the use of definite references (i.e., “the” dog vs. ”a” dog) in the descriptions of the cards. Furthermore, one of the benefits of using a social communication paradigm is that it allows an analysis of the interaction above and beyond basic task performance. For example, the quality of vmPFC patient interactions may be changed compared to healthy participants’ interactions. To get at this, we examined the interactions for the use of verbal play, the playful manipulation of language to make puns, tell jokes or funny stories, or engage in verbal banter or teasing. In our previous work, we found that healthy participants routinely used verbal play as part of the common ground they developed with their partners (Duff, Hengst, Tranel, & Cohen, 2009).
2. Methods
2.1. Participants
Seven participants (3 females) with bilateral ventromedial prefrontal cortex (vmPFC) damage participated in this study. These participants have been well characterized neuropsychologically and neuroanatomically, and all have well-documented post-morbid changes in emotion processing, decision-making, personality, and/or social and interpersonal functioning (see Table 1; Croft et al., 2010; Koenigs & Tranel, 2008; Young et al., 2010). However, they have relatively intact neuropsychological profiles, including mostly normal performances on standardized tests of speech, language, and visual discrimination (see Table 1 for demographic and neuropsychological details). Participants with a Wechsler Memory Scale General Memory Index (WMS-GMI) less than 70 were excluded (one vmPFC patient was excluded on this basis). As in our previous work (e.g., Barrash, Tranel & Anderson, 2010), the vmPFC was defined as the region that encompasses the medial orbital sector and the lower medial sector of the prefrontal lobes (Brodmann areas medial 11, 25, 12, ventromedial 10, and anteroventral 32). A map depicting the lesion overlap for six of the seven vmPFC participants is provided in Figure 1. Patient 3350 was not included in this map, because the lesion is difficult to transfer reliably into common brain space due to uncertainties about the lesion boundary. This patient has a bilateral vmPFC lesion that includes the mesial orbital cortex and lower mesial prefrontal cortex posteriorly, along with the white matter subjacent to these regions. Seven participants free of neurological or psychiatric conditions and matched pair-wise to each vmPFC participant on sex (3 females), age (mean=58.4±9.2), education (mean=13.8±2.0), and handedness, served as healthy comparison participants.
Table 1.
Demographic and neuropsychological characteristics of the vmPFC participants
| Participant | Sex | Education (Years) | Age (Years) | Chronicity (Years) | WAIS-III FSIQ | WMS-III GMI | BNT | Token Test | COWA | JLO | BDI | Acquired Personality Problems1 | Social and Interpersonal Functioning2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0318 | M | 14 | 69 | 24 | 143 | 123 | 60 | 44 | 54 | 30 | 0 | Yes (3) | 3 |
| 1983 | F | 13 | 46 | 11 | 108 | 74 | 58 | 44 | 51 | 24 | 7 | Yes (3) | 3 |
| 2352 | F | 14 | 60 | 8 | 106 | 109 | 54 | 44 | 34 | 27 | 1 | Yes (3) | 2 |
| 2391 | F | 13 | 63 | 7 | 109 | 132 | 57 | 43 | 59 | 31 | 4 | Yes (2) | 2 |
| 2577 | M | 12 | 69 | 8 | 84 | 96 | 55 | 44 | 44 | 19 | 7 | Yes (3) | 3 |
| 3032 | M | 12 | 52 | 4 | 102 | 108 | 57 | 43 | 32 | 24 | 3 | Yes (1) | 1 |
| 3350 | M | 18 | 57 | 3 | 118 | 108 | 52 | N/A | 40 | 28 | 3 | Yes (1) | 1 |
Note. M=male; F=female; Chronicity=years between lesion onset and the current study; WAIS-III FSIQ=Wechsler Adult Intelligence Scale-III Full Scale Intelligence Quotient; WMS-III GMI=Wechsler Memory Scale-III General Memory Index. BNT=Boston Naming Test; COWA=Controlled Oral Word Association Test; JLO=Judgment of Line Orientation Test; BDI=Beck Depression Inventory; N/A=not available. Impaired scores are bolded.
Acquired Personality Problems refer to whether or not the participant had acquired problems in personality functioning, as derived from data on the Iowa Scales of Personality Change. The numbers in parentheses denote degree of severity, where 1 = mild, 2 = moderate, and 3 = severe.
The extent of post-lesion change or impairment in aspects of social conduct and interpersonal functioning was rated on a three-point scale, with 1 = no change or impairment, 2 = moderate change or impairment, 3 = severe change or impairment.
Characterizations of Acquired Personality Problems and Social and Interpersonal Functioning were rendered by a board-certified clinical neuropsychologist who was blind to the hypothesis of the current study at the time the ratings were performed.
Figure 1.
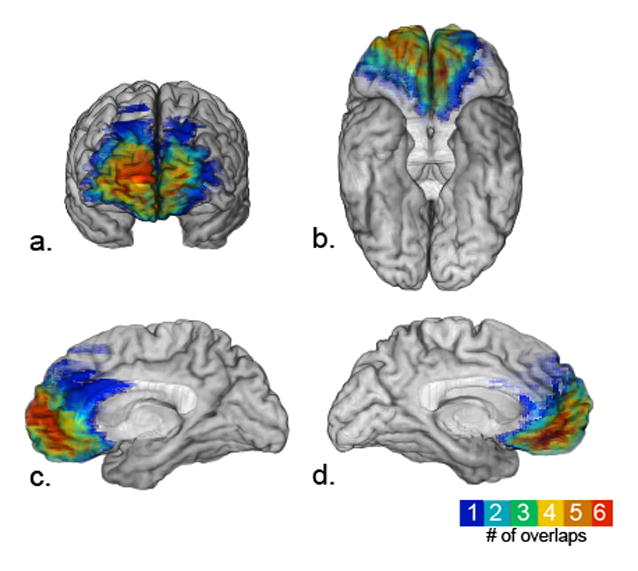
Lesion overlap map of six vmPFC participants. Displayed here are a.) frontal, b.) ventral, c.) right mesial, and d.) left mesial surfaces of the brain.
Each vmPFC and comparison participant completed the collaborative referencing with a familiar communication partner of their choice (e.g., spouse, friend, with at least 5 years of communication history; in our sample communication histories ranged from 10–63 years), free of neurological and psychiatric conditions. Familiar partners for the vmPFC participants included 4 spouses, 1 mother, 1 aunt, and 1 friend; familiar partners for the comparison participants included 5 spouses and 2 friends. The familiar partners for both groups were similar in terms of average age (vmPFC=58.9±14.0; comparison=59.9±10.0; t=0.15; p=0.88) and education (vmPFC=14.4±2.4; comparison=13.4±2.5; t=0.75; p=0.46). All participants gave informed written consent approved by the Institutional Review Board of the University of Iowa.
2.2. Collaborative referencing task procedure
Procedures for the collaborative referencing task followed Duff and colleagues (Duff et al., 2006; Duff et al., 2008). All participant pairs (vmPFC or matched comparisons and their familiar communication partners) performed the collaborative referencing task across four sessions on two consecutive days, with two sessions per day and six trials per session (total of 24 trials). Each session was separated by at least approximately 30 minutes.
During the collaborative referencing task, participants were given identical boards numbered 1–12 and identical sets of 12 cards with abstract Chinese tangrams (see Figure 2 for examples). A low barrier was placed between the pair, which allowed them to see each other’s faces, but hid their boards and tangrams. In order to best capture the perspective and learning of the target participants during the 24 trials, the vmPFC participants were given the role of “director” and their familiar partners were given the role of “matcher.” For every trial, the cards were placed in a predetermined, unique order on the director’s (vmPFC participant’s) board. The director verbally described which card was placed in each numbered spot, so the matcher could fill their numbered spots with the same card, so that at the end of the trial, both boards looked identical. Both participants were allowed to communicate freely, including the use of any facial expressions or gestures, and no restrictions were placed on the matcher’s communication. The participants terminated each trial when they believed they had placed all of the cards correctly. The time to complete each trial was recorded in seconds. Participants were told that the time was not as important as accuracy of the card placements.
Figure 2.

Two examples from the set of 12 tangrams used by the participants during the collaborative referencing task.
2.3. Data analysis
2.3.1. Transcribing the interactions
Sessions were videotaped and transcribed in their entirety including each of the 24 trials, and conversations between trials using a three-stage consensus procedure (see Duff et al., 2008). First, the original transcriber transcribed all utterances, audible sounds, and pause times from the audio portion of the taped interactions. In the second stage, the original transcriber used the videotape of the interactions to add card placements and gestures and make corrections to the audio content of the transcript. Finally, a consensus transcriber and the original transcriber viewed the video together and generated the final version of the transcript.
2.3.2. Coding communicative resources
As a preliminary means of characterizing the data set, we coded the communicative resources (words and interactional turns) used in each of the 24 trials and by both participants in a pair (the director and the matcher) following Duff and collegues (Duff et al., 2006; Duff et al., 2008). Interactional turns were defined as utterances produced by one individual before a change in speaker, and could be verbal, nonverbal (e.g., gesture or head nod alone), or both. When two individuals spoke simultaneously, each speaker’s utterance was counted as a turn.
Words were broadly defined with little emphasis placed on morphological or syntactic form. Consistent with our previous work (see Duff et al., 2008), in order to capture all aspects of the discourse, including verbal effort, each word in a false start was counted (e.g., the tri-triangle facing left= 5 words), fillers (i.e., uh, um) were counted as words (e.g., um it has = 3 words), contractions were counted as one word (e.g., can’t = 1 word), and verbal back-channel or continuer responses (i.e., uh huh, yeah, mhm) were each counted as one word (e.g., uh huh = 1 word).
2.3.3. Initial description word counts
One of the primary dependent variables that we have found to be most sensitive for assessing the ability to develop and use common ground is the initial description word count (Duff et al., 2006; Gupta et al., 2011a). The initial description is defined as a director’s (vmPFC or matched participants) first attempt at describing each of the 12 cards, edited to remove any words that did not directly relate to the referencing of the individual cards such as task management (e.g., the next one is; wait, what number are we on), mazing (e.g., exact repetition, abandoned phrases, false-starts, fillers), and discourse makers (e.g., okay, alright) (for more examples and details see Duff et al., 2006 Supplementary Methods). The remaining words in the initial description were then tallied.
2.3.4. Definite reference coding
The transition from using predominately indefinite references (e.g., looks like a person kneeling) to definite references (e.g., the angle) is an indication of the speaker’s confidence that the reference is part of shared knowledge, or common ground (Clark & Marshall, 1978; Clark & Wilkes-Gibbs, 1986). After identifying each initial description, the use of a definite reference in each description was coded. Using a previously developed coding protocol (Duff et al., 2011a; 2011b) based on Clark & Wilkes-Gibbs (1992) and Hengst (2003), each initial description was coded as an indefinite (e.g., looks like an angel; it’s got a big triangle) or definite referential expression (e.g., the barn; kicker; the dragon reading a book; the one reclining in the chair with his feet stickin’ out). While definite references were typically signaled by the use of definite articles (the) this was not always the case. We have noted that, particularly on later trials, when labels had become increasingly concise and streamlined, it is not uncommon for pairs to omit the article (e.g., windmill instead of the windmill) from definite initial descriptions (Duff et al., 2011a). Thus, in these instances, if the reference would have otherwise been coded as definite (e.g., windmill), they were coded as definite despite the omitted article. Importantly, not all references with omitted articles were coded as definite. Consistent with Clark’s coding scheme (Clark & Wilkes-Gibbs, 1986), initiating references that contained carrier phrases such as “looks like …” or “it has….”, whether or not it contained an article, were coded as indefinite (e.g., looks like somebody praying).
2.3.5. Verbal play
We assessed the use of verbal play to support the development and use of common ground and to assess the quality of the interactions. Following our previous work (Duff et al., 2009), playful episodes were identified and coded using descriptions of verbal play from the literature (e.g., Crystal, 1998; Sherzer, 2002) and a broad definition of verbal play from Hengst (2006). Verbal play was defined as instances of telling funny stories or jokes, playing with sounds or making puns, overt teasing of other or self-deprecating humor, use of marked or playful voices or registers, singing or song-like intonations, and use of sound effects and gestures. Episodes of verbal play were identified through a three-step process (primary coder, secondary coder, consensus) involving repeated viewings of the videotapes, supported by the transcripts, for all sessions across both participants (vmPFC/matched comparison participants and their partners) during the 24 collaborative referencing trials as well as the researcher during the interactions between trials.
An instance of verbal play is termed an episode. An episode can consist of single or multiple utterances sharing a common theme, while playful exchanges that were a series of unrelated episodes or playful exchanges on the same theme but that were temporally disconnected (across trials, sessions, or days), were counted as separate episodes. Each verbal play episode was characterized in terms of its communicative resources, functions, and interactional forms. Three types of resources were coded: verbal, prosodic, and gestural. Verbal resources included playing with sounds and meanings of words (e.g., Eleven’s going to heaven), playful names and nicknames (e.g., puppy fish), and expressions (e.g., M: Something’s not right here. D: Somethin’ fishy in Denmark?). Prosodic resources included sound effects (e.g., Like a cheerleader going ‘Ta Da!’) and singing (e.g., Movin’ on up, to the east side) as well as marked shifts in voicing and exaggerated prosody (e.g., Hey now, I’m the boss [mock angry voice]). Gestural resources included gestures that contributed significant meaning to episodes (e.g., dropped jaw in disbelief, skeptical raised eyebrows). Episodes could have multiple resources.
Each episode was categorized as having one of four communicative functions: narrative, teasing, referencing, and other. Narrative functions captured jokes and funny stories of everyday events (e.g., My husband actually asked me if we can get married on my birthday so he’d only have to remember the one date [laugh]). Teasing functions included competitive teasing, scolding, bragging, and self-deprecating comments (e.g., Aren’t you enjoying these cards?; Now if only I had X-Ray vision.) Referencing functions included playing with the sounds, words, and meanings of labels (e.g., The urineator. We could make a movie, like The Urineator.). The other functions documented playful episodes not captured by the above categories.
To examine the interactional form of playful episodes, two production forms were coded: simple and extended. Simple episodes were short, spanning just one to three contiguous turns in the form of either single-utterance episodes, or episodes consisting of a playful utterance and a response by one or more interlocutors. Extended episodes consisted of multiple, more than three, thematically related and contiguous utterances. Extended episodes included participants telling funny stories about everyday events and extended playful conversational exchanges and banter.
2.3.6. Reliability of coding
Reliability ratings were obtained for approximately 12% of the data (three trials randomly selected per pair). For the total word count, inter- and intra-rater reliabilities were 98% and 97%, respectively. For interactional turns, inter- and intra-rater reliabilities were 97% and 99%, respectively. For the initial description word count, inter- and intra-rater reliabilities were 97% and 98%, respectively. For the definite referencing coding, inter- and intra-rater reliabilities were 95% and 96% respectively. Finally, for the verbal play coding, inter-and intra-rater reliabilities respectively were 88%, and 85% for resource coding and 90% and 92% for function coding.
2.3.7. Statistical analyses
The three main dependent variables used to assess the development and use of common ground were the rate of reduction in time, the rate of reduction in words in the initial description of the cards, and the use of definite references across trials. For the first two of these, the rate of reduction in the measure is the critical index of learning; hence, the slopes of the rate of reduction will be compared using one-tailed t-tests (to test our hypothesis that vmPFC pairs will be impaired compared to normal comparison pairs). Effect sizes (Pearson’s r correlation) and 95% confidence intervals (CI) are reported. For the third measure (use of definite references), a repeated measures ANOVA will be used to assess changes across trials. As these three dependent measures are related, statistical tests will be corrected for multiple comparisons using a Bonferroni correction. Finally, for the verbal play analysis, since the resource and function coding categories are not independent of one another, nonparametric tests will be used to compare the proportion of verbal play episodes produced in each of the categories and the contrasts will be corrected for multiple comparisons using a Bonferroni correction.
3. Results
3.1. General characterization of performance
Before analyzing the dependent variables regarding the development and use of common ground, we characterized the interactions in broad terms by calculating the total number of words and turns, along with the accuracy of card placements.
3.1.1. Total words
Across the entire data set a total of 98,056 words were coded (vmPFC pairs = 57,463 words; comparison pairs = 40,593). The vmPFC pairs tended to produce more words (for vmPFC pairs, M=342.0±136.9 words; for comparison pairs, M=241.63±118.0 words), but the group means were not significantly different (t=1.4; p=0.16). Furthermore, we found that the partners of the vmPFC participants produced a similar proportion of words as the partners of the comparison participants. In vmPFC sessions, the partners produced 28.7% of words in the task, and comparison partners produced 27.8% of words in the task. This suggests that the vmPFC partners were not contributing disproportionately to the task, in terms of the number of words contributed in the sessions.
3.1.2. Turns
Across the entire data set (for all participants) a total of 14,763 interactional turns were coded (vmPFC pairs = 8,474 interaction turns; comparison pairs = 6,289). vmPFC pairs tended to require more turns across all trials, producing on average 50.4±15.2 turns, while comparison participants produced 37.4±7.1 turns, however this did not reach significance (t=2.0; p=0.06).
3.1.3. Accuracy
A common finding in this task is that participants tend to have high accuracy at placing the cards, reaching ceiling within the first several trials (e.g., Duff et al., 2006; Hupet & Chantraine, 1992). Participants in the current study were no different: across all trials, both groups performed similarly in terms of card placement accuracy, with vmPFC and comparison pairs respectively placing on average 11.8 and 11.8 of the 12 cards correctly (t=0.58; p=0.57) and both groups reached ceiling by Trial 2 or 3.
3.2. Learning and development of common ground
3.2.1. Time to completion
Across trials, both vmPFC pairs and comparison pairs showed similar decreases in the amount of time required to complete each trial (Figure 3). vmPFC pairs took on average 8:08±2:52 (min:sec) to complete Trial 1 and comparison pairs took 12:17±6:47 (min:sec). On the final trial, vmPFC pairs took on average 1:01±0:31 (min:sec) and comparison pairs took on average 0:43±0:22 (min:sec). Overall the slopes of the groups’ rate of reduction in time across all 24 trials did not differ significantly (vmPFC mean slope=−12.1; comparison mean slope=−10.9; Bonferroni corrected alpha=0.0125; t=0.76, p=0.23; 95% CI [−4.7, 7.1]; r=0.13). Given the tendency for the time-to-completion variable to asymptote by around Trial 6 (especially in the comparison participants), we followed up with a test of the groups’ rate of reduction during Trials 1–6. Although the comparison group had a faster mean rate of reduction in time during across these trials (comparison mean slope=−104.5; vmPFC mean slope=−59.5), this did not reach significance (Bonferroni corrected alpha=0.0125; t=2.1; p=0.03; 95% CI [−4.2, 98.0]; r=0.60); also, the comparison group had an unusually long time to completion on Trial 1, which contributed to this finding.
Figure 3.
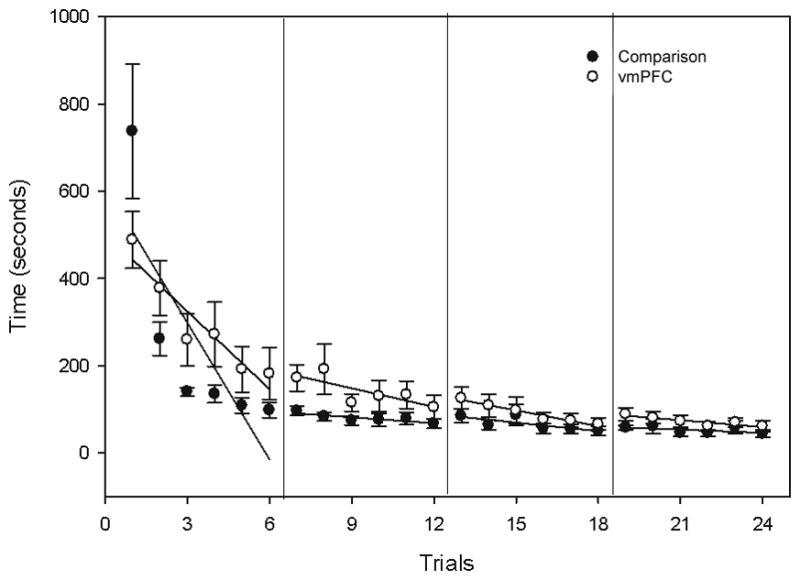
Across trials, participants with vmPFC damage and comparison participants display similar reductions in time required to complete each trial (shown with session-by-session linear trends). Error bars represent S.E.M.
3.2.2. Initial description word count
Both comparison pairs and vmPFC pairs displayed decreases in the number of words in the initial description of the card across trials (Figure 4). Comparison participants produced an average of 173.9±36.5 words on Trial 1, and 56.3±41.5 words on Trial 24. Similarly, vmPFC participants produced an average of 258.8±76.3 words on Trial 1 and 55.7±20.1 words on Trial 24. Overall the slopes of the groups’ rate of reduction in words did not differ significantly (comparison mean slope=−4.1; vmPFC mean slope=−5.9; Bonferroni corrected alpha=0.0125; t=2.1 p=0.03; 95% CI [−0.03, 3.8]; r=0.53). Participants in both groups developed unique labels for the cards that became increasingly concise across trials. For example, on Trial 1, a comparison participant described the tangram pictured on the right in Figure 2 as: “An alien lookin’ guy looks like he might be reading a book and his hands are really low like just barely above his feet … and he is holding on to a square shape.” This became simply “the reader” by the final trial. Similarly, a vmPFC participant described the same card on Trial 1 as: “A person reading a book, there’s a square in his hand. Looks like he’s standing up, he’s got a triangle at the top of his head … looks like he’s got two feet at the bottom.” This became simply “guy readin’ the book” on the final trial.
Figure 4.
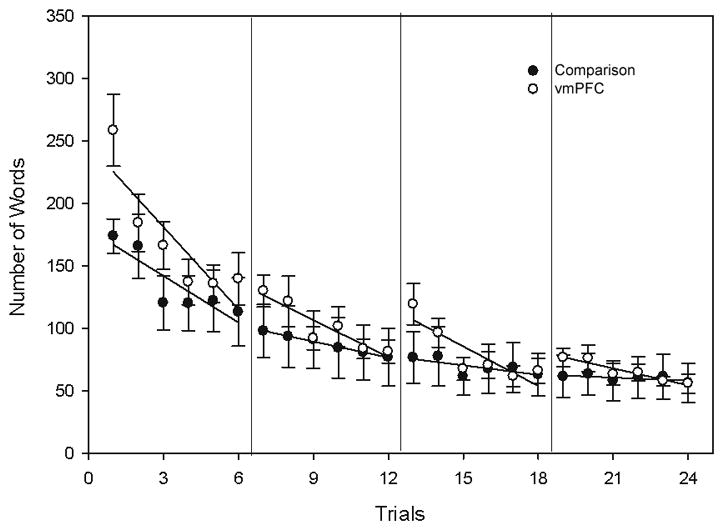
Across trials, participants with vmPFC damage and comparison participants both showed similar decreases in the total number of words used in the initial description of the cards, and the rates of reduction were not significantly different (shown with session-by-session linear trends). Error bars represent S.E.M.
3.2.3. Use of definite references
Across all trials, vmPFC participants produced a total of 2,009 card descriptions and 1,735 of those descriptions were definite references (86.4%). Comparison participants produced a total of 2,011 card descriptions and 1,704 of those were definite references (84.7%). Across sessions, both vmPFC pairs and comparison pairs steadily increased their production of definite references (Figure 5). A repeated measures ANOVA showed a significant effect of trials (F(6.1, 74.2)=31.3 p<.0001; Huynh-Feldt corrected) but no effect of group (F(1, 12)=0.04 p=.83) and no significant interaction between group and trials (F(6.1, 74.2)=0.75 p=.61; Huynh-Feldt corrected).
Figure 5.
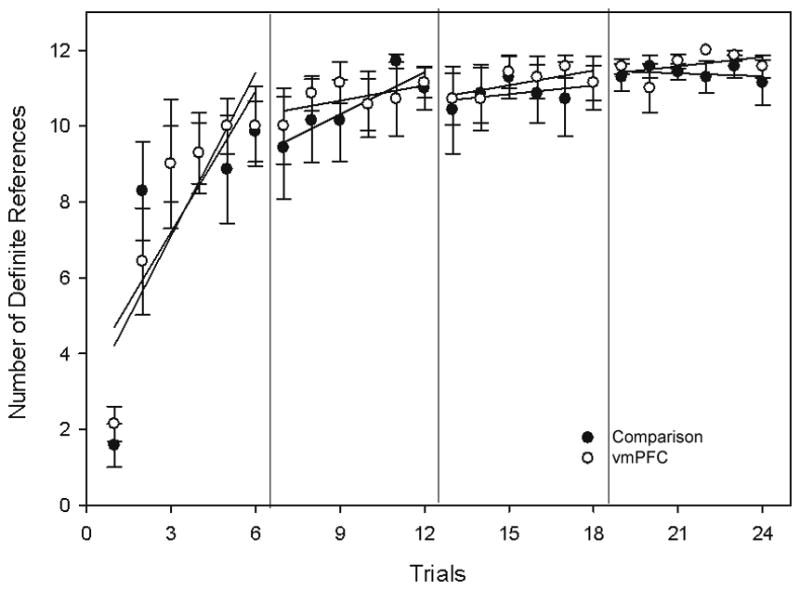
Comparison and vmPFC pairs display similar increases in the average number of definite references used to describe the cards across trials. Error bars represent S.E.M.
3.3. Verbal play
Across the entire data set 2,047 episodes of verbal play were identified, with 1,132 produced in the vmPFC interactions, and 915 produced in the comparison interactions. Looking at just those episodes produced by the directors, there were no significant differences as vmPFC participants produced 787 (69.5%) and comparison participants produced 536 (58.6%) of the total episodes (t=1.8, p=0.09; see Figure 6). In all sessions, participants in both groups used a full range of verbal, prosodic and gestural resources in the production of verbal play. Using a two-tailed Wilcoxon matched pairs signed rank test and a Bonferroni correction for multiple comparisons (alpha of .016), the distribution of episodes did not differ for vmPFC and comparison interactions: using one resource (verbal) the vmPFC and comparison group means were 25.8% and 42.5% (Z=1.85; p=0.06); using two resources, the group means were 39.9% and 32.1% (Z=1.84; p=0.06); and using all three resources (verbal, prosody, gesture) the group means were 34.3% and 25.4% (Z=1.01; p=0.31), respectively. The interactions were also similar in terms of the communicative functions of the verbal play episodes. Again, using a two-tailed Wilcoxon matched pairs signed rank test and a Bonferroni correction for multiple comparisons (alpha of .0125), vmPFC and comparison pairs produced 40.2% and 39.6% episodes coded as referencing (Z=0.17; p=0.86); 43.0% and 40.5% coded as teasing (Z=0.84; p=0.39); 3.4% and 6.0% coded as narrative (Z=0.51; p=0.61); and 13.4% and 13.9% coded as other (Z=1.13, p=0.18), respectively. Finally, the interactional form of the verbal play episodes in the interactions were very similar as 79.7% and 80.6% were coded as simple, and 20.3% and 19.4% were coded as extended for vmPFC and comparison interactions, respectively (t=0.99, p=0.34).
Figure 6.
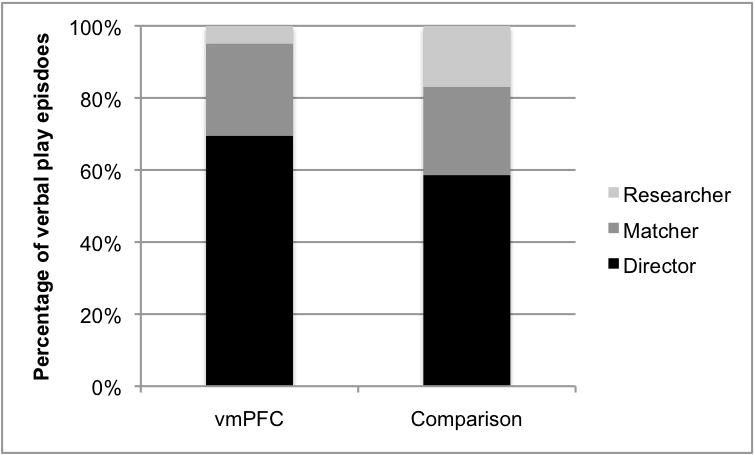
Percentage of verbal play episodes produced by group and participant.
4. Discussion
In this study, we sought to further understand the neural systems involved in the development of common ground in social interaction. Based on previous research implicating social and emotional cognitive and neural systems (e.g., Krauss & Fussell, 1996; Gupta et al., 2011a), we tested the hypothesis that the ventromedial prefrontal cortex (vmPFC) would be required for the acquisition and use of common ground in social interaction. Contrary to our hypothesis, participants with bilateral vmPFC damage were able to successfully develop common ground with a familiar partner, as they consistently used unique and precise labels to describe the cards that became more concise and simplified across time. This was reflected in the key measures of “common ground,” as across trials, vmPFC pairs showed significant reductions in both the amount of time required to complete the task and the number of words required to describe the cards, as well as increases the use of definite references (e.g., the angel) (Clark, 1992). Moreover, the performances of the vmPFC pairs were entirely similar to those of the healthy participant pairs. These results are striking in contrast to our previous findings. We have documented impairments in certain aspects of common ground in patients with bilateral hippocampal damage (e.g., the use of definite references, Duff et al., 2011a), and bilateral amygdala damage (rate of reduction in time, initial description word count, Gupta et al., 2011a). The current results suggest that the vmPFC is not critical for the development and use of common ground in social interaction, at least as measured in our task.
These results are also surprising given our previous work on the role of the amygdala in common ground and the functional and anatomical connections between the vmPFC and amygdala. In previous work, we found disruptions in the development and use of common ground in a patient with bilateral amygdala damage demonstrated by an impaired rate of reduction in time and the number of words used in the initial description of the task (Gupta et al., 2011a). In fact, the amygdala patient showed very little reduction in the words used to describe the cards across trials (18% reduction in words from Trial 1 to Trial 24). In contrast, in the current study, patients with bilateral vmPFC damage were able to develop and use common ground, and showed reductions in the number of words used to describe the cards that were similar to those in healthy participants (from Trial 1 to Trial 24; vmPFC=78.5% reduction in words; comparison=67.7% reduction in words). While the vmPFC and amygdala are part of a neural network involved in many of the same social and emotional processes (e.g., decision-making, moral updating; empathy; reward processing; Bechara, Damasio, Damasio, & Lee, 1999; Croft et al., 2009; Croft et al., 2010; Decety, 2011; Gottfried, O’Doherty, & Dolan, 2003; Gupta, Koscik, Bechara, & Tranel, 2011c), there are other processes for which each region makes distinct contributions. We (Gupta et al., 2011a) speculated that impairments in certain basic social processes dependent on the amygdala, including recognition of socially salient information, such as directing gaze to the face and understanding facial expressions representing complex mental states (e.g., confusion), may have contributed to SM’s impairments in developing common ground, however, the involvement of the vmPFC in these processes is less well established. The current study better clarifies to the unique contributions of the neural systems involved in common ground and different aspects of social interaction. Furthermore, the age of the patients at onset of lesion may contribute to our findings. SM’s lesion is presumed to have occurred during adolescence or earlier (Adolphs & Tranel, 2000), while all of the vmPFC participants have adult-onset lesions. Similar to the amygdala (e.g., Shaw et al., 2004), developmental lesions to the vmPFC tend to be more deleterious to social and emotional functioning than those that occur during adulthood (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999). This raises the interesting question as to whether developmental vmPFC lesions may disrupt the development and use of common ground—we predict that they would, and are currently beginning to test this hypothesis.
Importantly, the development of common ground is only one aspect of conversation and social interaction. Our analyses of the number of turns and rate of reduction in time did not yield significant group differences, although there were trends towards significance. But even assuming that such trends may be reflecting some (general) disruption in the interactions of the vmPFC pairs, we find it striking that the vmPFC patients were successful at developing common ground across all three dependent variables—thus, potential interactional disruptions do not seem to affect the patients’ overall ability to create and consistently use unique and precise labels that become shorter over time. However, it seems entirely possible that while the vmPFC participants were successful at the task and at developing common ground, the vmPFC pairs may require more collaborative effort and negotiation (e.g., more turns, more time) in order to complete the task, hinting at a more general interactional disruption.
We did attempt to capture other aspects of the interaction beyond task performance, e.g., by measuring “verbal play” or the use of playful language. However, we found no differences between the vmPFC and comparison group in the production or use of verbal play. The lack of group differences on this measure is interesting, as the research assistants who were blind to the aims of the study and participant groups often remarked that individual vmPFC participants tended to have odd, awkward and qualitatively less engaging interactions. This highlights an important distinction between successful task performance and normal social interaction: the current findings speak to the patients’ intact task performance vis-à-vis the development and use of common ground; however, there are very likely other aspects of social interaction that are affected by vmPFC damage but that are not adequately captured here. Indeed, in an analysis of their conversational discourse, we have found impairments in the ability of these same patients to converge, or become more similar, with their partner on linguistic variables such as length of utterances, disrupting their ability to have a reciprocal interaction (Gupta, Duff, & Tranel, 2011b). Furthermore, the analyses in the current study focused on verbal behavior, but there is obviously much more that occurs during social interaction than verbal interchanges. Indeed, we found that disruptions in nonverbal behaviors, such as the timing of gazes to the partner’s face at critical moments during the interaction, may contribute to deficiencies in this task (Gupta et al., 2011a). Future avenues of research could explore the use of nonverbal behaviors and other subtle effects of vmPFC damage on conversation and social interaction.
In the collaborative referencing task, participants must understand their partners’ perspective and knowledge about the cards in order to create efficient effective references and develop common ground. In fact, without feedback from the partner or in the absence of social interaction, research has shown that there is little to no reduction in the length of the card descriptions (Hupet & Chantraine, 1992). Theory of mind and perspective-taking type processes have been reported to be impaired in patients with damage to the vmPFC, however, there is debate in the literature regarding the exact role of the vmPFC. Theory of mind is traditionally defined as the ability to take another’s perspective and understand their mental state, including their feelings, beliefs, thoughts, and knowledge. However, it has been suggested that there may be two cognitively and neuroanatomically distinct aspects of theory of mind: an affective component and a cognitive component (Shamay-Tsoory, Tomer, Goldsher, Berger, & Aharon-Peretz, 2004). Affective theory of mind involves the ability to understand others’ feelings, while cognitive theory of mind involves the ability to understand another’s beliefs and knowledge. Recent findings from both functional imaging and lesion studies suggest that the vmPFC is important for affective theory of mind, and related constructs such as empathy (Beadle & Tranel, in press; Hynes et al., 2006; Shamay-Tsoory & Aharon-Peretz, 2007; Shamay-Tsoory, Tibi-Elhanany, & Aharon-Peretz, 2006). However, the role of the vmPFC in cognitive theory of mind is less clear and may involve more widespread involvement of the prefrontal cortex (such as dorsomedial prefrontal cortex, a common finding in functional imaging studies) (Gallagher & Frith, 2003; Lieberman, 2010; Shamay-Tsoory & Aharon-Peretz, 2007). While knowledge and understanding of another’s feelings are important components of social interaction, the development and use of common ground, as measured in this study, may place higher demands on the use of cognitive theory of mind to understand their partners’ knowledge about the task and card descriptions. vmPFC participants are able to successfully utilize this information suggesting that they have intact cognitive theory of mind. These results fit with current research suggesting a potential bifurcation of theory of mind processes and preserved cognitive of theory of mind in patients with vmPFC damage.
Although previous reports have noted striking impairments in social and emotional processes in real life and in a number of laboratory tasks, patients with vmPFC damage routinely engage in appropriate and successful social interactions. Their social difficulties are not a constant feature of their interactions and can be subtle. These patients have a wealth of preserved social knowledge, that coupled with normal intelligence and other cognitive abilities, allow them to function normally in many settings a lot of the time (Anderson, Barrash, Bechara, & Tranel, 2006; Saver & Damasio, 1991). When they have social failures, however, these impairments are devastating and are the types of errors that are often seen as relationship deal breakers (e.g., spending life savings on impractical business ventures, seeming emotionally cold or aloof, lacking or not exhibiting empathy for others). The collaborative referencing task used in this study is well structured and with their chosen partner, who in virtually all cases was a partner who has known them since before their brain injury, they know the patient well enough to overlook or compensate, and adjust for any deficiencies in their interactional skills. However, we found that the partners of the vmPFC participants produced a similar proportion of words as the comparison partners, and to address this further, we found that the vmPFC partners contribute similarly to the content of the final description used for the cards across trials, where the vmPFC partners initially suggested 6/84 of the final descriptions, and comparison partners suggested 9/84 of the descriptions, suggesting that the vmPFC partners are not contributing disproportionally to the task. However, we chose to use familiar partners in this work (and in our past work, e.g., Duff et al., 2006) in order to give patients the best possible chance at success, and to better understand how they interact with those people that they communicate with most often. Future research should attempt to characterize the social interactions of vmPFC patients in face-to-face communication without task restraints, such as a natural conversation with an unfamiliar partner, in order to better understand the role of the vmPFC in real world social interaction.
Certain limitations of this study should be noted, including the relatively small sample size. A larger sample of vmPFC participants may reveal subtle differences between the patients and comparison participants on various target dependent variables, e.g., assessing common ground. However, we note that in our data set, across measures, the vast majority (>95%) of the individual vmPFC patient data fell within one standard deviation of the comparison means. Also, future approaches to the operationalization of this construct could result in new ways to measure “common ground,” that may make any deficits in vmPFC patients apparent.
In sum, this study provides important insights into the neural systems involved in the development of common ground, and the intricacies of the role of the vmPFC in social interaction. Their similar performance to comparison participants on virtually all measures of this face-to-face communication task is particularly surprising, in light of the extensive literature suggesting the critical role of the vmPFC for social interaction. Future studies could use less structured laboratory tasks in order to better understand the precise contributions of the vmPFC to communication and social interaction.
Highlights.
Common ground, or mutual knowledge of shared information, employs theory of mind
Ventromedial prefrontal cortex (vmPFC) processes social/emotional information
We hypothesized that vmPFC damage would impair the use of common ground
However, vmPFC and comparisons showed similar development and use of common ground
Common ground places demands on cognitive theory of mind and may not require vmPFC
Acknowledgments
We thank the Duff Communication and Memory Laboratory for assistance with transcribing and coding the sessions. We thank Joel Bruss for creating the lesion overlap maps. The study was supported by NIDA R01 DA022549 and NINDS P50 NS19632 to DT; NIDCD F32DC008825 to MCD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D. Emotion recognition and the human amygdala. In: Aggleton J, editor. The Amygdala. 2. New York: Oxford University Press; 2000. pp. 587–630. [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12 (2):224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18(3):355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Beadle J, Tranel D. Social Neuroscience: A Neuropsychological perspective. In: Cacioppo J, Decety J, editors. Topics in Social Neuroscience. New York: Oxford University Press; in press. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony J, Blair R, Dolan R. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125(1):1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain. 2004;127(4):914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Body R. Decision making and somatic markers in conversation after traumatic brain injury. Aphasiology. 2007;21(3–4):394–408. [Google Scholar]
- Clark HH. Arenas of Language Use. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Clark HH. Using language. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Clark HH, Marshall CR. Reference diaries. In: Waltz David L., editor. Theoretical issues in natural language processing. Vol. 2. Association for Computing Machinery, New York; New York: 1978. pp. 57–63. [Google Scholar]
- Clark HH, Marshall CR. Definite reference and mutual knowledge. In: Joshi AK, Webber B, Sag I, editors. Elements of discourse understanding. Cambridge: Cambridge University Press; 1981. pp. 10–63. [Google Scholar]
- Clark HH, Murphy GL. Audience design in meaning and reference. In: LeNy J-F, Kintsch W, editors. Language and comprehension. Amsterdam: North-Holland: 1982. [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. Cognition. 1986;22(1):1–39. doi: 10.1016/0010-0277(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. In: Clark H, editor. Arenas of language use. Chicago, IL: The University of Chicago Press; 1992. pp. 107–143. [Google Scholar]
- Croft KE, Duff MC, Anderson SW, Adolphs R, Tranel D. Bilateral amygdala damage is associated with reduced updating of character judgments. Paper presented at the annual conference of the Society for Neuroscience; Chicago, IL. 2009. Oct, [Google Scholar]
- Croft KE, Duff MC, Kovach CK, Anderson SW, Adolphs R, Tranel D. Detestable or marvelous? Neuroanatomical correlates of character judgments. Neuropsychologia. 2010;48(6):1789–1801. doi: 10.1016/j.neuropsychologia.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal D. Language play. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Decety J. Dissecting the Neural Mechanisms Mediating Empathy. Emotion Review. 2011;3(1):92–108. [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23(2):744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Gupta R, Hengst JA, Tranel D, Cohen NJ. The Use of Definite References Signals Declarative Memory: Evidence From Patients With Hippocampal Amnesia. Psychological Science. 2011a;22(5):666–673. doi: 10.1177/0956797611404897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Gupta R, Tranel D, Cohen NJ. Distributed impact of cognitive-communication impairment: Disruptions in the use of definite references when speaking to individuals with amnesia. Aphasiology. 2011b;25(6):675–687. doi: 10.1080/02687038.2010.536841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nature Neuroscience. 2006;9(1):140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen NJ. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology. 2009;23(7–8):926–939. doi: 10.1080/02687030802533748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ. Neurological and neuropsychological bases of empathy. European Neurology. 1998;39(4):193–199. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Tranel D. Bilateral amygdala damage impairs the acquisition and use of common ground in social interaction. Neuropsychology. 2011a;25(2):137–146. doi: 10.1037/a0021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Tranel D. Focal ventromedial prefrontal cortex damage impairs convergence in discourse. Paper presented at the Clinical Aphasiology Conference.2011b. [Google Scholar]
- Gupta R, Koscik TR, Bechara A, Tranel D. The amygdala and decision-making. Neuropsychologia. 2011b;49(4):760–766. doi: 10.1016/j.neuropsychologia.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst J. Collaborative referencing between individuals with aphasia and routine communication partners. Journal of Speech, Language, and Hearing Research. 2003;46:831–848. doi: 10.1044/1092-4388(2003/065). [DOI] [PubMed] [Google Scholar]
- Hengst JA. “That mea :: n dog”: Linguistic mischief and verbal play as a communicative resource in aphasia. Aphasiology. 2006;20(2–4):312–326. [Google Scholar]
- Horton WS. The influence of partner-specific memory associations on language production: Evidence from picture naming. Language and Cognitive Processes. 2007;22:1114–1139. doi: 10.1080/01690960701402933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton WS, Gerrig RJ. The impact of memory demands on audience design during language production. Cognition. 2005;96(2):127–142. doi: 10.1016/j.cognition.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Hupet M, Chantraine Y. Changes in Repeated References - Collaboration or Repetition Effects. Journal of Psycholinguistic Research. 1992;21(6):485–496. [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Keysar B. Communication and miscommunication: The role of egocentric processes. Intercultural Pragmatics. 2007;4:71–84. [Google Scholar]
- Koenigs M, Tranel D. Prefrontal cortex damage abolishes brand-cued changes in cola preference. Soc Cogn Affect Neurosci. 2008;3(1):1–6. doi: 10.1093/scan/nsm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RM, Fussell SR. Social psychological approaches to the study of communication. In: Higgins ET, Kruglanski A, editors. Social psychology: Handbook of basic principles. New York: Guilford Press; 1996. [Google Scholar]
- Krauss RM, Glucksberg S. The development of communication: Competence as a function of age. Child Development. 1969;40:255–266. [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5. New York, NY: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161(7):1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Deficits in social knowledge following damage to ventromedial prefrontal cortex. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(1):66–74. doi: 10.1176/jnp.17.1.66. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45(13):3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Social Neuroscience. 2006;1(3–4):149–166. doi: 10.1080/17470910600985589. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Aharon-Peretz J. The neuroanatomical basis of understanding sarcasm and its relationship to social cognition. Neuropsychology. 2005;19(3):288–300. doi: 10.1037/0894-4105.19.3.288. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15(3):324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. Journal of Clinical and Experimental Neuropsychology. 2004;26(8):1113–1127. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- Shammi P, Stuss DT. Humour appreciation: a role of the right frontal lobe. Brain. 1999;122:657–666. doi: 10.1093/brain/122.4.657. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Sherzer J. Speech play and verbal art. Austin, TX: University of Texas Press; 2002. [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Straehle CA. “Samuel?” “Yes, dear?”. Teasing and conversational rapport. In: Tannen D, editor. Framing in discourse. New York: Oxford University Press; 1993. pp. 210–230. [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95(1):3–28. [PubMed] [Google Scholar]
- Stuss DT, Gallup GG, Jr, Alexander MP. The frontal lobes are necessary for ‘Theory of Mind’. Brain. 2001;124(2):279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Wilkes-Gibbs D, Clark HH. Coordinating beliefs in conversation. Journal of Memory and Language. 1992;31:183–194. [Google Scholar]
- Xi C, Zhu Y, Niu C, Zhu C, Lee TM, Tian Y, et al. Contributions of subregions of the prefrontal cortex to the theory of mind and decision making. Behavioral Brain Research. 2010;221(2):587–593. doi: 10.1016/j.bbr.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65(6):845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule G. Referential communication tasks. Mahwah, NJ: Lawrence Erlbaum Associates Inc; 1997. [Google Scholar]


