Abstract
Hypericum perforatum L. (St. John’s-wort, Hypericaceae) is a valuable medicinal plant species cultivated for pharmaceutical purposes. Although the chemical composition and pharmacological activities of H. perforatum have been well studied, no data are available concerning the influence of arbuscular mycorrhizal fungi (AMF) on this important herb. A laboratory experiment was therefore conducted in order to test three AMF inocula on H. perforatum with a view to show whether AMF could influence plant vitality (biomass and photosynthetic activity) and the production of the most valuable secondary metabolites, namely anthraquinone derivatives (hypericin and pseudohypericin) as well as the prenylated phloroglucinol—hyperforin. The following treatments were prepared: (1) control—sterile soil without AMF inoculation, (2) Rhizophagus intraradices (syn. Glomus intraradices), (3) Funneliformis mosseae (syn. Glomus mosseae), and (4) an AMF Mix which contained: Funneliformis constrictum (syn. Glomus constrictum), Funneliformis geosporum (syn. Glomus geosporum), F. mosseae, and R. intraradices. The application of R. intraradices inoculum resulted in the highest mycorrhizal colonization, whereas the lowest values of mycorrhizal parameters were detected in the AMF Mix. There were no statistically significant differences in H. perforatum shoot mass in any of the treatments. However, we found AMF species specificity in the stimulation of H. perforatum photosynthetic activity and the production of secondary metabolites. Inoculation with the AMF Mix resulted in higher photosynthetic performance index (PItotal) values in comparison to all the other treatments. The plants inoculated with R. intraradices and the AMF Mix were characterized by a higher concentration of hypericin and pseudohypericin in the shoots. However, no differences in the content of these metabolites were detected after the application of F. mosseae. In the case of hyperforin, no significant differences were found between the control plants and those inoculated with any of the AMF applied. The enhanced content of anthraquinone derivatives and, at the same time, better plant vitality suggest that the improved production of these metabolites was a result of the positive effect of the applied AMF strains on H. perforatum. This could be due to improved mineral nutrition or to AMF-induced changes in the phytohormonal balance. Our results are promising from the biotechnological point of view, i.e. the future inoculation of H. perforatum with AMF in order to improve the quality of medicinal plant raw material obtained from cultivation.
Keywords: AMF species specificity, Anthraquinone derivatives, Arbuscular mycorrhiza, Hyperforin, Photosynthetic performance index, St. John’s-wort
Introduction
Hypericum perforatum L. (St. John’s-wort; Hypericaceae) is a common perennial plant with a reputed medicinal value. This species is included in the European Pharmacopoeia (2008) and American Herbal Pharmacopoeia (1997–2005) as well as many other pharmaceutical monographs (Barnes et al. 2007). The aerial parts of H. perforatum contain anthraquinone derivatives (hypericin and pseudohypericin), flavonoids, prenylated phloroglucinols (hyperforin), tannins, phenolic acids, and volatile oil as major constituents (Barnes et al. 2007). H. perforatum is held to possess sedative and astringent properties. It has been utilized for excitability, neuralgia, anxiety and depression and as a nerve tonic. St. John’s-wort has a long history of traditional use in topical preparations for wound healing. H. perforatum is presently utilized extensively in homeopathic as well as in herbal products (Barnes et al. 2007; van Wyk and Wink 2008). At one time, the plant was collected only in the wild; however, due to the demand for high plant material quality for use in the herbal industry, as well as the need to standardize medicinal plant raw materials for pharmaceutical purposes, the species has been cultivated by introducing it into agriculture (van Wyk and Wink 2008).
To meet the increasing demand for plants utilized in the herbal industry, the emphasis in recent research has been on the development of new techniques to improve the yield and quality of plant material. One of the techniques for enhancing the biomass and standard of medicinal plants is the application of arbuscular mycorrhizal fungi (AMF) during their cultivation. AMF have been found to stimulate growth and improve pathogen, heavy metal, and salinity resistance, as well as to influence the level of secondary metabolites in plants (reviewed in Smith and Read 2008). Therefore, the practical use of these symbiotic soil microorganisms is proposed for agricultural (Hamel 1996; Feldmann et al. 2008; Gianinazzi et al. 2010), endangered (Gemma et al. 2002; Zubek et al. 2008, 2009; Bothe et al. 2010), and medicinal plant species (Kapoor et al. 2002a, b, 2007; Copetta et al. 2006; Khaosaad et al. 2006; Toussaint 2007; Toussaint et al. 2007; Zubek and Błaszkowski 2009; Ceccarelli et al. 2010; Zubek et al. 2010, 2011).
Although the chemical composition and pharmacological activities of H. perforatum have been well studied (Barnes et al. 2007; van Wyk and Wink 2008), and earlier observations indicate that H. perforatum is colonized by AMF (Wang and Qiu 2006; Zubek and Błaszkowski 2009), no data are available on the influence of AMF on this important medicinal plant species. The aim of our present laboratory experiment was therefore to test three AMF inocula on H. perforatum grown in sterile soil in order to show whether inoculation could influence plant vitality and the production of the most valuable secondary metabolites, namely anthraquinone derivatives (hypericin and pseudohypericin), as well as the prenylated phloroglucinol—hyperforin. The effects of AMF inoculation on H. perforatum were evaluated by physiological and phytochemical methods, namely by shoot mass, mycorrhizal colonization assessment, and HPLC measurements of the aforementioned secondary metabolite contents, as well as by biophysical methods, known as the JIP-test. This test translates the polyphasic chlorophyll a fluorescence transient OJIP exhibited by plants upon illumination into the biophysical parameters of the photosynthetic machinery, evaluating plants’ vitality. It has been successfully utilized for the evaluation of the role of AMF inoculation on plants, including those of medicinal importance (Tsimilli-Michael et al. 2000; Pinior et al. 2005; Biró et al. 2006; Strasser et al. 2007; Tsimilli-Michael and Strasser 2008; Zubek et al. 2009; Jurkiewicz et al. 2010; Zubek et al. 2010).
Materials and methods
AMF inocula
The inocula applied in the experiment were: (1) Rhizophagus intraradices (syn. Glomus intraradices N.C. Schenck & G.S. Smith) C. Walker & A. Schüßler (BEG140), (2) Funneliformis mosseae (syn. Glomus mosseae T.H. Nicolson & Gerd.) C. Walker & A. Schüßler (BEG12), and (3) an AMF Mix inoculum which contained the following isolates: Funneliformis constrictum (syn. Glomus constrictum Trappe) C. Walker & A. Schüßler (262-5 C. Walker), Funneliformis geosporum (syn. Glomus geosporum T.H. Nicolson & Gerd.) C. Walker & A. Schüßler (UNIJAG.PL.12-2), F. mosseae (BEG12), and R. intraradices (BEG 140). The fungal species names are after Schüßler and Walker (2010). The fungi were multiplied on a sterile substratum of sand: expanded volcanic clay, and rock phosphate—3:1, v/v; 50 g L−1, using Plantago lanceolata L. as the host plant. Dried inocula containing P. lanceolata roots colonized to over than 80% of the root length with no other fungal endophytes present, extraradical mycelium, and spores were used in the experiment.
Plant material
The seeds of H. perforatum L. var. Topaz were obtained from the Institute of Natural Fibres and Medicinal Plants, Poznań. They were germinated on wet filter paper in Petri dishes at room temperature and daylight.
Experiment set-up
One-week-old H. perforatum seedlings were transferred into 1-L pots. There were two pots for each treatment, with seven individual plants per pot. Each pot contained 900 ml of substratum, i.e. sterile commercially available soil which was expanded using volcanic clay (5:1, v/v; respectively). The soil was sterilized twice at 90°C for 1 h at 24-h intervals, and it was then sprayed with distilled water for 2 weeks before using in the experiment. The chemical properties of the soil were pH 5.9, N 1.6%, C 39.9%, P 1,506.7 mg kg−1, K 663.3 mg kg−1, and Ca 2,436.7 mg kg−1. The following treatments were prepared: (1) control—sterile soil without AMF inoculation, (2) R. intraradices BEG140, (3) F. mosseae BEG12, and (4) the AMF Mix. Dried inocula were mixed with the soil, using 30 g per pot. The pots were kept under greenhouse conditions at 22 ± 2°C and the following light regime: 100–110 μmol PAR photons × m−2 × s−1. The pots were kept in sealed Sigma-Aldrich Sunbags for the first month, after which the bags were opened. The cultures were watered one time per week.
After 4 months of H. perforatum growth, chlorophyll (Chl) a fluorescence measurements were conducted, and the plants were then harvested. The roots were stained to visualize AMF mycelium for the mycorrhizal colonization assessment, and the shoots were dried at room temperature and used for the evaluation of plant biomass and secondary metabolite contents. The shoots were weighed using a Radwag WPA 60/c/1 electronic analytical balance with the precision of 0.0001 g. The aforementioned analyses and assessments were performed on each of the individual plants—in total, 14 per treatment. Additional data were obtained in the case of Chl a fluorescence measurement (see below).
Evaluation of the plants’ vitality
Measurement of Chl a fluorescence transient OJIP
Chl a fluorescence transients OJIP were measured with a HandyPEA-fluorimeter (Hansatech Instruments Ltd., King’s Lynn Norfolk, PE30 4NE, UK). The transients were induced by a red light (peak at 650 nm) of 3,000 μmol photons m−2 s−1 provided by an array of three light-emitting diodes and recorded for 1 s with 12 bit resolution. The data acquisition was conducted every 10 μs, in the interval from 10 μs to 0.3 ms, every 0.1 ms (0.3–3 ms), every 1 ms (3–30 ms), every 10 ms (30–300 ms) and every 100 ms (300 ms to 1 s). The measurements were conducted on fully expanded leaves, still attached to the plants, which were dark-adapted for 30 min prior to measuring. Measurements were performed on one leaf of each individual plant. Where it was possible, i.e. when the leaves were at the same developmental stage, additional measurement on a single plant was performed, giving a total of 20 replicates for each treatment.
The JIP-test
For each treatment, the average OJIP fluorescence transient was analysed according to the JIP-test (Strasser et al. 2004), using the “Biolyzer” software produced by the Laboratory of Bioenergetics at the University of Geneva, Switzerland. The parameter chosen for presentation was the performance index (PItotal), which evaluates the overall photosynthetic performance; for the analytical description, see Tsimilli-Michael and Strasser (2008). PI represents an index combining functional and structural criteria of the photosystem (PS) II functioning and is based on several independent parameters used to characterize the responses of PSII to diverse environmental factors (Tsimilli-Michael and Strasser 2008).
Determination of AMF colonization
The roots were stained in accordance with the Phillips and Hayman (1970) protocol, with minor modifications as incorporated by Zubek et al. (2010). After staining, the roots were cut into ca. 1-cm fragments, then mounted on slides in glycerol:lactic acid (4:1, v:v), and analysed using a Nikon Eclipse 80i light microscope with Nomarski interference contrast optics. The mycorrhizal colonization assessment was carried out in line with the Trouvelot method (Trouvelot et al. 1986). The parameters analysed were mycorrhizal frequency (F), relative mycorrhizal root length (M), and relative arbuscular richness (A).
Estimation of secondary metabolite contents
The dried shoots were stored in the dark at room temperature until required for analysis. The sample preparation and quantification of anthraquinone derivatives and the prenylated phloroglucinol—hyperforin—were carried out using high-performance liquid chromatography—HPLC FLD and HPLC DAD techniques for hypericin and pseudohypericin and for hyperforin, respectively (de los Reyes and Koda 2002). The identification of the compounds under analysis was based on the comparison of their retention times and spectral parameters with hypericin, pseudohypericin and hyperforin standards obtained from PhytoLab GmbH & Co. KG.
Statistical analysis
The data obtained from mycorrhizal colonization assessments were analysed by means of the nonparametric Kruskal–Wallis test (p < 0.05). The analysis of biomass, secondary metabolite contents and photosynthetic performance index values were conducted using a one-way analysis of variance. The significance of differences between treatments was tested following Tukey (p < 0.05). The analyses were carried out using Statsoft’s STATISTICA version 9.
Results
AMF colonization
The roots of the plants from all AMF treatments were colonized by AMF mycelium with arbuscules. Other root endophytes were not detected in the material under investigation. The roots of H. perforatum from the control treatments were devoid of fungi. In the case of mycorrhizal frequency (F), no statistically significant differences were found between the treatments. The application of R. intraradices inoculum resulted in the highest mean mycorrhizal colonization rate (M) and arbuscule richness (A). The lowest values of mycorrhizal parameters (M, A) were detected after the AMF Mix application. Statistically significant differences were only found between these two extreme mean values (Fig. 1).
Fig. 1.

Mycorrhizal colonization (percentages; mean ± SD) of H. perforatum roots; AMF treatments: R.intra. R. intraradices (BEG140), F.moss. F. mosseae (BEG12), Mix inoculum containing F. constrictum (262-5 C. Walker), F. geosporum (UNIJAG.PL.12-2), F. mosseae (BEG12), and R. intraradices (BEG 140); mycorrhizal parameters: F mycorrhizal frequency, M relative mycorrhizal root length, A relative arbuscular richness. The different letters above the bars indicate statistically significant differences (p < 0.05)
Shoot mass
There were no statistically significant differences in H. perforatum shoot mass in any of the treatments (Fig. 2). Only a slight tendency towards higher biomass production was observed after the AMF inoculation in comparison to the control treatment.
Fig. 2.
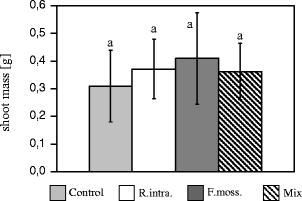
H. perforatum shoot dry mass (grams; mean ± SD) of the control and AMF treatments presented in Fig. 1; no statistically significant differences were found between treatments (p > 0.05)
Photosynthetic performance index
The application of R. intraradices and F. mosseae had no significant impact on the photosynthetic performance (expressed in PItotal) of H. perforatum comparing to the control plants, whereas inoculation with the AMF Mix resulted in significantly higher PItotal values in comparison to all the other treatments (Fig. 3).
Fig. 3.
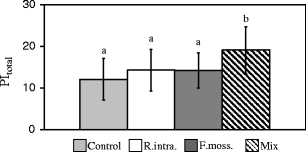
The performance index (PI; mean ± SD) of H. perforatum for the treatments presented in Fig. 2. The different letters above the bars indicate statistically significant differences (p < 0.05)
Anthraquinone derivative and hyperforin shoot contents
The plants inoculated with R. intraradices and the AMF Mix were characterized by a higher concentration of the two studied anthraquinone derivatives in the shoots in comparison to the control plants. However, no differences were detected in hypericin and pseudohypericin concentrations after the application of F. mosseae (Fig. 4). In the case of hyperforin, no statistically significant differences were found between the control plants and those inoculated with any of the AMF applied (Fig. 5).
Fig. 4.
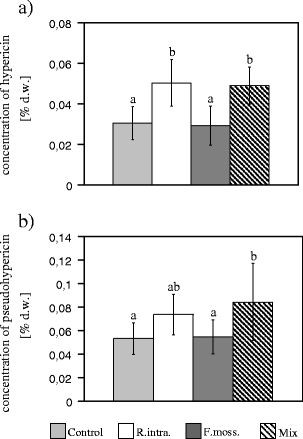
Anthraquinone derivative concentrations: hypericin (a) and pseudohypericin (b) (percentages of dry weight; mean ± SD) in the H. perforatum aerial parts from the treatments presented in Fig. 2. The different letters above the bars indicate statistically significant differences (p < 0.05)
Fig. 5.
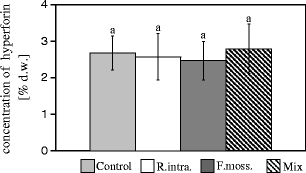
Hyperforin concentrations (percentages of dry weight; mean ± SD) in the H. perforatum aerial parts from the treatments presented in Fig. 2; no statistically significant differences were found between treatments (p > 0.05)
Discussion
Mycorrhizal colonization may induce quantitative and/or qualitative changes in plant secondary metabolite contents such as alkaloids (Abu-Zeyad et al. 1999; Rojas-Andrade et al. 2003); terpenoids (Maier et al. 1997, 1999; Fester et al. 1999; Akiyama and Hayashi 2002; Kapoor et al. 2002a, b, 2007; Copetta et al. 2006; Jurkiewicz et al. 2010), including carotenoids (Fester et al. 2002) and thymol derivatives (Zubek et al. 2010); glucosinolates (Vierheilig et al. 2000); phenolic compounds such as flavonoids (Morandi et al. 1984; Larose et al. 2002) and phenolic acids (Toussaint et al. 2007, 2008; Ceccarelli et al. 2010; Jurkiewicz et al. 2010). The mechanism of AMF-induced alterations in the phytochemical concentration in roots and/or shoots can be multidirectional (Toussaint 2007). Firstly, the modification of compound concentrations such as flavonoids and alkaloids in roots may be the consequence of signalling mechanisms between the host plant and fungi (Larose et al. 2002; Rojas-Andrade et al. 2003). Secondly, the alterations in the secondary metabolite balance can also result from plant response to AMF colonization. In general, the production of phenolic compounds and terpenoids, the components of essential oils, is considered as a defensive response to fungal colonization/infection. Given the fungicide properties of several constituents of essential oils, and the increased production of these metabolites in mycorrhizal plants, it has been suggested that they could be synthesized as a defensive response to AMF presence (Copetta et al. 2006). Thirdly, the enhanced synthesis of secondary metabolites in mycorrhizal plants, namely terpenoids and phenolic acids, may reside in better phosphorus and/or nitrogen nutrition owing to AMF (Kapoor et al. 2002a, b; Toussaint et al. 2007). Finally, an association between the alterations in secondary metabolite contents, namely terpenoids, and the changes in phytohormone levels in plants induced by AMF has been posited (Copetta et al. 2006; Kapoor et al. 2007; Toussaint 2007; Toussaint et al. 2007).
In the present study, we report, to the best of our knowledge for the first time, the AMF influence on the content of anthraquinone derivatives and hyperforin in plants. The enhanced hypericin and pseudohypericin shoot concentrations of the R. intraradices and AMF Mix treatments, as well as the parallel stimulation of H. perforatum photosynthetic performance after the AMF Mix inoculation, may suggest positive plant response to the inocula applied. This could be the result of improved plant phosphorus and/or nitrogen nutrition due to symbiosis. A similar tendency was found in the studies conducted by Zubek et al. (2010) concerning the response of Inula ensifolia L. to AMF. An increase in the quantity of thymol derivatives in roots was found after Rhizophagus clarus (Glomus clarum) inoculation, and at the same time, good plant vitality indicated by the high values of photosynthetic performance index was observed. Moreover, the decreased production of these metabolites and the lowest PI were observed in R. intraradices (G. intraradices) treatments (Zubek et al. 2010). In addition, a second possible interpretation of our results could be that the AMF applied influenced phytohormone contents differently, and changes thus occurred in the physiology of H. perforatum. However, in order to fully support both of the mechanisms posited here, further studies are planned involving the parallel analysis of AMF influence on anthraquinone derivatives, phytohormones, and P and N contents in both the roots and shoots of H. perforatum. In this investigation, our focus was on the therapeutic compound contents in the aerial parts, which is important for the medicinal value of H. perforatum and the possible future application of AMF in the cultivation of this species for pharmaceutical purposes.
In our experiment, commercially available soil with a relatively high phosphorus content was used. This could have decreased mycorrhizal colonization levels, and as a consequence, the effects of AMF on the plants might have been less pronounced. High soil P content usually results in low AMF colonization (Smith and Read 2008). For instance, Duan et al. (2010) found low colonization levels in maize, soybean, and wheat grown on fertilized soils. However, high levels of colonization in soils high in P and the apparent insensitivity of AMF colonization to increased P content have also been reported (Vosátka 1995; Ryan and Ash 1999). Furthermore, the plants were grown in a soil relatively high in P, and these conditions might have led to a reduction in the role of AMF in enhancing P acquisition. In view of this fact, the aforementioned mechanism whereby AMF influence the secondary metabolism of H. perforatum by improving plant nutritional status seems to be less possible.
The inoculation of H. perforatum with R. intraradices and the AMF Mix enhanced the concentration of anthraquinone derivatives, with no differences being noted in the F. mosseae treatment. This is in accordance with recent investigations which have revealed that various fungal strains induced different changes in the production of several groups of secondary metabolites in the same plant species (Kapoor et al. 2002a, b; Copetta et al. 2006; Khaosaad et al. 2006; Kapoor et al. 2007; Toussaint et al. 2007; Ceccarelli et al. 2010; Jurkiewicz et al. 2010; Zubek et al. 2010). For example, Jurkiewicz et al. (2010) found that total phenolic acid accumulation was enhanced in the roots and leaves of Arnica montana L. after application of AMF isolates originating from one of the plant’s natural stands with no significant influence of other inoculants. Similarly, Ceccarelli et al. (2010) proved that R. intraradices was more effective in the stimulation of total phenolic content in the leaves of Cynara cardunculus L. var. scolymus than in those of F. mosseae (G. mosseae).
In our studies, the lowest mycorrhizal colonization rates of H. perforatum roots were observed after the application of the AMF Mix. At the same time, the enhanced content of anthraquinone derivatives in shoots and the highest photosynthetic performance index values were found in the case of plants inoculated with the AMF Mix. As earlier studies have shown, the extent of mycorrhizal colonization is not necessarily correlated with the effects of AMF on plants (Kapoor et al. 2002a; Toussaint et al. 2007; Smith and Read 2008). Experimental and field data acquired by Feldmann et al. (2009) showed that AMF symbiosis was the most effective when root colonization ranged between 20% and 30%. In the studies conducted by Toussaint et al. (2007), even a relatively low level of colonization by F. mosseae had a considerable effect on Ocimum basilicum physiology, with increased caffeic acid content in shoots. However, a positive correlation was found between fungal colonization rate and the content of castanospermine, an alkaloid of the indolizidine type, in Castanospermum australe seeds (Abu-Zeyad et al. 1999).
The enhanced H. perforatum performance and the improved production of anthraquinone derivatives after the AMF Mix inoculation in comparison to the single AMF treatments could result from AMF species complementarity. Direct evidence for such a mechanism among AMF species was provided by Jansa et al. (2008). The authors found that leek colonized by a mixture of Claroideoglomus claroideum (Glomus claroideum) and R. intraradices acquired more phosphorus than with either of the two AMF separately. However, Janoušková et al. (2009) found that inoculation with a mixture of C. claroideum and R. intraradices brought no additional benefit to the host plants under study in comparison to single treatments. In addition, the plant growth depression observed after inoculation with C. claroideum persisted in the AMF Mix treatment. In our experimental approach, the better stimulation of H. perforatum performance in the case of the AMF Mix application could result not only from the potential complementarity of F. mosseae and R. intraradices but also from the additional presence of F. constrictum and/or F. geosporum in this treatment. The presence of these two strains and their symbiotic potential might have reinforced the positive response of the plant to inoculation. Furthermore, both plants and fungal strains can differ in their responsiveness and in the input of nutrients that influence the final effect of the symbiosis. For instance, in the studies conducted by Grace et al. (2009), F. geosporum (G. geosporum) has been shown to be a much less effective root colonizer and P contributor to the plant symbiont than R. intraradices. The presence of such a fungus could result in the reinforced production of the secondary metabolites that maintain the fungal colonization at the low level, while simultaneously allowing the increased performance of H. perforatum that might be due to the presence of R. intraradices. The evidence that plants colonized by AMF regulate further colonization by AMF through altered secondary metabolite production, namely root exudates, was provided by Pinior et al. (1999). The interactions between different AMF species and host plants are little understood at present, but the data available to date seem to support the view that the manipulation of rhizosphere communities may be of importance in developing more sustainable agriculture and more effective production of medicinal plant materials.
The inoculation of H. perforatum with Mix inoculum seems to be the best solution for improving both plant performance and the quality of medicinal plant raw material obtained from cultivation for pharmaceutical purposes. However, large-scale field experiments are required in order to support our laboratory investigations before an attempt can be made to utilize AMF in H. perforatum agricultural systems. Nevertheless, there is a need for the cultivation process to be carried out in accordance with the ecological approach, i.e. with low chemical inputs, such as fertilizers and pesticides, in order to both produce medicinal plant raw materials devoid of contaminants and avoid soil pollution. Therefore, the exploitation and possible application of soil microorganisms such as AMF could greatly benefit the herbal industry (Copetta et al. 2006; Toussaint 2007; Zubek and Błaszkowski 2009; Ceccarelli et al. 2010; Zubek et al. 2010, 2011).
Acknowledgements
The present research was financially supported by the Polish Ministry of Science and Higher Education, project no. N N304 381939 (2010–2013). Dr. hab. K. Seidler-Łożykowska (Institute of Natural Fibres and Medicinal Plants, Poznań) is acknowledged for providing us with the seeds of H. perforatum.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abu-Zeyad R, Khan AG, Khoo C. Occurrence of arbuscular mycorrhiza in Castanospermum australe A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza. 1999;9:111–117. [Google Scholar]
- Akiyama K, Hayashi H. Arbuscular mycorrhizal fungus-promoted accumulation of two new triterpenoids in cucumber roots. Biosci Biotechnol Biochem. 2002;66:762–769. doi: 10.1271/bbb.66.762. [DOI] [PubMed] [Google Scholar]
- American Herbal Pharmacopoeia and Therapeutic Compendium (1997–2005). Analytical, quality control and therapeutic monographs. Santa Cruz, California. American Herbal Pharmacopoeia
- Barnes J, Anderson LA, Philipson JD (2007) Herbal medicines 3rd edn., London Chicago Pharmaceutical Press
- Biró B, Köves-Péchy K, Tsimilli-Michael M, Strasser RJ. Role of beneficial microsymbionts on the plant performance and plant fitness. In: Mukerji KG, Manoharachary C, Singh J, editors. Microbial activity in the rhizosphere, Soil Biology vol. 7 (Varma A—series editor) Berlin: Springer; 2006. pp. 265–296. [Google Scholar]
- Bothe H, Turnau K, Regvar M. The potential role of arbuscular mycorrhizal fungi in protecting endangered plants and habitats. Mycorrhiza. 2010;20:445–457. doi: 10.1007/s00572-010-0332-4. [DOI] [PubMed] [Google Scholar]
- Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, Giovannetti M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil. 2010;335:311–323. doi: 10.1007/s11104-010-0417-z. [DOI] [Google Scholar]
- Copetta A, Lingua G, Berta G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza. 2006;16:485–494. doi: 10.1007/s00572-006-0065-6. [DOI] [PubMed] [Google Scholar]
- de los Reyes G, Koda RT. Determining hyperforin and hypericin content in eight brands of St. John’s wort. Am J Health-Syst Pharm. 2002;59:545–547. doi: 10.1093/ajhp/59.6.545. [DOI] [PubMed] [Google Scholar]
- Duan T, Shen Y, Facelli E, Smith SE, Nan Z. New agricultural practices in the Loess Plateau of China do not reduce colonisation by arbuscular mycorrhizal or root invading fungi and do not carry a yield penalty. Plant Soil. 2010;331:265–275. doi: 10.1007/s11104-009-0251-3. [DOI] [Google Scholar]
- European Pharmacopoeia 6th edn. Vol 2. (2008) Council of Europe, Strasburg
- Feldmann F, Hallmann J, Wagner S, Long X-Q, Schneider C, Hutter I, Ceipek B, Fan J, Zheng X, Wang C, Feng G. Mycorrhizal fungi as biological components of integrated cucumber production (BIOMYC)—promising results for mycorrhizal technology transfer to horticultural practice. In: Feldmann F, Kapulnik Y, Baar J, editors. Mycorrhiza works. Braunschweig: Deutsche Phytomedizinische Gesellschaft; 2008. pp. 25–38. [Google Scholar]
- Feldmann F, Gillessen M, Hutter I, Schneider C. Should we breed for effective mycorrhiza symbioses? In: Feldmann F, Alford DV, Furk C, editors. Crop plant resistance to biotic and abiotic factors. Current potential and future demands. Braunschweig: Deutsche Phytomedizinische Gesellschaft; 2009. pp. 507–522. [Google Scholar]
- Fester T, Maier W, Strack D. Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza. 1999;8:241–246. doi: 10.1007/s005720050240. [DOI] [Google Scholar]
- Fester T, Schmidt D, Lohse S, Walter MH, Giuliano G, Bramley PM, Fraser PD, Hause B, Strack D. Stimulation of carotenoid metabolism in arbuscular mycorrhizal roots. Planta. 2002;216:148–154. doi: 10.1007/s00425-002-0917-z. [DOI] [PubMed] [Google Scholar]
- Gemma JN, Koske RE, Habte M. Mycorrhizal dependency of some endemic and endangered Hawaiian plant species. Am J Bot. 2002;89:337–345. doi: 10.3732/ajb.89.2.337. [DOI] [PubMed] [Google Scholar]
- Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 2009;181:938–949. doi: 10.1111/j.1469-8137.2008.02720.x. [DOI] [PubMed] [Google Scholar]
- Hamel C. Prospects and problems pertaining to the management of arbuscular mycorrhizae in agriculture. Agr Ecosyst Environ. 1996;60:197–210. doi: 10.1016/S0167-8809(96)01071-7. [DOI] [Google Scholar]
- Janoušková M, Seddas P, Mrnka L, van Tuinen D, Dvořáčková A, Tollot M, Gianinazzi-Pearson V, Vosátka M, Gollotte A. Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza. 2009;19:393–402. doi: 10.1007/s00572-009-0243-4. [DOI] [PubMed] [Google Scholar]
- Jansa J, Smith FA, Smith SE. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008;177:779–789. doi: 10.1111/j.1469-8137.2007.02294.x. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz A, Ryszka P, Anielska T, Waligórski P, Białońska D, Góralska K, Tsimilli-Michael M, Turnau K. Optimization of culture conditions of Arnica montana L.: effects of mycorrhizal fungi and competing plants. Mycorrhiza. 2010;20:293–306. doi: 10.1007/s00572-009-0280-z. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Giri B, Mukerji KG. Glomus macrocarpum: a potential bioinoculant to improve essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague) World J Microbiol Biotechnol. 2002;18:459–463. doi: 10.1023/A:1015522100497. [DOI] [Google Scholar]
- Kapoor R, Giri B, Mukerji KG. Mycorrhization of coriander (Coriandrum sativum L.) to enhance the concentration and quality of essential oil. J Sci Food Agric. 2002;82:339–342. doi: 10.1002/jsfa.1039. [DOI] [Google Scholar]
- Kapoor R, Chaudhary V, Bhatnagar AK. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza. 2007;17:581–587. doi: 10.1007/s00572-007-0135-4. [DOI] [PubMed] [Google Scholar]
- Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae) Mycorrhiza. 2006;16:443–446. doi: 10.1007/s00572-006-0062-9. [DOI] [PubMed] [Google Scholar]
- Larose G, Chênevert R, Moutoglis P, Gagné S, Piché Y, Vierheilig H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J Plant Physiol. 2002;159:1329–1339. doi: 10.1078/0176-1617-00896. [DOI] [Google Scholar]
- Maier W, Hammer K, Dammann U, Schulz B, Strack D. Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta. 1997;202:36–42. doi: 10.1007/s004250050100. [DOI] [Google Scholar]
- Maier W, Schmidt J, Wray V, Walter MH, Strack D. The arbuscular mycorrhizal fungus, Glomus intraradices, induces the accumulation of cyclohexenone derivatives in tobacco roots. Planta. 1999;207:620–623. doi: 10.1007/s004250050526. [DOI] [Google Scholar]
- Morandi D, Bailey JA, Gianinazzi-Pearson V. Isoflavonoid accumulation in soybean roots infected with vesicular-arbuscular mycorrhizal fungi. Physiol Plant Pathol. 1984;24:357–364. doi: 10.1016/0048-4059(84)90009-2. [DOI] [Google Scholar]
- Phillips J, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc. 1970;55:158–161. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- Pinior A, Wyss U, Piché Y, Vierheilig H. Plants colonized by AM fungi regulate further root colonization by AM fungi through altered root exudation. Can J Bot. 1999;77:891–897. [Google Scholar]
- Pinior A, Grunewaldt-Stöcker G, von Alten H, Strasser RJ. Mycorrhizal impact on drought stress tolerance of rose plants probed by chlorophyll a fluorescence, proline content and visual scoring. Mycorrhiza. 2005;15:596–605. doi: 10.1007/s00572-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Rojas-Andrade R, Cerda-Garcia-Rojas CM, Frias-Hernández JT, Dendooven L, Olalde-Portugal V, Ramos-Valdivia AC. Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza. 2003;13:49–52. doi: 10.1007/s00572-002-0201-x. [DOI] [PubMed] [Google Scholar]
- Ryan MH, Ash J. Effects of phosphorus and nitrogen on growth of pasture plants and VAM fungi in SE Australian soils with contrasting fertiliser histories (conventional and biodynamic) Agric Ecosyst Environ. 1999;73:51–62. doi: 10.1016/S0167-8809(99)00014-6. [DOI] [Google Scholar]
- Schüßler A, Walker C (2010) The Glomeromycota. A species list with new families and genera. Gloucester, England, http://www.lrz.de/∼schuessler/amphylo/species_infos/higher_taxa/funneliformis_claroideoglomus_rhizophagus_redeckera.pdf
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3. London: Academic; 2008. [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, editors. Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration Series (Govindjee—Series Editor) vol 19. Rotterdam: Kluwer Academic Publishers; 2004. pp. 321–362. [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Dangre D, Rai M. Biophysical phenomics reveals functional building blocks of plants systems biology: a case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In: Varma A, Oelmuller R, editors. Advanced techniques in soil biology. Springer, Germany: Soil Biology Series; 2007. pp. 220–221. [Google Scholar]
- Toussaint JP. Investigating physiological changes in the aerial parts of AM plants: what do we know and where should we be heading? Mycorrhiza. 2007;17:349–353. doi: 10.1007/s00572-007-0133-6. [DOI] [PubMed] [Google Scholar]
- Toussaint JP, Smith FA, Smith SE. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza. 2007;17:291–297. doi: 10.1007/s00572-006-0104-3. [DOI] [PubMed] [Google Scholar]
- Toussaint JP, Kraml M, Nell M, Smith FA, Smith SE, Steinkellner S, Schmiderer C, Vierheilig H, Novak J. Effect of Glomus mosseae on concentration of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f.sp. basilica. Plant Pathol. 2008;57:1109–1116. doi: 10.1111/j.1365-3059.2008.01895.x. [DOI] [Google Scholar]
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S, editors. Physiological and genetical aspects of mycorrhizae. Paris: INRA; 1986. pp. 217–221. [Google Scholar]
- Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants’ vitality: applications in detecting and evaluating the impact of mycorrhization on host plants. In: Varma A (ed.) Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics (3rd edn). Springer, pp 679–703
- Tsimilli-Michael M, Eggenberg P, Biró B, Köves-Pechy K, Vörös I, Strasser RJ. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol. 2000;15:169–182. doi: 10.1016/S0929-1393(00)00093-7. [DOI] [Google Scholar]
- van Wyk BE, Wink M. Medicinal plants of the world. Polish. Wrocław (in Polish): Medpharm Polska; 2008. [Google Scholar]
- Vierheilig H, Bennett R, Kiddle G, Kaldorf M, Ludwig-Müller J. Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol. 2000;146:343–352. doi: 10.1046/j.1469-8137.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- Vosátka M. Influence of inoculation with arbuscular mycorrhizal fungi on the growth and mycorrhizal infection of transplanted onion. Agric Ecosyst Environ. 1995;53:151–159. doi: 10.1016/0167-8809(94)00563-T. [DOI] [Google Scholar]
- Wang B, Qiu YL. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 2006;16:299–363. doi: 10.1007/s00572-005-0033-6. [DOI] [PubMed] [Google Scholar]
- Zubek S, Błaszkowski J. Medicinal plants as hosts of arbuscular mycorrhizal fungi and dark septate endophytes. Phytochem Rev. 2009;8:571–580. doi: 10.1007/s11101-009-9135-7. [DOI] [Google Scholar]
- Zubek S, Turnau K, Błaszkowski J. Arbuscular mycorrhiza of endemic and endangered plants from the Tatra Mts. Acta Soc Bot Pol. 2008;77:149–156. [Google Scholar]
- Zubek S, Turnau K, Tsimilli-Michael M, Strasser RJ. Response of endangered plant species to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Mycorrhiza. 2009;19:113–123. doi: 10.1007/s00572-008-0209-y. [DOI] [PubMed] [Google Scholar]
- Zubek S, Stojakowska A, Anielska T, Turnau K. Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza. 2010;20:497–504. doi: 10.1007/s00572-010-0306-6. [DOI] [PubMed] [Google Scholar]
- Zubek S, Błaszkowski J, Mleczko P (2011) Arbuscular mycorrhizal and dark septate endophyte associations of medicinal plants. Acta Soc Bot Pol 80(3) (in press)


