Abstract
In the study we examined the production of cytotonic and cytotoxic toxins and the presence of a type III secretion system (TTSS) in 64 Aeromonas spp. strains isolated from fecal specimens of patients with gastroenteritis. We observed that contact of the bacteria with host epithelial cells is a prerequisite for their cytotoxicity at 3 h incubation. Cell-contact cytotoxic activity of the strains was strongly associated with the presence of the TTSS. Culture supernatants of the strains induced low cytotoxicity effects at the same time of incubation. Cell-free supernatants of 61 (95%) isolates expressed cytotoxic activity which caused the destruction of HEp-2 cells at 24 h. Moreover, 44% strains were cytotonic towards CHO cells and 46% of strains invaded epithelial cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s10482-011-9627-5) contains supplementary material, which is available to authorized users.
Keywords: Aeromonas spp., Cytotoxicity, Type III secretion system, Invasion
Introduction
Some Aeromonas species are opportunistic pathogens that have been implicated as etiological agents of human diseases. The most common infection is gastroenteritis, mainly in young, elderly or immunocompromised patients. The clinical symptoms of the infection varied from watery, self-limited diarrhea to chronic intestinal or cholera-like and dysentery-like disease. The most serious complications potentially resulting from gastroenteritis is ileal ulceration, inflammatory bowel disease, cholangitis, cirrhosis and peritonitis, which may result in septicemia and mortality (Janda and Abbott 2010; Parker and Shaw 2011). The most prevailing species isolated from patients with gastroenteritis is Aeromonas caviae, followed by Aeromonas hydrophila and Aeromonas veronii biotype sobria (von Gravaenitz 2007). Epidemiological studies suggested that there were differences in the predominating species depending on geographical area. Strains of A. caviae were predominantly isolated from children with diarrhea in Europe (Szczuka and Kaznowski 2004). Strains of A. hydrophila predominated in Brazil, Thailand and Bangladesh (von Gravaenitz 2007).
The pathogenicity of Aeromonas spp. is complex and multifactorial, with the involvement of multiple potential virulence factors. These bacteria produce a variety of biologically active extracellular products similar to the virulence factors of enteropathogenic bacteria (von Gravaenitz 2007; Janda and Abbott 2010). They include lipopolysaccharide, fimbriae, flagellae, proteases nucleases, and siderophores. Their role in pathogenesis has not been elucidated. Some strains possess type III secretion systems (TTSS) that can deliver virulence factors directly into the host cell. The contribution of TTSS to bacterial virulence has been proven for fish pathogens Aeromonas salmonicida (Burr et al. 2002) and A. hydrophila AH-1 (Yu et al. 2004). There exists a broad clinical spectrum of diseases caused by TTSS-containing pathogens for example infections with enteropathogenic Escherichia coli, Shigella, Salmonella, and Yersinia species result in serious intestinal diseases (Coburn et al. 2007).
Some bacteria cause diarrhea by production of enterotoxins or by invasion of the gastrointestinal epithelium (Janda and Abbott 2010). Two categories of enterotoxins, cytotoxic and cytotonic, have been discovered in culture filtrates of Aeromonas spp. isolates (Krzymińska et al. 2003; von Gravaenitz 2007; Janda and Abbott 2010). The cytotoxic enterotoxins cause extensive damage to epithelia. The toxins include heat-labile and stable enterotoxins with hemolytic and cytotoxic activities, the pore-forming toxin aerolysin and different α- and β-hemolysins (von Gravaenitz 2007; Galindo et al. 2006). Aeromonas spp. strains produce also cytotonic enterotoxins that, like cholera toxin, cause increase in the level of cAMP in intestinal epithelial cells (Galindo et al. 2006).
To further understand the role of TTSS of Aeromonas spp. strains in virulence and host-pathogen interactions, we compared cytotoxic activity and invasion ability of strains with and without TTSS genes.
Materials and methods
Bacterial strains
Sixty-four Aeromonas spp. strains were used in the study: 17 A. veronii biotype sobria, 43 A. caviae and 4 A. hydrophila (Table 1). The strains originated from the Aeromonas spp. collection of the Department of Microbiology A. Mickiewicz University, Poznań, Poland (MPU A). They were recovered from fecal samples of patients suffering from gastroenteritis and were identified on the basis of their phenotypic properties, confirmed by DNA–DNA hybridization (Szczuka and Kaznowski 2004) and by the 16S rDNA RFLP method described previously by Figueras et al. (2000). The strains were stored at −75°C in brain heart infusion broth (BHI, Difco) containing 50% (v/v) glycerol. As a negative control, E. coli K-12 C600 was included. For cytotoxic and cytotonic assay, bacterial strains were cultured in tryptic soy broth supplemented with 0.6% yeast extract at 37°C for 24 h. Bacterial supernatants were filter sterilized through 0.22 μm-pore size filters as described by Krzymińska et al. (2003).
Table 1.
Aeromonas spp. strains used in the study
| Aeromonas species (total number) | Strain no. |
|---|---|
| A.veronii biotype sobria (17) | MPU A382, 387, 389, 391, 392, 524-530, 532, 533, 551-553 |
| A. caviae (43) | MPU A375-380, 383-386, 388, 390, 393, 500-512, 514, 515-523, 544-550 |
| A. hydrophila (4) | MPU A540-543 |
Cell lines and culture condition
Chinese hamster ovary (CHO), human epidermoid carcinoma cells from the larynx (HEp-2) and African monkey kidney (Vero) cells were cultured in Eagle minimum essential medium (MEM, Sigma) with 5% fetal calf serum (FCS) containing 2 mM glutamine, penicillin (50 U/ml), streptomycin (100 μg/ml) and 1 mg/ml of nystatin (Krzymińska et al. 2003, 2010). The epithelial cells were seeded with 100 μl of suspension in MEM containing 1 × 103 cells per well and incubated at 37°C in an atmosphere with 5% CO2.
Bacterial cell-contact cytotoxicity
We tested whether contact with host cells is essential to the Aeromonas spp. cytotoxicity. HEp-2 cells were cultivated on 6-well plates (Nunc) for 24 h and the monolayer was incubated with bacterial suspension in phosphate buffered saline (PBS, Biomed) and diluted 1:10 in MEM to give a multiplicity of infection (MOI) of 100 bacteria per one HEp-2 cell, meaning that 1 × 104/ml of the epithelial cells were incubated with approximately 1 × 106/ml of bacteria at 37º C for 3 h in a 5% CO2. Similarly non-pathogenic E. coli K-12 C600 was prepared. Morphological changes were evaluated by phase-contrast microscopy over a 1-4 h period. Next, the bacteria were removed and viability of infected cells was assessed by MTT assay as previously described (Krzymińska et al. 2009b). To test the importance of bacteria-host cell contact in cytotoxicity, we performed cocultures of the bacteria and HEp-2 cells by using transwell inserts with 0.2 μm pore size (Nunc). HEp-2 cells were cultured in the lower chamber. Next day the bacteria cells at MOI 100 were added in the upper chamber and incubated for 3 h. Assays were performed in triplicate in two separate experiments for each isolate. Epithelial cells were also incubated with MEM as a negative control.
Cytotoxic and cytotonic activity of bacterial culture supernatants to epithelial cells
The assay was performed according to Krzymińska et al. (2003). Twofold serial dilutions in PBS (from 1:2 to 1:128) of culture filtrates of Aeromonas spp. and non-pathogenic E. coli K-12 C600 were added to the wells of tissue culture plates containing confluent CHO and HEp-2 monolayers in the culture medium and incubated for 24 h at 37°C. The results were observed under an inverted microscope. All tests were performed in duplicate. Microscopic examination of the cells affected by cytotoxic activity of cell-free supernatants revealed a number of changes: rounding and shrinking of cells, followed by detachment, loss of cytoplasmic extensions, dissorganization of the cell monolayer. Cytotonic activity was revealed as elongation of CHO cells. The cytotoxic and cytotonic titre of each isolate was calculated by determining the reciprocal of the highest dilution of culture filtrates which produced cytopathic or cytotonic effects.
Aeromonas spp. invasion of HEp-2 cells
Bacterial invasion was quantified using a gentamicin survival assay (Krzymińska et al. 2003) with modifications. Aeromonas spp. and non-pathogenic E. coli K-12 C600 strains were cultured on tryptic soy agar (TSA, Difco) at 37°C for 24 h. The bacterial inoculum was made to 0.5 of McFarland scale and diluted 1:10 in MEM to give a concentration about 1 × 105 per ml. The number of bacteria was counted by serial dilutions in PBS and CFU determination. HEp-2 cells were incubated in MEM without gentamicin for 24 h. Infection was performed at a MOI of 10. After 3 h of infection the medium was replaced by that containing 100 μg/ml of gentamicin for 2 h at 37°C to kill extracellular bacteria. After washing three-times in PBS, the cells were incubated in lysing medium containing 0.01 M NaH2PO4, 0.1% Tween 20 (v/v), 0.025% trypsin (w/v) pH 8.0 for 30 min. The released bacteria were enumerated by plating 100 μl of the lysate on TSA. The number of invaded bacteria was expressed as the Invasion Index (InI) i.e. the percentage of intracellular bacteria after gentamicin treatment in comparison to inoculum. The monolayer was infected separately with an invasive strain of Yersinia enterocolitica O:8/1B (pYV+) and non-pathogenic E. coli K-12 C600.
Detection of type III secretion system
A PCR assay was performed to detect the ascV gene in the genomes of Aeromonas spp. strains. Genomic DNA was isolated using the Genomic Mini kit (A&A Biotechnology, Poland). The amplifications were performed in a C1000 thermal cycler (BioRad) in a 25-μl volume with 200 ng of template DNA, 2.5 μl of 10 × PCR buffer with MgCl2 and NH4(SO4)2, 0.6 mM of primers ASCV-F (5′-GTAARCAGATGAGTATCGATGG-3′) and ASCV-R (5′-GAGACSCGGGTGACGATAAT-3′) (Yu et al., 2004), 200 mM of dNTP mix and 1 U of Hi-Fi Taq polymerase (Novazym, Poland). The PCR reaction consisted of an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation (94°C, 30 s), annealing (58°C, 45 s) and extension (72°C, 60 s), and one final extension at 72°C for 5 min. The PCR products were separated in a 1.5% agarose gel, stained with ethidium bromide, visualized under UV and digitalized with a Bio-Print V.99 system (Vilbert-Lourmat, France). The experiments were performed in duplicate. To exclude false positive results, the amplicons were sequenced in a 3130xl Genetic Analyzer (Applied Biosystems). The sequences were then compared against the GenBank database using Basic Local Alignment Search Tool (BLAST).
Scanning electron microscopy
Samples for scanning electron microscopy were prepared according to Krzymińska et al. (2009b). Bacteria grown overnight on BHI agar at 37°C were harvested, suspended in phosphate-buffered saline (PBS) to a density of 1 on the McFarland scale and diluted 1:100 in MEM. The bacterial suspension was incubated with a monolayer of HEp-2 cells for 45 min at 37°C and then passaged to the next well containing cells. The passages were repeated four times. The bacteria were then fixed in 1% formaldehyde for 1 h on ice, washed in PBS and placed on a glass coverslip. The samples were fixed in 2% glutaraldehyde for 5 min at room temperature, washed in TE buffer and dehydrated in a series of acetone solutions. The samples were critical point-dried, coated with gold and observed under EVO 40 (Zeiss) scanning electron microscope.
Statistical analysis
All values of cytotoxicity and InI are the average of at least two separate experiments in triplicate. One-way analysis of variance (ANOVA) and comparison of mean values using Tukey’s HSD test at significance level P < 0.05 were performed. Association between occurrence of TTSS genes and cytotoxic titre of bacterial supernatants, cell contact cytotoxicity and InI was determined with Mann–Whitney U test. Statistical analyses were done with Statistica 9.1 (StatSoft).
Results
Aeromonas spp. cell-contact cytotoxic activity
A quantitative assay was developed to characterize cell-contact cytotoxic activity of the strains. The results showed that bacterial cells were able to lyse epithelial cells within 4 h of incubation (Table 2). HEp-2 cells which encountered live Aeromonas spp. cells became rounded, following detachment from the bottom of the culture plate. The cytotoxic activity ranged between 1.4 and 89.3%. The highest level of activity (above 78%) was observed for 18% of A. veronii biotype sobria strains and 33% of A. caviae strains. Cytotoxic activity of E. coli K-12 C200 reached 1.9%. No cytotoxicity could be observed when bacterial cells were not allowed contact with epithelial cells in tissue culture inserts. These results suggest that close contact of the strains with host cells is a prerequisite to the cytotoxicity. At 4 h incubation, low cytotoxic activity (below 20% of damaged cells) occurred with bacterial culture supernatant which suggests that the strains produced extracellular toxins. The highest extracellular activity ranged from 11.9 to 15.2% for 16 (25%) of the strains. No cytotoxicity was observed after contact with culture supernatants of 28% of Aeromonas spp. and non-pathogenic E. coli K-12 C200 strains.
Table 2.
Cell-contact cytotoxic activity of Aeromonas spp. strains to HEp-2
| Cytotoxicity rangea | Aeromonas species (% per species) | Strain no. |
|---|---|---|
| 78.7–89.3b | A. hydrophila (50) | MPU A540, 542 |
| A. veronii biotype sobria (18) | MPU A524, 533, 391 | |
| MPU A522, 508, 511, 514, 501, 509, 549, 503, 512, 502, 546, 548, 504, 507 | ||
| A. caviae (33) | ||
| 69.4–76.8 | A. hydrophila (25) | MPU A541 |
| A. veronii biotype sobria (12) | MPU A389, 532 | |
| A. caviae (16) | MPU A506, 547, 550, 386, 500, 510, 545 | |
| 61.2–67.9 | A. veronii biotype sobria (29) | MPU A530, 552, 527, 529, 553 |
| 11.7–19.3 | A. veronii biotype sobria (18) | MPU A551, 526, 525 |
| 5.7–10.6 | A. caviae (19) | MPU A515, 520, 383, 393, 388, 521, 516, 519 |
| A. hydrophila (25) | MPU A543 | |
| 1.4–4.6 | A. veronii biotype | MPU A528, 382, 387, 392 |
| sobria (23) | MPU A544, 517, 518, 375, 523, 505, 390, 377, | |
| A. caviae (33) | 385, 384, 376, 378, 380, 379 |
aThe percentage of cytotoxicity was determined by MTT assay at 4 h after infection
bMeans in the group did not differ significantly at P < 0.05 according Tukey’s HSD test
Cytotoxic and cytotonic activity of cell-free supernatants
At 24 h, all A. veronii biotype sobria strains, A. hydrophila strains and 93% of A. caviae supernatants were found to be cytotoxic to HEp-2 cells (Table 3). Cytopathic effect caused by activity of cytotoxic enterotoxins was detected by rounding and shrinkage of the cells. The highest cytotoxic titre ranging from 128 to 64 was observed for 71% of A. veronii biotype sobria and 30% of A. caviae strains. Preheating (56°C for 20 min) of the culture supernatants caused a decrease in the activity. The supernatant of E. coli K-12 C200, the negative control, was not cytotoxic to epithelial cells. Six strains had also cytotonic activity with titres ranging from 32 to 1 (Table 4). The activity caused elongation of CHO cells.
Table 3.
Cytotoxic activity of A. hydrophila, A. caviae and A. veronii biotype sobria cell-free supernatants to HEp-2 cells
| Cytotoxic titre rangea | Aeromonas species (% per pecies) | Strain no. |
|---|---|---|
| 64–128 | A.veronii biotype sobria (71) | MPU A387, 525, 526, 528, 533, 524, 527, 529, 530, 532, 552, 553 |
| A. caviae (30) | MPU A500, 506, 508, 515, 501-505, 507, 509, 511, 512 | |
| A. hydrophila (25) | MPU A540 | |
| 1–32 | A. veronii biotype sobria (29) | MPU A382, 391, 392, 389, 551 |
| MPU A388, 393, 384, 390, 517, 521-523,546-550, 375, 376, 378, 379, 383, 385, 545, 377, 380, 519, 544, 514, 510, 516, | ||
| A. caviae (63) | ||
| A. hydrophila (75) | MPU A541, 542, 543 | |
| 0 | A. caviae (7) | MPU A386, 518, 520 |
aThe reciprocal of the highest dilution yielding rounding, detachment and destruction of 50% of HEp-2 cells at 24 h after infection
Table 4.
Cytotonic activity of Aeromonas spp. cell-free supernatants to CHO cells
| Cytotonic titre rangea | Aeromonas species (% per species) | Strain no. |
|---|---|---|
| 32–64 | A. veronii biotype sobria (23) | MPU A525, 526, 528, 551 |
| A. caviae (30) | MPU A514, 515, 508, 510, 500, 501, 503 | |
| A. hydrophila (25) | MPU A543 | |
| 1–16 | A. veronii biotype sobria (12) | MPU A389, 391 |
| A. caviae (17) | MPU A519, 523, 516, 517 | |
| A. hydrophila (25) | MPU A542 | |
| 0 | A. veronii biotype sobria (41) | MPU A382, 387, 392, 527, 533, 552, 553 |
| A. caviae (39) | MPU A505, 506, 507, 511, 512, 518, 520-522 | |
| A. hydrophila (25) | MPU A540 | |
| DM | A. veronii biotype sobria (23) | MPU A524, 529, 530, 532 |
| A. caviae (13) | MPU A502, 504, 509 | |
| A. hydrophila (25) | MPU A541 |
DM destruction of CHO monolayer. Cytotonic activity of 20 A. caviae strains was reported in earlier studies (Krzymińska et al. 2003)
aThe reciprocal of the highest dilution causing elongation of CHO cells at 24 h after infection
Aeromonas spp. invasion of epithelial cells
Invasion of human epithelial cells by Aeromonas spp. strains was investigated in a quantitative gentamicin survival assay (Table 5). Preliminary experiments showed that all strains were gentamicin sensitive and unable to grow in media containing 100 μg/ml of the antibiotic. Fifteen of 43 strains revealed InI above 0.2% and were classified as invasive, according to the criteria of Shaw et al. (1995). Two strains of A. veronii biotype sobria showed the highest invasion activity with the index ranging between 15.8–49.6%, whereas that of invasive Y. enterocolitica O:8/1B reached 64.3%. Twenty-three (53%) strains showed the lowest invasion index below 0.2%, comparable to that of nonpathogenic negative control. The negative control of E. coli K-12 C600 strain had an InI of 0.013 ± 0.003%. Six strains caused destruction of HEp-2 monolayers, making the assay impossible to conduct.
Table 5.
Invasion of HEp-2 cells by Aeromonas spp. strains
| Invasion index rangea | Aeromonas species (% per species) | Strain no. |
|---|---|---|
| 15.8–49.6 | A. veronii biotype sobria (12) | MPU A533, 532 |
| 0.86–1.22 | A. veronii biotype sobria (12) | MPU A391, 528 |
| 0.21–0.65 | A. veronii biotype sobria (18) | MPU A382, 526, 387 |
| A. hydrophila (75) | MPU A543, 542, 540 | |
| A. caviae (22) | MPU A503, 501, 500, 523, 511 | |
| 0.01–0.08 | A. veronii biotype sobria (29) | MPU A527, 525, 392, 529, 530 |
| A. caviae (39) | MPU A522, 509, 504, 519, 521, 516, 506, 512, 502, | |
| 0 | A. veronii biotype sobria (12) | MPU A389, 551 |
| A. caviae (30) | MPU A508, 510, 514, 515, 517, 518, 520 | |
| DM | A. veronii biotype sobria (18) | MPU A552, 553, 524 |
| A. hydrophila (25) | MPU A541 | |
| A. caviae (9) | MPU A505, 507 |
DM destruction of HEp-2 monolayer. Invasion of HEp-2 cells by 20 A. caviae strains was reported in earlier studies (Krzymińska et al. 2003)
aThe percentage of intracelullar bacteria after gentamicin treatment in comparison to initial inoculum
TTSS
To check the Aeromonas spp. isolates for the presence of a TTSS, we conducted a PCR assay for detection of the ascV gene, which encodes a highly conserved inner membrane component characteristic of TTSS (Stuber et al. 2003). Altogether, 29 isolates (45.3%) (Table 6) were positive for the presence of the ascV gene, yielding a 331-bp PCR product (Supplementary Fig. S1).
Table 6.
Presence of the TTSS genes
| Species (% per species) | Strain no. |
|---|---|
| A. veronii biotype sobria (29) | MPU A389, 391, 524, 532, 533 |
| A. caviae (49) | MPU A386, 500-504, 506-512, 514, 522, 545-550 |
| A. hydrophila (75) | MPU A540, 541, 542, |
The presence of TTSS genes was strongly associated with cell-contact cytotoxicity (P < 0.001) but not with InI (P = 0.56) and cytotoxic activity of cell-free bacterial supernatants (P = 0.14).
Scanning electron microscopy
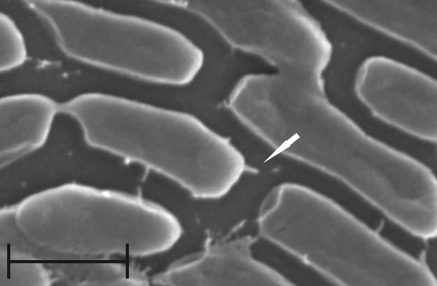
Needle-like structures on the cells of Aeromonas spp. strains grown in conditions promoting the expression of TTSS were observed in scanning electron microscopy (Fig. 1). They were 40-nm-wide, had 130–200 nm in length, and were present only in strains that were positive for the presence of ascV gene. The number of these appendages ranged from one to four per cell. They were absent in strains lacking the ascV gene.
Fig. 1.
Needle-like structures (white arrow) on the surface of Aeromonas hydrophila MPU A541 cells (scale bar 1 μm)
Discussion
The results of the present study determined association between the presence of a TTSS (as defined by the presence of the ascV gene) and cell-contact cytotoxicity in A. caviae, A. veronii biotype sobria and A. hydrophila strains.
Bacterial pathogens have evolved extraordinary mechanisms to efficiently infect host organisms. Among these, a majority of pathogens do so by delivering virulence factors into host cells to impair host defence. The interaction of enteropathogens with epithelial cells is the first stage of successive bacterial invasion of the host (Shames and Finlay 2010). We demonstrated that one of the virulence pathways of Aeromonas spp. strains provoked contact-dependent cytotoxicity. In the present study, 46 strains (72%) displayed this activity, whereas little or no cytotoxicity was detected in culture supernatant of the strains at 4 h-incubation. The results indicated that Aeromonas spp. strains promote epithelial cell lysis by a virulence factor associated with bacterial cells and extracellular toxins. The cytotoxic activity appears to require the TTSS, which demands close contact between infecting bacteria and the host cell to secrete and deliver effector proteins directly into the cytosol of the host cell. We observed strong association between the incidence of the TTSS gene selected and the cell contact cytotoxicity. Aeromonas spp. strains which harboured the TTSS gene caused destruction of more than 69% of HEp-2 cells at 4 h post infection. Although there were no statistically significant differences in the distribution of the TTSS ascV gene among the three species, we found that 49% of A. caviae strains had ascV, which is much more than the values (0–12.5%) reported by Chacón et al. (2004), Vilches et al. (2004) and Pablos et al. (2010) for clinical A. caviae isolates. TTSS form needle-like structures that likely become inserted into the epithelial cell membrane to create pores. The width of the appendages (ca. 40 nm) found on the surface of Aeromonas spp. (Fig. 1) cells is similar to the 30-70 nm reported by Chakravortty et al. (2005) for Salmonella enterica and to that for structures described earlier by us for Enterobacter cloacae (Krzymińska et al. 2009b). This suggests that the needle has a sheath, like that of Salmonella enterica (Chakravortty et al. 2005); although, on the other hand, it cannot be definitely concluded from the morphology alone that the appendages are actually part of a TTSS. Zhou et al. (2009) have suggested that, in some cases, pore formation results in the entry of small ions and water into the cytosol of host cells leading to cellular lysis. TTSS-dependent lysis of epithelial cells has been observed in a number of bacteria. Zhou et al. (2009) have reported that Vibrio parahaemolyticus type III secretion system 1 induces cytotoxicity for mammalian epithelial cells. They have suggested that bacterial contact is required for TTSS 1-induced cytotoxicity. Strains of Salmonella typhimurium induce rapid programmed cell death of host cells. The process depends on the incidence of functional TTSS (Waterman and Holden 2003). We observed low cytotoxic activity of Aeromonas spp. cell-free culture supernatant at 4 h after infection that suggested production of extracellular toxins. The highest activity of the toxins was observed at 24 h of incubation. The highest cytotoxic titre range was observed for 12 of 17 A. veronii biotype sobria strains, 13 of 43 A. caviae strains and 1 of 4 A. hydrophila isolates. The strains, as with many enteropathogens, displayed a variety of virulence factors involved in the infection process, showing the ability to damage host tissues as well as to evade the host defence system. One of the most potent cytotoxic factors produced by Aeromonas spp. strains is a cytotoxic enterotoxin which has been previously isolated and characterized in our laboratory (Krzymińska et al. 2006). The toxin has cytotoxic, cytotonic and hemolytic activities and induces apoptosis of epithelial cells and macrophages (Krzymińska et al. 2009a, b, 2011). We have demonstrated that the interaction of the cytolytic enterotoxin isolated from A. veronii biotype sobria with the epithelial cells resulted in the highest generation of reactive oxygen species (ROS) and nitric oxide radicals (NO·) and caused the highest cytotoxicity. We observed that increased accumulation of intracellular ROS leads to a loss of mitochondrial membrane potential (ΔΨm). Sha et al. (2005) have suggested that the cytolytic enterotoxin (Act) produced by A. hydrophila strains is the only cytotoxic factor present in bacterial culture supernatants and is responsible for host cell damage and death, once secreted in sufficient quantities. Cytotonic activity of 20 A. caviae strains (MPU A375-393 and MPU 544-550) to CHO cells was reported in earlier studies (Krzymińska et al. 2003) with 75% of strains considered to be cytotonic. In the present study, six strains (26%) had cytotonic activity.
Invasion into epithelial cells may play a significant role in Aeromonas spp. colonization and pathogenesis. The InI of 20 A. caviae (MPU A375-393 and 544-550) strains was analyzed in earlier studies (Krzymińska et al. 2003) with 25% strains found to be invasive. In the present study, 35% of the strains were invasive with InI higher than that of the non-pathogenic control. The highest index, comparable to that of Y. enterocolitica O:8/1B, was observed for two A. veronii biotype sobria strains. We observed that 14 strains (22%) with TTSS genes were invasive to epithelial cells. Coburn et al. (2007) suggested that TTSS-dependent virulence could subvert the host cytoskeleton through interactions with filamentous and globular actin. TTSS-containing pathogens could modify the levels of phosphoinositides that anchor the actin cytoskeleton to the plasma membrane that facilitate bacterial invasion.
Our results show that one of the possible mechanisms of Aeromonas spp. pathogenesis is production of a TTSS that induces lysis of host epithelial cells. We showed that the presence of TTSS could mediate cell-contact cytotoxicity, destruction of host cells and contribute to tissue damage which is necessary for bacterial invasion of other sites within the host.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1. Agarose gel electrophoresis of the PCR products of ascV gene of Aeromonas caviae, A. hydrophila and A. veronii biotype sobria. Lanes marked by strain numbers (TIFF 548 kb)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Burr SE, Stuber K, Wahli T, Frey J. Evidence for a type III secretion system in Aeromonas salmonicida subsp. salmonicida. J Bacteriol. 2002;184:5966–5970. doi: 10.1128/JB.184.21.5966-5970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón MR, Soler L, Groisman EA, Guarro J, Figueras MJ. Type III secretion system genes in clinical Aeromonas isolates. J Clin Microbiol. 2004;42:1285–1287. doi: 10.1128/JCM.42.3.1285-1287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravortty D, Rohde M, Jäger L, Deiwick J, Hensel M. Formation of a novel surface structures encoded by Salmonella Pathogenicity Island 2. EMBO J. 2005;24:2043–2052. doi: 10.1038/sj.emboj.7600676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn B, Sekirov I, Finlay B. Type III secretion systems and disease. Clin Microbiol Rev. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueras MJ, Soler L, Chacon MR, Guarro J, Martinez-Murcia AJ. Extended method for discrimination of Aeromonas spp. by 16S rDNA RFLP analysis. Int J Syst Evol Microbiol. 2000;50:2069–2073. doi: 10.1099/00207713-50-6-2069. [DOI] [PubMed] [Google Scholar]
- Galindo CL, Sha J, Fadl AA, Pillai LL, Chopra AK. Host immune responses to Aeromonas virulence factors. Curr Immunol Rev. 2006;2:13–26. doi: 10.2174/157339506775471910. [DOI] [Google Scholar]
- Janda MJ, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity and infection. Clin Microbiol Rev. 2010;23:35–75. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzymińska S, Kaznowski A, Lindner K, Mnichowska M. Enteropathogenic activity and invasion of HEp-2 cells by Aeromonas caviae isolates. Acta Microbiol Pol. 2003;152:277–283. [PubMed] [Google Scholar]
- Krzymińska S, Kaznowski A, Spychała H. Purification and characterization of cytolytic toxins produced by Aeromonas hydrophila and Aeromonas veronii biotype sobria strains. Polish J Microbiol. 2006;55:37–42. [PubMed] [Google Scholar]
- Krzymińska S, Kaznowski A, Chodysz M. Aeromonas spp induced apoptosis of murine macrophages. Curr Microbiol. 2009;58:252–257. doi: 10.1007/s00284-008-9316-4. [DOI] [PubMed] [Google Scholar]
- Krzymińska S, Mokracka J, Koczura R, Kaznowski A. Cytotoxic activity of Enterobacter cloacae human isolates. FEMS Immunol Med Microbiol. 2009;56:248–252. doi: 10.1111/j.1574-695X.2009.00572.x. [DOI] [PubMed] [Google Scholar]
- Krzymińska S, Koczura R, Mokracka J, Puton T, Kaznowski A. Isolates of the Enterobactercloacae complex induce apoptosis of human intestinal epithelial cells. Microb Pathog. 2010;49:83–89. doi: 10.1016/j.micpath.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Krzymińska S, Tańska A, Kaznowski A. Aeromonas spp. induces apoptosis of epithelial cells through an oxidant dependent activation of the mitochondrial pathway. J Med Microbiol. 2011;60:889–898. doi: 10.1099/jmm.0.030833-0. [DOI] [PubMed] [Google Scholar]
- Pablos M, Remacha MA, Rodríguez-Calleja JM, Santos JA, Otero A, García-Lopez ML. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur J Clin Microbiol Infect Dis. 2010;29:1163–1172. doi: 10.1007/s10096-010-0982-3. [DOI] [PubMed] [Google Scholar]
- Parker JL, Shaw JG. Aeromonas spp clinical microbiology and disease. J Infect. 2011;62:109–118. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Sha J, Pillai L, Fadl AA, Galindo CL, Erova TE, Chopra AK. The type III secretion system and cytotoxic enterotoxin alter the virulence of A hydrophila. Infect Immun. 2005;73:6446–6457. doi: 10.1128/IAI.73.10.6446-6457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames SR, Finlay BB. Breaking the stereotypes: virulence factor-mediated protection of host cells in bacterial pathogenesis. PloS Pathog. 2010;6:1–3. doi: 10.1371/journal.ppat.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JG, Thornley JP, Palmer J, Geary I. Invasion of tissue culture cells by A.caviae. Med Microbiol Lett. 1995;4:333–342. [Google Scholar]
- Stuber K, Burr SE, Braun M, Wahli T, Frey J. Type III secretion system genes in Aeromonas salmonicida subsp salmonicida are located on a large thermolabile virulence plasmid. J Clin Microbiol. 2003;41:3854–3856. doi: 10.1128/JCM.41.8.3854-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczuka E, Kaznowski A. Typing of clinical and environmental Aeromonas sp strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive introgenic consensus sequence. J Clin Microbiol. 2004;42:220–228. doi: 10.1128/JCM.42.1.220-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilches S, Urgell C, Merino S, Chacón MR, Soler L, Castro-Escarpulli G, Figueras MJ, Tomás JM. Complete type III secretion system of a mesophilic Aeromonas hydrophila strain. Appl Environ Microbiol. 2004;70:6914–6919. doi: 10.1128/AEM.70.11.6914-6919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gravaenitz A. The role of Aeromonas in diarrhea: a review. Infection. 2007;35:59–64. doi: 10.1007/s15010-007-6243-4. [DOI] [PubMed] [Google Scholar]
- Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5:501–511. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- Yu HB, Srinivasa Rao PS, Lee HC, Vilches S, Merino S, Tomas JM, Leung KY. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect Immun. 2004;72:1248–1256. doi: 10.1128/IAI.72.3.1248-1256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Konkel ME, Call DR. Type III secretion system 1 of Vibrio parahaemolyticus induces oncosis in both epithelial and monocytic cell lines. Microbiol. 2009;155:837–851. doi: 10.1099/mic.0.024919-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Agarose gel electrophoresis of the PCR products of ascV gene of Aeromonas caviae, A. hydrophila and A. veronii biotype sobria. Lanes marked by strain numbers (TIFF 548 kb)