Abstract
Purpose
We investigated the association between serum levels of 25-hydroxyvitamin D (25-OHD) and risk of death in Norwegian cancer patients.
Methods
The study population was 658 patients with cancers of the breast (n = 251), colon (n = 52), lung (n = 210), and lymphoma (n = 145), obtained from JANUS, a population-based serum bank in Norway. Serum samples were collected within 90 days of cancer diagnosis and were analyzed for 25-OHD. Patients were diagnosed during 1984–2004 and were followed for death throughout 2008. We used Cox regression models to assess the relationship between serum 25-OHD and risk of death.
Results
Three hundred and ninety-nine patients died during follow-up, of whom 343 (86%) died from cancer. Adjusted for sex, age at diagnosis, and season of blood sampling, patients with 25-OHD levels below 46 nmol/L at diagnosis experienced shorter survival. Compared to patients in the lowest quartile of serum 25-OHD, the risk of cancer death among patients in the highest quartile was significantly reduced (HR 0.36 95% CI 0.27, 0.51). The estimated change in risk of cancer death was most pronounced between the first and the second quartile. The associations between 25-OHD levels and survival were observed for all four cancers.
Conclusions
Higher circulating serum levels of 25-OHD were positively associated with the survival for cancers of the breast, colon, lung, and lymphoma.
Keywords: Serum, Vitamin D, Survival, Cancer patients
Introduction
Vitamin D is increasingly recognized as an important, modifiable factor in the natural history of several cancers. Vitamin D synthesis begins when sunlight converts 7-dehydrocholesterol in the skin to vitamin D3 [cholecalciferol]. Vitamin D3 and/or vitamin D2 [ergocalciferol] can also be obtained from the diet. The liver hydroxylates vitamin D3 to form 25-hydroxyvitamin D (25-OHD), the storage form of vitamin D, and the accepted measure of an individual’s vitamin D status [1]. The active form of vitamin D results from a second hydroxylation of 25-OHD in the 1-α position to form 1α,25-Dihydroxyvitamin D (1,25(OH)2D). Although the “classic” site of 1,25(OH)2D synthesis is the kidney, many non-renal tissues, including the prostate, colon, and breast, express 1-α hydroxylase (CYP27B1) and synthesize 1,25(OH)2D from 25-OHD in an autocrine fashion [1]. Unlike the kidney, where the hydroxylation of 25-OHD is tightly regulated, the hydroxylation of 25-OHD in non-renal organs appears to be substrate dependent, suggesting why the non-classical actions of vitamin D are related to increased serum levels of 25-OHD [2, 3].
In Norway, a country where serum vitamin D levels vary markedly between summer and winter, a 15–25% lower risk of dying from breast, colon, and prostate cancer was reported for patients diagnosed during summer or fall, when vitamin D levels are typically higher, compared to patients diagnosed during the winter [4]. Similar results were observed for Norwegian patients with lymphoma [5] and lung cancer [6]. However, an important limitation of these studies is that they are ecologic, i.e., they are based on data at the level of the population rather than individual.
More recently, the association between vitamin D and cancer survival has been investigated using individual serum levels of 25-OHD, the accepted measure of an individual’s vitamin D status [7]. Positive associations between serum 25-OHD and survival have been reported for studies including colorectal [8, 9], breast [10], and prostate cancer [11] and for non-Hodgkin’s lymphoma [12] and chronic lymphocytic leukemia [13]. Although many studies report a positive association between 25-OHD and survival, it is possible that this association, at least in part, reflects an influence of advanced cancer on serum 25-OHD levels rather than a beneficial effect of serum 25-OHD levels on survival. For example, Palmieri et al. [14] reported significantly higher serum levels of 25-OHD in early than in later-stage breast cancers. These findings emphasize the need to address potential confounding by disease stage in the studies of vitamin D and survival.
The present study examined the relationship between serum levels of 25-OHD and the risk of death in Norwegian patients with breast cancer, colon cancer, lung cancer, and lymphoma, using individual serum samples collected at the time of diagnosis. Results were examined controlling for disease stage, as provided by clinical information in the cancer registry. These four cancers were selected a priori, based on positive results observed for survival from these cancers in ecologic studies in Norway.
Materials and methods
Patients and serum samples
The JANUS serum bank, established in 1973, includes serum samples from more than 330,000 healthy donors, of whom 91% were recruited from population-based health examinations of participants in the 35–49 year age-group. The health examinations were performed in several Norwegian counties during 1973–2005 and had an attendance rate of 88 percent. The remainder of the JANUS cohort (9%) consists of Red Cross blood donors (http://www.kreftregisteret.no/en/Research/Janus-Serum-Bank/). Examinees and donors who subsequently developed cancer and were admitted to the Norwegian Radium hospital for the treatment donated an additional serum sample to JANUS at the time of diagnosis. In the present study, this last sample was used for 658 white patients. The cancer types studied were as follows: breast (n = 251), colon (n = 52), lung (n = 210), and lymphoma (n = 145). All patients were diagnosed during the period 1973–2007. All cancers were verified pathologically, by histology and/or cytology, as appropriate. Four percent of the patients were diagnosed and treated in the 1970s, 17% in the 1980s, 31% in the 1990s, and 48% during 2001–2007. The serum samples were collected within 90 days of cancer diagnosis. Patients who were not alive 30 days after the date of serum collection were excluded. Seventy-five μl of serum was drawn from each patient’s sample for analysis of 25-OHD, which was performed using a competitive radioimmunoassay (RIA) (DiaSorin, Stillwater, MN). All the serum samples were analyzed at the same time in August 2009 (total coefficient of variation was 12%). Before any statistical analyses were performed, patients were categorized according to quartiles of 25-OHD for the total group of cancer patients: <46, 46–61, 62–81, or >81 nmol/L. In a sub-analysis, the quartiles were defined for each specific type of cancer.
A linkage between the Cancer Registry of Norway and the JANUS serum bank was made via the unique personal identification number (PIN) that identifies each Norwegian citizen [15]. Information about the cancer, date of birth, and sex was retrieved from the population-based Cancer Registry. Data on cause of death were obtained from the National Death Registry. The underlying cause, stated on the death certificate, was used. The codes for stage of disease at the time of diagnosis have been changed during the 20 years of patient inclusion into this study. Therefore, a re-coding was performed on the bases of clinical and pathological information about the patients in the Cancer Registry by an experienced medical coder. Cancer stage at the time of diagnosis was coded as: local, regional metastasis, distant metastasis, and unknown. The patients’ 25-OHD serum level was unknown to the coder. Season of blood sampling was defined as Winter: December–February; Spring: March–May; Summer: June–August, or Fall: September–November.
Statistical analyses
The patients were followed from the date of diagnosis until date of death, migration, or the end of follow-up (31 December 2008), whichever occurred first. Cox proportional hazard regression models were used to assess the relationship between serum 25-OHD and the risk of death of cancer-specific mortality and overall mortality (all causes). Hazard rates of death (HR) were estimated adjusting for sex, age, and season of blood sampling. Additional stratified analyses were conducted within disease stage. Kaplan–Meier survival curves with log-rank tests were used to illustrate the cumulative survival by follow-up time. All statistical analyses were conducted using SPSS [16].
Results
Table 1 presents the sex distribution, median age at diagnosis, and median time between 25-OHD measurement and diagnosis. The study population was relatively young, with a median age of 56.5 years at the time of cancer diagnosis. The median time between diagnosis and measurement of serum 25-OHD was 37 days (Inter-quartile range; 20–56 days). A regression analysis of the association between calendar year of diagnosis and 25-OHD level showed a weak downward trend during 1973–2007 of 0.07 nmol/L per year.
Table 1.
Characteristics of the cancer patients at the time of diagnosis
| Characteristics | Breast | Colon | Lung | Lymphoma | Total |
|---|---|---|---|---|---|
| Number | |||||
| Female/male | 251/0 | 20/32 | 78/132 | 52/93 | 401/257 |
| Age (years) | |||||
| Median | 53.6 | 59.1 | 59.0 | 56.3 | 56.5 |
| Range | (36–75) | (32–75) | (42–82) | (37–79) | (32–82) |
| Days between serum collection and diagnosis* | |||||
| Median | 33.0 | 30.5 | 45.0 | 41.0 | 37.0 |
| Range | (−85, 90) | (−82, 87) | (−86, 90) | (−90, 88) | (−90, 90) |
| Stage, number | |||||
| Local | 67 | 9 | 24 | ||
| Regional | 74 | 17 | 82 | ||
| Distant | 24 | 24 | 94 | ||
| Unknown | 86 | 2 | 9 | ||
* Negative value means before and positive means after diagnosis
During the follow-up period, 399 patients died, of whom 343 (86%) from cancer. This proportion of deaths from cancer is in accordance with the expected proportion based on death rates from the total Norwegian population (85%), when cancer type, age at diagnosis, sex, and time of follow-up are taken into account.
Cancer-specific mortality
Table 2 (Model I) shows the hazard ratio of dying from cancer among patients with breast, colon, lung, and lymphoma by quartile of serum 25-OHD. A significantly poorer prognosis is seen for patients with 25-OHD levels below 46 nmol/L at diagnosis. The association between 25-OHD and death rate, adjusted for sex, age at diagnosis, and season of blood sampling, is shown in Model II. Both age at diagnosis and sex were significantly associated with prognosis, but the adjustment did not materially influence the association between 25-OHD and prognosis.
Table 2.
Hazard ratio (HR) and 95% confidence intervals (95% CI) of dying from cancer in patients with cancer of breast, colon, prostate, or lymphoma, in quartile groups of serum 25-OHD
| Model I | Model II** | Model III*** | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
|
1. Quartile (<46 nmol/L) 109/161* |
1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
|
2. Quartile (46–61 nmol/L) 93/170* |
0.58 | (0.44, 0.77) | 0.66 | (0.50, 0.87) | 0.70 | (0.51, 0.94) |
|
3. Quartile (62–81 nmol/L) 75/162* |
0.45 | (0.34, 0.61) | 0.46 | (0.34, 0.62) | 0.46 | (0.34, 0.62) |
|
4. Quartile (>81 nmol/L) 66/165* |
0.35 | (0.35, 0.48) | 0.36 | (0.26, 0.50) | 0.36 | (0.25, 0.49) |
| p for trend | p < 0.01 | p < 0.01 | p < 0.01 | |||
* Number of fatal cases/number of patients
** Adjusted for sex, age at diagnosis and season of blood sampling
*** Adjusted for sex, age at diagnosis, season of blood sampling, time between serum sampling and 25-OHD measurement, and stage of the disease at the time of diagnosis
Model III shows the results when adding the variables, stage of disease at the time of diagnosis and time between serum sampling and 25-OHD measurement to the adjustment list. The adjustments did not change the hazard ratios.
Table 3A shows the association between quartiles of serum 25-OHD and risk of cancer-specific death for each type of cancer, adjusted for sex, age at diagnosis, and season of blood sampling. Although survival times differ markedly among the four cancers, a similar pattern exists for all the cancers, with a poorer prognosis for the lowest 25-OHD level. A further adjustment for stage of disease revealed estimates that deviate only minimally from what is presented for breast and lung cancer. The colon cancer group consists of 52 patients only, and a non-significant trend was suggested both before and after adjustment for stage (data not shown).
Table 3.
Hazard ratio (HR) and 95% confidence intervals (95% CI) of dying from the actual cancer disease by quartile groups of 25-OHD for all cancer patients combined (A) and by cancer-specific quartile groups of 25-OHD (B)
| Breast | Colon | Lung | Lymphoma | |
|---|---|---|---|---|
| HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | |
| (A) | ||||
|
1. Quartile (<46 nmol/L) |
1.00 reference 22/45* |
1.00 reference 8/13* |
1.00 reference 59/63* |
1.00 reference 20/40* |
|
2. Quartile (46–61 nmol/L) |
0.60 (0.33, 1.09) 22/62* |
0.53 (0.17, 1.64) 6/12* |
0.39 (0.26, 0.58) 49/62* |
0.61 (0.31, 1.20) 16/34* |
|
3. Quartile (62–81 nmol/L) |
0.43 (0.22, 0.82) 16/58* |
0.60 (0.20, 1.79) 8/17* |
0.34 (0.22, 0.53) 36/44* |
0.44 (0.22, 0.88) 15/43* |
|
4. Quartile (>81 nmol/L) |
0.41 (0.22, 0.78) 22/86* |
0.37 (0.08, 1.81) 4/10* |
0.18 (0.11, 0.29) 29/41* |
0.38 (0.17, 0.83) 11/28* |
| p for trend | p = 0.01 | p = 0.27 | p < 0.01 | p = 0.02 |
| (B) | ||||
| 1. Quartile*** |
1.00 reference 22/45* |
1.00 reference 8/13* |
1.00 reference 59/63* |
1.00 reference 20/40* |
| 2. Quartile*** |
0.47 (0.26, 0.85) 22/62* |
0.46 (0.15, 1.48) 6/12* |
0.37 (0.24, 0.57) 49/62* |
0.71 (0.36, 1.40) 16/34* |
| 3. Quartile*** |
0.53 (0.29, 0.95) 16/58* |
0.73 (0.25, 2.15) 8/17* |
0.35 (0.23, 0.54) 36/44* |
0.45 (0.21, 0.94) 15/43* |
| 4. Quartile*** |
0.42 (0.21, 0.82) 22/86* |
0.20 (0.04, 1.10) 4/10* |
0.18 (0.11, 0.29) 29/41* |
0.39 (0.18, 0.83) 11/28* |
| p for trend | p = 0.01 | p = 0.16 | p < 0.01 | p = 0.01 |
* Number of fatal cases/Number of patients
** Adjusted for sex, age at diagnosis and season of blood sampling
*** Quartile limits: Breast 50, 67, 86 nmol/L; Colon 44, 56, 77 nmol/L; Lung 41, 56, 76 nmol/L; Lymphoma 44, 60, 77 nmol/L
Since the quartiles for all four types of cancer combined differ from the quartiles defined for each specific type of cancer, an additional analysis was carried out. Table 3B shows the hazard ratios for each type of cancer, using cancer-specific quartile groups. The results were, however, similar.
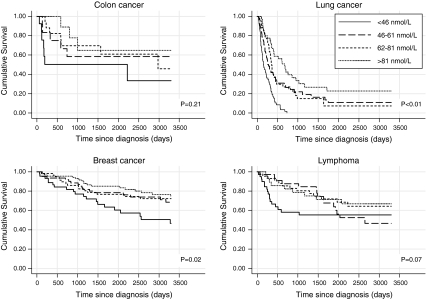
The Kaplan–Meier plots in Fig. 1 demonstrate that 25-OHD levels are associated with substantial differences in length of survival. For example, for lung cancer, the estimated median survival is 5.3 months (95% CI 3.1, 7.5) in the lowest quartile of 25-OHD and 22.6 months (95% CI 15.6, 29.6) in the highest quartile.
Fig. 1.
The cumulative survival curves for quartile groups of serum 25-OHD, for patients with cancer of breast, colon, lung, and lymphoma
Information about disease stage at the time of diagnosis was available for breast, colon, and lung cancer. Table 4 shows the risk of dying from cancer stratified by disease stage.
Table 4.
Hazard ratio (HR) and 95% confidence intervals (95% CI) of dying from the actual disease in breast, colon, and lung cancer patients stratified by stage of disease at the time of diagnosis, in quartile groups of 25-OHD
| Localized | Regional | Distant | Unknown | |
|---|---|---|---|---|
| HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | |
|
1. Quartile (<46 nmol/L) |
1.00 reference 4/12* |
1.00 reference 31/43* |
1.00 reference 43/49* |
1.00 reference 11/17* |
|
2. Quartile (46–61 nmol/L) |
0.39 (0.10, 1.59) 8/29* |
0.48 (0.27, 0.83) 23/46* |
0.73 (0.45, 1.20) 31/35* |
0.69 (0.30, 1.58) 15/26* |
|
3. Quartile (62–81 nmol/L) |
0.34 (0.09, 1.36) 6/24* |
0.39 (0.22, 0.71) 20/36* |
0.39 (0.23, 0.66) 25/33* |
0.42 (0.16, 1.05) 9/26* |
|
4. Quartile (>81 nmol/L) |
0.15 (0.03, 0.89) 4/35* |
0.29 (0.15, 0.53) 20/49* |
0.38 (0.22, 0.66) 20/25* |
0.39 (0.16, 0.98) 11/28* |
| p for trend | p = 0.06 | p < 0.01 | p < 0.01 | p = 0.04 |
* Number of fatal cases/Number of patients
** Adjusted for sex, age at diagnosis, and season of blood sampling
Because some patients might have received treatment before their blood draw, we examined the possibility that treatment could influence the vitamin D levels. We repeated the analyses in Tables 2, 3, 4, and 5 adding an indicator for blood draw before or after the day of diagnosis. The results were unchanged (data not shown).
Table 5.
Hazard ratio (HR) and 95% confidence intervals (95% CI) of dying from all causes, in cancer-specific quartile groups of 25-OHD
| Breast | Colon | Lung | Lymphoma | |
|---|---|---|---|---|
| HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | HR (95% CI)** | |
|
1. Quartile (<46 nmol/L) |
1.00 reference 27/45* |
1.00 reference 11/13* |
1.00 reference 63/63* |
1.00 reference 24/40* |
|
2. Quartile (46–61 nmol/L) |
0.55 (0.32, 0.95) 25/62* |
0.48 (0.18, 1.29) 8/12* |
0.40 (0.28, 0.59) 55/62* |
0.59 (0.32, 1.11) 18/34* |
|
3. Quartile (62–81 nmol/L) |
0.41 (0.23, 0.74) 20/58* |
0.61 (0.23, 1.59) 11/17* |
0.34 (0.22, 0.52) 38/44* |
0.46 (0.25, 0.86) 20/43* |
|
4. Quartile (>81 nmol/L) |
0.37 (0.21, 0.67) 26/86* |
0.40 (0.10, 1.60) 6/10* |
0.19 (0.12, 0.30) 34/41* |
0.33 (0.16, 0.69) 13/28* |
| p for trend | p < 0.01 | p = 0.23 | p < 0.01 | p < 0.01 |
* Number of fatal cases/Number of patients
** Adjusted for sex, age at diagnosis and season of blood sampling
Overall mortality
Among the 658 patients included in the study, only 56 died from causes other than the types of cancer under study. Results from analyses performed with all-cause mortality as the endpoint are shown in Table 5, which revealed similar results as for cancer-specific death (see Table 3A).
Discussion
We observed strong positive associations between serum levels of 25-OHD and prognosis for this young group of patients diagnosed with cancers of the breast, colon, lung, and lymphoma. For all cancers combined, the risk of dying during follow-up among patients in the highest quartile of 25-OHD was 36% (95% CI, 26%, 49%) of the risk observed among patients in the lowest quartile.
The relationship between serum 25-OHD levels and cancer survival has been examined in several studies, including our own [17]. The present analyses extend our previous findings on patients with prostate cancer. Our findings for the four cancer types are consistent with those of several recent reports. For example, Ng and colleagues [8] studied the relationship between 25-OHD levels and survival in 304 patients with colorectal cancer. They observed a significantly decreased hazard ratio (HR 0.52, 95% CI, 0.29, 0.94) for overall survival when patients in the highest quartile of 25-OHD were compared with patients in the lowest quartile. A non-significant trend toward improved colorectal cancer-specific mortality also was seen. The average interval between blood draw and cancer diagnosis in that study was 6 years.
Two studies have investigated the association between circulating 25-OHD levels, measured at the time of diagnosis, and prognosis in patients with non-small cell lung cancer, one in early-stage patients and the other in patients with advanced disease [18, 19]. A positive association between 25-OHD levels and survival was suggested for patients with early-stage disease, whereas no clear association was demonstrated for advanced stages. In a study of 512 early-stage breast cancer cases by Goodwin et al. [10], women with vitamin D levels <50 nmol/L had a significantly increased risk of distant recurrence and death compared to women with 25-OHD levels ≥50 nmol/L.
Our result for all types of lymphoma combined is comparable with the results for the solid tumors studied and is consistent with the results for two studies by Drake et al. [12] and Shanafelt et al. [13], respectively. These authors defined insufficient vitamin D status as 25-OHD levels <62.5 nmol/L and found a poorer prognosis for this group among patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia.
It is important to distinguish studies of serum vitamin D and cancer survival from studies of serum vitamin D and cancer incidence and/or cancer mortality in populations. With the exception of studies of colorectal cancer, where prospective incidence/mortality studies have generally shown protective effect of serum 25-OHD, most studies of serum 25-OHD and the risk of cancer have not shown convincing evidence of risk reduction and some have shown apparent increases in cancer risk [20]. One explanation for the discrepancy in the results of these studies concerns the effects of vitamin D on different stages in the carcinogenic process. Laboratory studies have convincingly shown effects of the hormonal form of vitamin D on cancer promotion and progression [21]. However, few data are available on the possible effects of vitamin D on cancer initiation (i.e., the initial events in causing cell transformation from normal to malignant phenotype). Thus, it is conceivable that vitamin D does not affect cancer initiation and therefore does not influence epidemiologic studies of cancer incidence. Conversely, the established effects of vitamin D on tumor promotion may underlie the positive associations observed in epidemiologic studies of cancer survival.
There are several possible explanations for the observed associations between higher serum levels of 25-OHD and improved cancer survival. These include chance, selection bias, confounding, effect of the cancer on 25-OHD levels (“reverse causality”), and effect of serum 25-OHD levels on survival. The sample size, and the similar association pattern observed for the four cancer types examined, makes chance an unlikely explanation. Because our sample is population-based with no loss to follow-up, selection bias can largely be excluded. We do not have information about lifestyle factors that are correlated to 25-OHD and that might influence survival (see Jacobs et al. [22]). Obvious factors, in particular for breast and colon cancer, would be physical activity and body mass index (BMI). In the breast cancer study by Goodwin et al. [10], information about BMI was included in the analyses. The results did not show any essential influence on the strength of the association between 25-OHD and prognosis. Even when adjusting for strong prognostic factors for breast cancer and some treatment information, only a limited attenuation was seen. Ng et al. [8] included information on BMI and post-diagnostic physical activity in their colorectal study, but no strong influence on the association between 25-OHD and survival was observed.
It has been suggested that genetic factors may account for a substantial proportion of the variability in serum 25-OHD [23, 24], and thus genetics could confound an association between serum 25-OHD and survival. However, the same genetic factors then need to be causally linked to the case fatality of the cancer. Because the positive associations between 25-OHD and cancer survival seen in the present study are strong, the association between a potential confounder and 25-OHD and the association between the potential confounder and the cancer survival rate must also be strong [25]. Thus far, studies on the heritability of vitamin D have not provided sufficient information to permit conclusions about the role of genetic factors in cancer survival [24].
An effect of cancer lowering the serum level of 25-OHD (“reverse causality”) is possible. In line with studies suggesting lower vitamin D levels among hospitalized or elderly persons, we may hypothesize that cancer also may influence the serum level of 25-OHD (see Bandeira et al. [26]). We consider that reverse causality is unlikely to be a major explanation for our findings for several reasons. First, patients with less than 1 month of survival after blood draw, for whom low levels of 25-OHD could reflect poor health, were excluded. In addition, we conducted additional analyses in which patients with less than 2 and less than 3 months of survival time were excluded. The association between 25-OHD quartiles and prognosis was almost unchanged (data not shown). Second, for diseases like breast cancer, the functional status at the time of diagnosis is often unimpaired, and the mean survival is relatively long. Moreover, as shown in Table 4, positive associations between serum levels of 25-OHD and prognosis of solid tumors are seen within each stage of disease at the time of diagnosis (although, as expected, the number of fatal cases is limited for patients with localized cancer). This observation weakens the argument for reverse causality. It is noteworthy in this regard that Fang et al. recently reported that 25-OHD levels were significantly associated with survival from prostate cancer when serum levels were determined prior to the diagnosis of cancer [11].
Alternately, it is conceivable that higher serum levels of serum 25-OHD are causally related to improved cancer survival. A significant protective effect for serum 25-OHD on mortality from all causes has recently been reported in the National Health and Nutrition Examination Survey, a population-based study in the United States [27]. This interpretation is plausible mechanistically because laboratory studies demonstrate pleiotropic anti-cancer effects of vitamin D (1,25(OH)2D) on many tumor types [8–14, 17, 28]. An improvement in survival from cancer in patients given 25-OHD has yet to be demonstrated in a randomized trial, however.
Table 3 shows that, for all types of cancer studied, patients with vitamin D levels in the lowest quartile experienced the shortest survival. The strength of the associations for the four cancer types was similar. The “cut point” for vitamin D sufficiency optimum for non-skeletal health is the subject of intense debate [29]. Whether 25-OHD levels below 50 nmol/L would be the best definition of deficiency is unclear. However, our results are comparable with those reported by Goodwin et al. [10], who also used a cut point of 50 nmol/L.
Our study has limitations, e.g., only one measurement of vitamin D status was obtained. Although the use of a single measurement potentially makes the study vulnerable to dilution effects, results of a recent population-based study demonstrate that the intra-individual variation in a single serum measurement of 25-OHD is low and that a single measurement is an accurate measurement of an individual’s long-term vitamin D status [30]. Thus, any random variation introduced by imprecision associated with single measurement would reduce the magnitude of the hazard ratios observed. The long storage time of the serum before 25-OHD measurement could also be a weakness. However, the strength of the association persisted when storage time before the analyses was included in the analyses (Table 2, Model III).
Treatment information was not available. Secular changes in the treatment conceivably could influence survival rates if, over the time course of this study, newer treatments were significantly better than older treatments and if serum levels of 25-OHD increased over time. However, this type of confounding would require improvement in treatment to have occurred for all four types of cancer under study. Survival rates, especially from lung cancer, have not changed appreciably in Norway (http://www.kreftregisteret.no\cin2009english). Moreover, our analysis indicates that 25-OHD levels actually decreased slightly by calendar year of diagnosis (i.e., changed in a conservative direction).
Conversely, this study is population-based and has several other strengths. For example, the use of date of diagnosis as the start of follow-up minimizes the possibility that the associations observed between vitamin D status and survival are due to reverse causality. The ability to control for disease stage is an important advantage as it minimizes the possibility that the apparent low survival hazard is a result of advanced disease lowering the serum levels of 25-OHD. Additionally, no potential selection has been introduced as all donors in JANUS who subsequently developed cancer and were admitted to the Norwegian Radium hospital donated a serum sample to the JANUS serum bank.
In conclusion, the present study demonstrates positive associations between circulating serum levels of 25-OHD, measured at the time of diagnosis, and length of survival for patients with cancer of breast, colon, lung, and lymphoma. These findings confirm previous ecologic data on vitamin D and cancer survival in Norway and add to a growing body of literature, indicating that serum levels of 25-OHD are positively associated with cancer survival. Intervention studies of vitamin D administration among cancer patients will be required to determine whether these observations are causal.
Acknowledgments
We address sincere thanks to JANUS serum bank, which made this study possible. The Norwegian Cancer Society is gratefully acknowledged for grant support, and we also address our thanks to Ms. Anikken Kristiansen, at the Hormone Laboratory, skillful technical assistance.
Permissions
The study was recommended by the National Committee for Research Ethics, Norway, and approved by the Norwegian Data Inspectorate, the Directorate for Health and Social Affairs, the JANUS committee, and the Protocol Committee at the Norwegian Radium hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trump DL, Hershberger PA, Bernardi RJ, Ahmed S, Muindi J, Fakih M, et al. Antitumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90:519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 3.Townsend K, Evans KN, Campbell MH, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1-alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;2:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 5.Porojnicu AC, Robsahm TE, Ree AH, Moan J. Season of prognosis is a prognostic factor in Hodgkin’s lymphoma: a possible role of sun-induced vitamin D. Br J Cancer. 2005;93:571–574. doi: 10.1038/sj.bjc.6602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porojnicu AC, Robsahm TE, Dahlback A, Berg JP, Christiani D, Bruland OS, et al. Seasonal and geographical variations in lung cancer prognosis in Norway. Does vitamin D from the sun play any role? Lung Cancer. 2007;55:263–270. doi: 10.1016/j.lungcan.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 8.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25-hydroxvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;36:2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 9.Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 11.Fang F, Kasperzyk J, Shui I, Hendrickson W, Hollis BW, Fall K, et al. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS One. 2011;6:e18625. doi: 10.1371/journal.pone.0018625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia (CLL) Blood. 2011;117:1492–1498. doi: 10.1182/blood-2010-07-295683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmieri C, MacGregor T, Girgis S, Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006;59:1334–1336. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 16.SPSS for Windows, version 15.0 (2007) Chicago, Illnois, Spss Inc
- 17.Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25 (OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heist RS, Zhou W, Wang Z, Liu G, Neuberg D, Su L, et al. Circulating 25-hydroxyvitamin D, VDR Polymorphisms, and survival in advancer non-small cell lung cancer. J Clin Oncol. 2008;26:5596–5602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Heist RS, Liu G, Asomaning K, Neuberg DS, Hollis BW, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small lung cancer patients. J Clin Oncol. 2007;25:479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer: Vitamin D and Cancer (2010) ISBN 978 92 832 2446 4
- 21.Krishnan AV, Feldman D. Mechanisms of the anticancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs ET, Martinez ME, Jurutka PW. Vitamin D: marker or mechanism of action? Cancer Epidemiol Biomarkers Prev. 2011;20:585–590. doi: 10.1158/1055-9965.EPI-10-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D’Agostino RB, Sr, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karohl C, Su S, Kumari M, Tangpricha V, Veledar E, Vaccarino V, et al. Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr. 2010;92:1393–1398. doi: 10.3945/ajcn.2010.30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bross IDJ. Pertinence of an extraneous variable. J Chron Dis. 1967;20:487–495. doi: 10.1016/0021-9681(67)90080-X. [DOI] [PubMed] [Google Scholar]
- 26.Bandeira F, Griz L Dreyer P, Eufrazino C, Bandeira C, Freese E (2006) Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metab 220; 50/4:640–646 [DOI] [PubMed]
- 27.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2009;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hines SL, Jorn HKS, Tompson KM, Larson JM. Breast cancer survivors and vitamin D: a review. Nutrition. 2010;26:255–262. doi: 10.1016/j.nut.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39:381–395. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19:927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]