Abstract
HERG (human ether-à-go-go-related gene) K+ currents fulfill important ionic functions in cardiac and other excitable cells. In addition, HERG channels influence cell growth and migration in various types of tumor cells. The mechanisms underlying these functions are still not resolved. Here, we investigated the role of HERG channels for cell growth in a cell line (SW2) derived from small cell lung cancer (SCLC), a malignant variant of lung cancer. The two HERG1 isoforms (HERG1a, HERG1b) as well as HERG2 and HERG3 are expressed in SW2 cells. Inhibition of HERG currents by acute or sustained application of E-4031, a specific ERG channel blocker, depolarized SW2 cells by 10–15 mV. This result indicated that HERG K+ conductance contributes considerably to the maintenance of the resting potential of about −45 mV. Blockage of HERG channels by E-4031 for up to 72 h did not affect cell proliferation. In contrast, siRNA-induced inhibition of HERG1 protein expression decreased cell proliferation by about 50%. Reduction of HERG1 protein expression was confirmed by Western blots. HERG current was almost absent in SW2 cells transfected with siRNA against HERG1. Qualitatively similar results were obtained in three other SCLC cell lines (OH1, OH3, H82), suggesting that the HERG1 channel protein is involved in SCLC cell growth, whereas the ion-conducting function of HERG1 seems not to be important for cell growth.
Keywords: HERG, Small cell lung cancer, Cell proliferation, Oncogenic potential, Protein expression, Membrane currents
Introduction
Small cell lung cancer (SCLC) derived from a primitive neuroectodermal stem cell is one of the most aggressive forms of cancer, with a median survival time from diagnosis of only months [23]. The poor prognosis of SCLC patients is due to the fact that SCLC metastasizes early and develops resistance towards chemo- and radiotherapy rapidly. This unfavorable prognosis stimulated the search for molecular mechanisms involved in SCLC initiation, progression, and treatment.
K+ channels are involved in the generation of the resting potential and repolarization of the action potential [10]. In addition to these ion-conducting functions, some types of K+ channels have additional cellular functions by influencing cell proliferation and migration. Voltage-dependent Kv1.3 channels for example are involved in the physiological immune response of lymphocytes comprising cell proliferation and differentiation [13, 15]. In some tumors, Kv channels are overexpressed as compared with the normal tissue and augment uncontrolled cell proliferation and metastasis, like in breast [11] and prostate [42] cancer cells. In a number of tumors, it had been demonstrated that pharmacological blockage of K+ currents inhibits cell proliferation. Despite intense research, little is known about the role which K+ channels are playing in the molecular mechanisms underlying malignant transformation from controlled physiological to uncontrolled pathological cell growth, and little is known about the mechanisms leading to inhibition of cell growth by the pharmacological blockage of K+ channels [3].
Within the large group of K+ channels, ether-à-go-go (EAG) K+ channels are known for their oncogenic potential [30, 55]. The EAG ion channel family consists of three subfamilies: EAG (ether-à-go-go; Kv10), ERG (EAG-related gene; Kv11), and ELK (EAG-like; Kv12) [8]. Attempts to clone the human EAG channel led to the detection of the human ERG (HERG) channel [50]. In the heart, the rapidly activating component of the delayed rectifying K+ current (I Kr) is mediated by HERG1 [32]. Blockage of IKr induces a prolongation of the heart action potential thereby increasing the risk of the occurrence of life-threatening arrhythmias (LQT2) [37]. In neurons, neuroendocrine and smooth muscle cells, HERG channels mediate subthreshold currents, since they activate near the resting potential and therefore modulate cell excitability [17, 19, 36].
Pharmacological blockage of HERG channels with selective blockers significantly reduces cell proliferation in primary leukemia cells [31], colon cancer cell lines [24], and other tumors [5, 40]. In the human neuroblastoma cell line SH-SY5Y [57] and melanoma cells [1], not only pharmacological ERG channel blockage but also inhibition of HERG channel protein expression effectively reduces cell proliferation. In the present work, we show that cell proliferation of SCLC cells is reduced by siRNA-induced inhibition of HERG1 channel protein expression, whereas blockage of HERG current by E-4031 does not influence cell growth. This unexpected result indicates that, in contrast to many other malignancies, in SCLC cells, the HERG channel protein itself is connected to cell proliferation by an intracellular pathway which is independent from ion flux through the HERG channel.
Methods
SCLC cell lines
SCLC cell lines (SW2, OH1, OH3, H82; kindly provided by Prof. Dr. U. Zangemeister-Wittke, University of Bern) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco Invitrogen, Carlsbad, USA) and maintained in a humidified atmosphere of 5% CO2 in air at 37°C. For electrophysiological recordings, cells were plated in 35 mm plastic culture dishes (Nunc) coated with poly-d-lysine (Sigma).
Cell proliferation assay
Cells of SCLC cell lines were incubated with a combined solution of the tetrazolium compound sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) and the electron coupling reagent PMS (N-methyl dibenzopyrazine methyl sulfate) (Roche, Mannheim, Germany). The quantity of formazan formed by reduction of the XTT reagent by viable proliferating cells was determined. The absorbance of formazan at 490 nm was directly proportional to the proliferation rate in the culture (Roche). To investigate the effect of E-4031 on cell proliferation, samples of SCLC cells were seeded at a density of 160,000 cells/ml into 96-well culture plates (Sarstedt) and incubated with 1 or 5 μM E-4031 for 24 to 72 h. Stocks of E-4031 were prepared in distilled water. Each concentration was tested in triplicate, and each experiment was repeated independently two or three times. Data are presented as means ± SEM. The statistically significant change in cell proliferation was tested by using the t-test with a Bonferroni correction.
Electrophysiology
Membrane currents were recorded in the whole-cell configuration of the patch-clamp technique, using an EPC9 patch-clamp amplifier and Pulse 8.65 software (HEKA Elektronik, Lambrecht, Germany). The patch electrodes were made from 1.5 mm diameter borosilicate glass capillaries. After filling with intracellular solution, the pipette resistance was 2–4 MΩ. Series resistance errors were compensated for by at least 70 %. All experiments were carried out at room temperature (21–23°C). HERG K+ current density was calculated by dividing the leak subtracted peak tail current amplitude measured at −100 mV by the cell capacitance as estimated from the compensation of the slow capacitance component with the patch clamp amplifier. Data were analyzed with PulseFit (HEKA) and Sigma Plot (SPSS, Chicago, IL, USA). Data are presented as means ± SEM. Availability curves were fitted with the Boltzmann equation using Sigma Plot. Student’s two-tailed paired or unpaired t-test was performed to test for significance (Excel, Microsoft).
The extracellular Ringer’s solution contained (in millimolars): 140 NaCl, 5 KCl, 0.8 MgCl2, 1 CaCl2, 10 HEPES, 5 glucose, pH adjusted to 7.3 with NaOH. The 40 mM K+ bath solution contained (in millimolars): 100 NaCl, 40 KCl, 2 MgCl2, 1 CaCl2, 10 HEPES, 5 glucose, pH adjusted to 7.3 with NaOH. Tetrodotoxin (1 μM; TTX; Biotrend, Cologne, Germany) was added to the external 40 mM KCl solution. Pipette solution contained (in millimolars): 140 KCl, 2 MgCl2, 1 CaCl2, 2.5 EGTA, 10 HEPES (66 nM free Ca2+, calculated using Eqcal, Biosoft), pH adjusted to 7.3 with KOH. E-4031 was a gift of Eisai (Tokyo, Japan).
RT-PCR of SCLC cells
RNAs were extracted from SCLC cells using RNAzol™ B (AGS, Heidelberg, Germany). DNase digestion was performed routinely before preparing cDNA. Reverse transcription and RT-PCRs with 40 cycles of amplification were performed as previously described [56]. Amplified DNA fragments were analyzed by agarose gel electrophoresis. All RT-PCRs were performed at least two times, and all DNA fragments were verified by sequencing. In negative controls, H2O was used instead of cDNA as template.
For the PCR amplifications, the following oligonucleotide primer sequences were used (accession no. of the GenBank database is given in parentheses):
HERG1, nucleotides 1398–1950 (accession no. U04270); forward 5′CTTCAAGGCCGTGTGGGACT3′; reverse 5′CAGGTTGTGCAGCCAGCCGA3′
HERG1a, nucleotides 173–603 (accession no. U04270); forward 5′ATGGGCTCAGGATGCCGGTG3′; reverse 5′GGACCCCACCATGTCCTTCT3′
HERG1b, nucleotides 327–1073 (accession no. NM_172057); forward 5′ATGGCGGCCCCAGCCGGGAA3′; reverse 5′CAGGTTGTGCAGCCAGCCGA3′
HERG2, nucleotides 2507–2993 (accession no. AF311913); forward 5′GATGAACAGGCTGGAGTCCC3′; reverse 5′GTGGCCCCAACTCCCTGCAA3′
HERG3, nucleotides 2114–2670 (accession no. AF032897); forward 5′GGAACTGCCAGGTACCACAT3′; reverse 5′GTTAGAAAGTGATCAGAAAA3′
siRNA silencing
The silencing reactions were carried out with siHERG Silencer® pre-designed siRNA (Ambion #AM16708, Austin, USA) against HERG1 with the following sequences: sense, GAUAGGCAAACCCUACAACtt; antisense, GUUGUAGGGUUUGCCUAUCtg.
In addition, we used a siRNA expression vector, pSilencer 2.0-U6, which includes a RNA polymerase III promoter (U6, Ambion) to drive expression of a short hairpin RNA that mimics the structure of a siRNA (shRNA-U6) [46]. Transfection was performed with a mix of two constructs, one against nucleotide sequence 2697–2715, the other one against nucleotide sequence 3182–3200 of r-ERG1. For transfection reactions, 60–190 nM of siHERG in the total culture volume was used. Negative controls were transfection reactions without siRNA or with the non-specific BLOCK-iT™ fluorescent oligo (Invitrogen) with the end concentration of 40–75 nM in the total cell culture volume. Transfection was carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In brief, SCLC cells were plated in culture medium in 96-well plates 24 h before transfection. The si(HERG) Silencer® pre-designed siRNA, pSilencer 2.0-U6, or BlockiT™ were diluted with opti-MEM and incubated for 5 min. In a second tube, Lipofectamine 2000 was diluted with opti-MEM and also incubated for 5 min. Both dilutions were combined and incubated for another 20 min before adding to the SCLC cells. For the proliferation studies, 6.4 × 103 cells in 80 μl culture medium per 96 microtiter well were used, and for the transfection reaction for Western blot analysis 3.0 × 105 cells in 6 ml culture medium in 50-ml flasks (24 cm2).
For electrophysiological recordings, SW2 cells were plated in 35 mm Nunc dishes coated with poly-d-lysine and transfected as described above using siRNA at end concentrations of 40 nM for BLOCK-iT and 60 nM for siHERG. In these experiments, cells were co-transfected with cDNA encoding EGFP-N1 (subcloned in pcDNA3; 0.8 ng/μl; Clontech) to identify successfully transfected cells.
Western blotting
To obtain cell lysate, SW2 cells were washed with cold phosphate-buffered saline, collected and homogenized in lysis buffer (25 mM Tris–HCl, 250 mM sucrose, 20 mM EDTA; protease inhibitors: 2 μg ml−1 leupeptin, aprotinin, and pepstatin A (Sigma); 0.1 mM phenylmethylsulfonyl fluoride (Sigma), 2 mM Na3O4V (Merck-Calbiochem), pH 7.2) and incubated on ice for 10 min. The lysate was centrifuged for 5 min at 800 g, and the cell debris was removed. For immunoblotting, the ERG1 antibody (Chemicon) targeted against the C terminus of the human correlate of ERG1, HERG1 (aa 1145–1159) [33] was used with the ECL Plus Western Blotting Detection System (Amersham). The antibody against β-actin was purchased from Sigma.
Results
HERG currents in SW2 cells
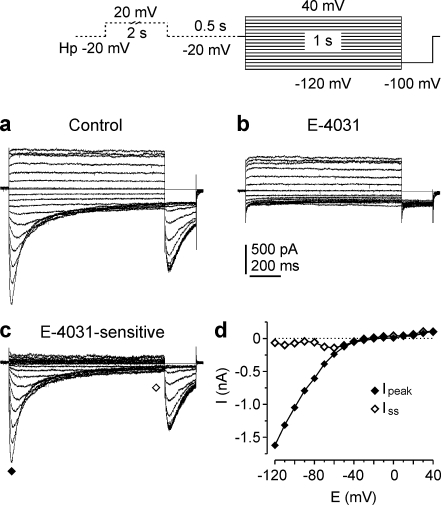
Membrane currents were recorded in SW2 cells by using experimental conditions and pulse protocols optimized to detect native ERG K+ currents [6, 20]. The external K+ concentration was increased to 40 mM, since in addition to an increase of the driving force for K+ at negative membrane potentials the ERG channel conductance is larger in an elevated external K+ concentration [41, 45]. The holding potential was shifted to a depolarized potential (−20 mV) to maximally activate HERG channels. The transient and part of the steady-state currents of the membrane currents shown in Fig. 1a were blocked by 10 μM E-4031 (Fig. 1b), a specific HERG channel blocker [44, 52]. HERG currents were isolated as E-4031-sensitive currents (Fig. 1c) by subtracting membrane currents recorded in the presence of E-4031 from control currents [7, 38]. The current-potential relation was constructed by plotting the amplitudes of the transient and steady-state HERG currents against test potential. At potentials more positive than −40 mV, HERG currents had tiny current amplitudes, whereas at potentials more negative than −40 mV transient inward currents occurred. Their amplitudes increased with more negative potentials demonstrating the strong inwardly rectifying properties of the HERG current (Fig.1d). These transient currents as well as the tail currents recorded with the constant −100 mV pulse exhibited the characteristic “hook”-like shape caused by fast recovery from HERG channel inactivation and subsequent slow deactivation. The tail current amplitudes increased with more positive prepulses until a constant current amplitude was reached at prepulses more positive than −30 mV. HERG currents were recorded in SW2 cells as well as in the three other SCLC cell lines investigated (OH1, OH3, H82; see Table 1). HERG current properties of OH1, OH3, and H82 cells were similar to those of SW2 cells (data not shown). Since the percentage of cells containing HERG currents was higher in SW2 cells (88%) than in the other cell lines (Table 1), this study focused on SW2 cells. However, some of the crucial experiments were also done on OH1, OH3, and H82 cells to test whether the results obtained in SW2 cells were similar in the four SCLC cell lines.
Fig. 1.
ERG currents in SW2 cells. Membrane currents of a SW2 cell recorded in a solution containing 40 mM K+ under control conditions (a) and in the presence of 10 μM E-4031 (b). From a holding potential of −20 mV a 2-s depolarizing prepulse to 20 mV was followed by a 0.5-s gap and 1-s test pulses to potentials between 40 and −120 mV in 10-mV increments. During a final 200 ms pulse to −100 mV tail currents were recorded (see pulse diagram). c E-4031-sensitive currents, obtained by subtracting the membrane currents shown in b from the control currents in a. Symbols denote where peak inward and outward currents (filled diamond) and steady-state currents (open diamond) were determined. d Current–potential curves, amplitudes of peak and steady-state currents of the E-4031-sensitive currents (c) were plotted versus test pulse potential. Data points were connected by continuous lines
Table 1.
E-4031 does not affect cell proliferation
| Cell line | IHERG (pA/pF), n/n+ | Cell proliferation | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| SW2 | 31.7 ± 2.5, 22/25 | Control, 1 μM E-4031; 5 μM E-4031 | 1.00 ± 0.05; 0.92 ± 0.03; 0.99 ± 0.02 | 1.00 ± 0.05; 0.86 ± 0.04; 0.90 ± 0.06 | 1.00 ± 0.03; 0.89 ± 0.06; 0.84 ± 0.04 |
| OH1 | 13.2 ± 2.8, 5/7 | Control, 1 μM E-4031; 5 μM E-4031 | 1.00 ± 0.01; 0.98 ± 0.02; 1.00 ± 0.05 | 1.00 ± 0.06; 0.96 ± 0.06; 0.84 ± 0.05 | 1.00 ± 0.02; 0.96 ± 0.02; 0.92 ± 0.05 |
| OH3 | 34.5 ± 2.7, 4/6 | Control, 1 μM E-4031; 5 μM E-4031 | 1.00 ± 0.03; 0.97 ± 0.04; 0.92 ± 0.01 | 1.00 ± 0.08; 0.93 ± 0.07; 0.92 ± 0.09 | 1.00 ± 0.05; 0.85 ± 0.03; 0.91 ± 0.05 |
| H82 | 12.7 ± 0.8, 3/5 | Control, 1 μM E-4031; 5 μM E-4031 | 1.00 ± 0.02; 1.04 ± 0.14; 0.97 ± 0.09 | 1.00 ± 0.10; 1.03 ± 0.18; 1.37 ± 0.10 | 1.00 ± 0.05; 1.11 ± 0.20; 1.09 ± 0.13 |
Cell proliferation of four SCLC cell lines (SW2, OH1, OH3, H82) measured after 24, 48, and 72 h in the presence of 1 or 5 μM E-4031 normalized to the corresponding control cells in the absence of E-4031 (mean ± SEM). Each mean value was calculated from six to nine measurements. There was no statistically significant change in cell proliferation as compared with the control cells by using the t-test and the Bonferroni correction
n number of cells containing HERG current, n+ total number of cells investigated
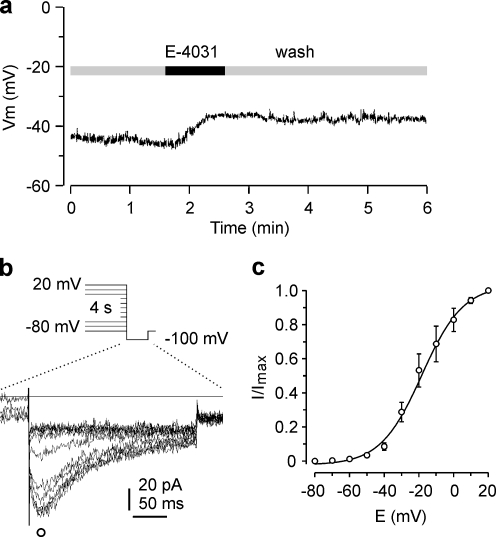
The resting potential of SW2 cells varied between −40 and −59 mV (−47 ± 2 mV; n = 8) and was depolarized by 13 ± 2 mV after application of 1 μM E-4031. Upon wash-out, no recovery was observed within 3 min (Fig. 2a) in accordance with previous observations [52]. To determine the degree of HERG channel activation near the resting potential, peak tail current amplitudes elicited with the activation protocol (see legend of Fig. 2) were measured. As in the current clamp experiments, the external solution was Ringer’s solution. From the activation curve (Fig. 2c), it can be seen that, at the resting potential of SW2 cells of about −45 mV, a fraction of HERG channel conductance was already activated, explaining its contribution to the maintenance of the resting potential.
Fig. 2.
E-4031 depolarizes the resting potential. a Recording of resting potential in a SW2 cell. Application of 3 μM E-4031 induced a depolarization which was not reversible within the recording time. b Superimposed tail current recordings elicited with a pulse to −100 mV, preceded by 4-s test pulses to potentials between 20 and −80 mV from a holding potential of −80 mV. Extracellular solution: Ringer’s with 5 mM K+. c Activation curve. Tail current amplitudes as shown in b were normalized (n = 6), averaged, plotted versus the preceding test pulse potential and fitted with a Boltzmann function, yielding V 0.5 = −18.5 mV and k = 11.9 mV
No effect of HERG channel blockage by E-4031 on cell proliferation
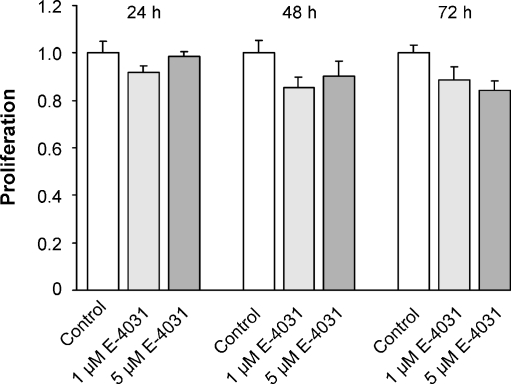
To study the effect of HERG channel blockage by E-4031 on SW2 cell proliferation, SW2 cells were incubated with 1 or 5 μM E-4031 for 24, 48, or 72 h in the culture medium. As shown in Fig. 3 and Table 1, there was no statistically significant change in the proliferation of SW2 cells as compared with the controls. Since there was a tendency to smaller values of cell proliferation after 72 h E-4031 incubation, we performed an additional experiment in which SW2 cells were incubated with E-4031 for 72 h. As compared with the controls (n = 21) incubation with 1 μM E-4031 yielded a normalized value of cell proliferation of 1.07 (n = 12) and with 5 μM E-4031 of 1.08 (n = 12). Both differences were statistically not significant. Cell proliferation was also not reduced by E-4031 in the other SCLC cell lines (OH1, OH3, H82; Table 1). This result was astonishing, since HERG channel-containing tumor cells so far investigated were sensitive to pharmacological HERG channel blockage [5, 29, 55].
Fig. 3.
No influence of ERG channel blockage by E-4031 on SW2 cell proliferation. SW2 cells were incubated without (control) and with E-4031 (1 and 5 μM) for 24, 48, and 72 h. As compared with controls, no significant change in cell proliferation was detected. Cell proliferation was normalized to control values
To test whether E-4031 was still effective after an incubation time of 72 h, a solution of 5 μM E-4031 in culture medium was stored in the incubator for 72 h. From this solution, a 100 nM E-4031 solution was prepared using an extracellular solution containing 40 mM KCl. We used 100 nM E-4031, since this is the lowest concentration which fully blocks ERG channels in GH3 cells [52]. This concentration blocked HERG currents in four of four SW2 cells (Fig. 4a) demonstrating that E-4031 was not degraded or metabolized after 72 h in culture medium at 37°C. To exclude the possibility that such a large percentage of E-4031 was bound to the proteins of the culture medium [25, 51] that the free E-4031 concentration was insufficient to produce an effective block of ERG channels during the incubation period, we perfused SW2 cells with culture medium containing 1 μM E-4031 which had been incubated for 72 h in the presence of SW2 cells. As shown in Fig. 4b, the HERG current was totally blocked under these conditions (n = 5). Since about 70% of E-4031 is bound to the protein in the culture medium [51], the remaining 30% of “free” E-4031 is sufficient to induce a full blockage of HERG channels. Perfusion of SW2 cells with preincubated culture medium in the absence of E-4031 showed no significant decrease of the transient peak HERG current amplitude, measured at −120 mV (107.0 ± 5.0% of the control current amplitude measured in Ringer’s solution; n = 4).
Fig. 4.
E-4031 remains effective after 3 days in culture. a Membrane currents recorded with 500-ms test pulses using the pulse protocol shown in Fig. 1 before (control) and after application of 100 nM E-4031. External solution contained 40 mM K+. The E-4031 solution was prepared from an E-4031 stock solution incubated for 3 days at 37°C in the culture medium. b Membrane currents recorded in Ringer’s solution containing 5 mM K+ and after perfusion of SW2 cells with preincubated culture medium containing 1 μM E-4031. The same pulse protocol was used as shown in Fig. 1 (illustrated current traces were obtained with test pulse potentials between −20 and −120 mV in steps of −10 mV)
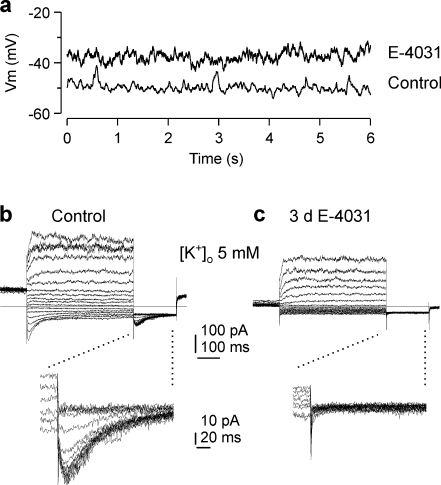
The E-4031-induced depolarization of the resting potential was discussed as one explanation for the reduction of cell proliferation in other tumors [28, 47]. To exclude the possibility that during sustained application of E-4031 the initial depolarization was removed or compensated for, we measured the resting potential in SW2 cells after the continued presence of E-4031 for 24 and 72 h. After 24 h in culture with 5 μM E-4031, SW2 cells were depolarized (−36 ± 3 mV; n = 11) as compared with control cells without E-4031 (−48 ± 3 mV; n = 7; p = 0.016). SW2 cells incubated with 5 μM E-4031 for 72 h were also depolarized (−29 ± 4 mV; n = 9) as compared with control cells (−50 ± 1 mV; n = 12; p < 0.001). Fig. 5a shows an example of the recording of the resting potential in an untreated control cell and in a cell which had been incubated together with 5 μM E-4031 for 72 h. In subsequent voltage clamp recordings, typical HERG currents were visible in all control cells (Fig. 5b), despite the small HERG channel conductance in Ringer’s solution. In contrast, HERG current was absent in the SW2 cells pretreated with E-4031. From these experiments, it was concluded that the resting potential of SW2 cells remained depolarized for at least 72 h in the presence of E-4031 and that this sustained depolarization did not affect cell proliferation. However, since the resting potential of tumor cells containing ERG channels varies with the stage of the cell cycle, part of the differences in the resting potential in the presence and absence of E-4031 could be due to the fact that SW2 cells were not synchronized in a certain stage of the cell cycle [4].
Fig. 5.
SW2 cells incubated with E-4031 for 3 days remain depolarized. a Resting potential of a control SW2 cell (control) and a SW2 cell which had been staying in culture in the presence of 5 μM E-4031 for 72 h (E-4031). The Ringer’s solution in which cells which had been incubated with E-4031 were recorded contained 2% of the 72 h preincubated 5 μM E-4031 culture medium. b, c Membrane currents of the control cell (b) and the cell preincubated with 5 μM E-4031 (c) shown in a were recorded with the pulse protocol illustrated in Fig. 1 (test pulse duration 500 ms). Tail current recordings zoomed
Inhibition of HERG1 protein expression by siRNA reduced cell proliferation
To study whether inhibition of HERG channel protein expression would reduce SW2 cell proliferation, we first determined which members of the HERG channel family were expressed in SCLC cells. Using RT-PCR, we found transcripts of the two splice variants HERG1a and HERG1b. In addition, HERG2 and HERG3 were detected in SW2 cells and in the three other SCLC cell lines (OH1, OH3, H82; Fig. 6a–c). To inhibit HERG1 expression, SW2 cells were transfected with siRNA with sequences of sense and antisense directed against both ERG1 isoforms (see “Methods”). This treatment abolished HERG current (Fig. 7c) whereas HERG currents were still recorded in SW2 cells transfected with EGFP alone (Fig. 7a; control) or together with siBlockiT (Fig. 7b). In four out of five SW2 cells transfected with siHERG and EGFP, no HERG current was recorded, in one SW2 cell a small amplitude HERG current was present (4.2 pA/pF; Fig.7d). HERG current density (pA/pF) of EGFP-expressing cells measured 48 to 58 h after transfection (pulse protocol as in Fig. 1) was 13.7 ± 8.5 pA/pF (n = 5) and 18.2 ± 8.1 pA/pF (n = 5) in cells transfected with siBlockiT. This result indicated that siRNA against ERG1 was very effective in cells successfully transfected. Since transfection with siRNA against ERG1 almost abolished HERG current, we assumed that HERG current in SW2 cells was predominantly mediated by HERG1. We therefore did not investigate the effect of siRNA against ERG2 and ERG3.
Fig. 6.
SW2 cells express multiple ERG subunits. Using RT-PCR, mRNA transcripts for HERG1a and HERG1b (a) and for HERG1, HERG2, and HERG3 were detected in four SCLC cell lines (SW2, OH1, OH3, H82) (b, c)
Fig. 7.
Inhibition of HERG currents in SW2 cells transfected with siRNA against ERG1. Membrane currents recorded from a SW2 cell transfected with EGFP alone (control) (a), and from SW2 cells transfected with siBlockiT (b) or siHERG1 (c). d Mean ERG current density measured in control cells and in cells treated with siBlockiT or siRNA against ERG1 (siHERG1). Five cells in each group
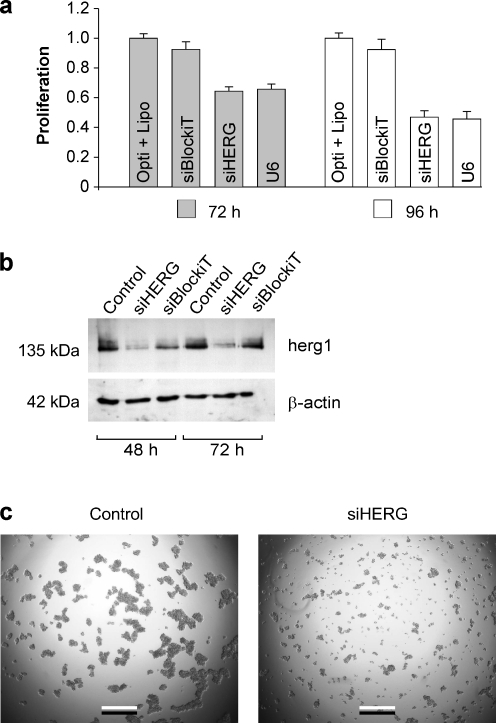
To investigate whether siRNA against ERG1 would decrease cell proliferation, SW2 cells were transfected with siRNA against ERG1 (siHERG1). Cell proliferation was significantly reduced by about 40% after 72 h and by about 60% after 96 h after siRNA transfection as compared with control cells (Fig. 8a; Table 2). Control cells were incubated with Lipofectamine alone or transfected with the nonspecific siBlockiT. In the same experiment, i.e., using the same controls, cell growth was reduced to a similar degree as with siHERG1 by inhibition of HERG channel protein expression with the short hairpin RNA against ERG1 (shRNA-U6) (Fig. 8a). No change in cell growth was observed after transfection with siBLOCKiT (Table 2). In two independent additional experiments using the same experimental design, the inhibiting effect of siRNA and shRNA-U6 on cell growth was confirmed (Table 2). The reduction of HERG1 protein content by siRNA was verified by Western blotting of SW2 cells after incubation for 48 and 72 h (Fig. 8b). The two photomicrographs (Fig. 8c “a” and “b”) show the decrease in SW2 cell growth and density in the presence of siHERG. In another set of experiments, we compared the effect of siRNA against ERG1 in SW2 cells with that in the other three cell lines. In all SCLC cell lines investigated, cell proliferation was significantly reduced by siRNA against ERG1 (Table 3). Whereas transfection of SW2, OH1, and H82 cells with siBlockiT yielded no significant change in cell growth, transfection of OH3 cells with siBlockiT significantly reduced cell proliferation. However, the decrease in siHERG1-induced cell proliferation was still significantly larger than that induced by siBlockiT (p < 0.001). Since the transfection rate was not 100%, we assume that the reduction in HERG protein content and reduction of cell proliferation would be larger if the transfection would be more efficient.
Fig. 8.
Inhibition of HERG channel protein expression decreases cell proliferation. a Data from SW2 cells transfected with siHERG for 72 h (dark columns) and 96 h (white columns). Control cells were treated with Lipofectamine and Opti-MEM only or transfected with siBlockiT. Cell proliferation was inhibited following transfection with siRNA against ERG1 (siHERG) and shRNA-U6 against ERG1 (U6). Cell proliferation was normalized to control values. For quantitative details, see Table 2. b Western blot of HERG1 protein levels in SW2 cells treated with siHERG and siBlockiT for 48 and 72 h, respectively. β-actin was used as a loading control. c Photomicrographs of SW2 cells grown in control medium or in the presence of siHERG1 for 72 h. Scale bars, 500 μm
Table 2.
Inhibition of HERG1 channel protein expression reduces cell proliferation
| Control | siBlockiT | siHERG | U6 | |
|---|---|---|---|---|
| 72 h | 1.00 ± 0.03; 1.00 ± 0.03; 1.00 ± 0.04 | 0.93 ± 0.05; 1.05 ± 0.08; 1.04 ± 0.08 | 0.64 ± 0.03***; 0.59 ± 0.03***; 0.65 ± 0.03*** | 0.66 ± 0.03***; 0.74 ± 0.02**; 0.87 ± 0.02* |
| 96 h | 1.00 ± 0.04; 1.00 ± 0.03; 1.00 ± 0.04 | 0.92 ± 0.07; 0.92 ± 0.05; 1.02 ± 0.03 | 0.47 ± 0.04***; 0.52 ± 0.04***; 0.77 ± 0.06** | 0.46 ± 0.05***; 0.74 ± 0.07**; 0.71 ± 0.08** |
Cell proliferation of SW2 cells measured after 72 and 96 h in control cells incubated with Lipofectamine only (control), siBlockiT, Lipofectamine + siHERG1, and shRNA against ERG1 (U6) (mean ± SEM). Each mean value was calculated from six measurements and normalized to the corresponding control. t-Test with Bonferroni correction was used to test for statistically significant changes as compared with the control cells
*p < 0.05, **p < 0.01,***p < 0.001
Table 3.
Inhibition of HERG1 channel protein expression reduces cell proliferation in SCLC cells
| Cell line | Control | siBlockiT | siHERG1 |
|---|---|---|---|
| SW2 | 1.00 ± 0.06 | 0.97 ± 0.05 | 0.42 ± 0.06*** |
| OH1 | 1.00 ± 0.08 | 0.84 ± 0.08 | 0.73 ± 0.07* |
| OH3 | 1.00 ± 0.09 | 0.49 ± 0.03*** | 0.21 ± 0.03*** |
| H82 | 1.00 ± 0.06 | 0.82 ± 0.05 | 0.36 ± 0.03*** |
Cell proliferation measured in four SCLC cell lines (SW2, OH1, OH3, H82) after incubation with Lipofectamine alone (control), siBlockiT, and siRNA against ERG1 (siHERG1) for 96 h (mean ± SEM). Each mean value was calculated from 12 measurements and normalized to the mean value of the corresponding control. The level of statistical significance as compared with the controls was calculated by using the t-test with Bonferroni correction
*p < 0.05, **p < 0.01, ***p < 0.001 are the levels of significance
Discussion
We found that, in SW2 cells as well as in three other SCLC cell lines (OH1, OH3, H82), HERG1 channels are expressed and that HERG2 and HERG3 transcripts were also present. Selective pharmacological blockage of HERG currents with E-4031 did not influence SCLC cell proliferation. However, siRNA inhibition of HERG1 protein expression almost abolished HERG currents and strongly reduced SCLC cell proliferation. These results suggest that HERG currents in these tumor cells are predominantly mediated by HERG1 channels and that the presence of HERG1 channel protein is important for cell growth, whereas the conductive function of HERG channels does not influence cell proliferation of SCLC cells.
Pharmacological blockage of Kv channels and cell growth
So far, the main emphasis in the discussion about the oncogenic potential of ERG K+ channels was concentrated on the observation that pharmacological blockage of K+ channels reduced cell growth. In 1996, Wonderlin and Strobl [54] reported that pharmacological blockage of K+ channels with tetraethylammonium-chloride (TEA), quinidine, or 4-aminopyridine (4-AP) reduces cell proliferation in healthy cells (lymphocytes and Schwann cells) as well as in tumor cells (melanoma and breast cancer cells). Since their review, the list of normal tissue, tumor cell lines, and primary tumors which show reduced cell growth upon pharmacological blockage of K+ channels has grown [5, 28, 47, 55]. However, the mechanisms underlying the inhibitory effects of pharmacologically blocked K+ channels on cell growth, migration, or invasiveness remain still elusive. Several hypotheses have been discussed. First, it had been argued that the depolarization induced by K+ channel block or K+ channel activity itself could be important, either because of the ion flux through the channel pore or due to the accompanying local changes in the K+ concentration. Second, it was assumed that movements of parts of the K+ channel protein during gating could influence neighboring scaffold proteins or proteins involved in the cell cycle [18]. Previously, it has been shown that, even in some SCLC cell lines (H69, H128, H146) different from those studied in this project, incubation with TEA or 4-AP inhibited cell growth. Since both substances are affecting a broad band of K+ channels including ERG channels [35, 48], it is not clear which type of K+ channel is responsible for this inhibition [26, 27].
ERG channels and cell growth
As compared with normal tissue, ERG K+ channels are either overexpressed or newly expressed in primary tumors of various histogenesis [11, 34]. Selective pharmacological blockage of ERG channels reduced cell proliferation and tumor invasiveness in almost all tumor cells so far investigated, e.g., in hematopoetic malignancies [31, 43], colorectal carcinomas [16], and melanoma cells [1]. The function of HERG channels in tumor cells was thought to contribute to the maintenance of a more depolarized membrane potential, thereby permitting an easier passage through the cell cycle, since depolarization is needed for cell cycle progression [29]. In human primary acute myeloid leukemia, the amount of expression of the two isoforms HERG1a and HERG1b is strongly cell cycle-dependent, with HERG1a predominantly expressed during the G1 phase and HERG1b in the S phase, thereby influencing the relative amount of HERG channel heteromers or homomers [14]. Since in SCLC cells, at least four HERG subunits are expressed (HERG1a, HERG1b, HERG2, and HERG3), they could form heteromeric channels [53]. This heteromeric channel formation is also possible for human prolactinoma cells [9]. However, we do not know to which degree heteromeric HERG channels are formed and whether all or only HERG1 channel subunits support cell proliferation. So far, the literature has been dealing predominantly with HERG1 and its two main isoforms. Our present data support the importance of HERG1 in SCLC cells, especially our result that siRNA against HERG1, i.e., the two isoforms of HERG1, strongly reduced HERG current, although transcripts of HERG2 and HERG3 subunits were also expressed.
It is still a mystery which role ERG channels play in the malignant transformation from a normal cell to a tumor cell and which functional role they play for tumor growth, invasiveness, and formation of metastases. In melanoma and SH-SY5Y neuroblastoma cells, proliferation and migration of cells are attenuated by both, pharmacological HERG channel blockage with E-4031 and siRNA-induced inhibition of HERG protein expression [1, 57]. In contrast, our data show that pharmacological blockage of HERG channels in four defined SCLC cell lines does not influence cell proliferation, whereas cell proliferation is reduced by a decrease in the cellular content of the HERG channel protein. Since not all SCLC cells were transfected with siRNA against HERG1, the decrease of HERG channel protein and probably the ensuing degree of cell growth reduction would have been larger with a higher transfection rate. Probably, the transfection rate could be increased by using viral transduction [2]. We did not analyze the function of the HERG protein in the cellular processes underlying cell growth. It could be possible that the HERG channel protein is necessary for cell cycle regulation [39]. ERG channel proteins have also been shown to interact with integrins, thereby regulating cell survival and migration [3]. In neuroblastoma cells, ERG channels are activated upon integrin-mediated adhesion of the cells to extracellular matrix molecules like fibronectin and vitronectin [21]. This important functional linkage between ERG channels and integrins may explain the involvement of ERG channels in cell migration. Other proteins presumably involved in mitogenesis have been reported to bind to HERG1 channels, like the adaptor protein 14-3-3 [22], Src tyrosine kinase [12], or the TNF-α receptor [49]. Preliminary experiments in our laboratory have shown that there is also a linkage between the cell adhesion molecule NCAM and HERG1 in SW2 cells (unpublished data by Kleene, Schumacher, Schwarz).
HERG channel blockers have been used to reduce cell growth in some types of tumors [5, 40, 55]. From our experiments, it can be inferred that pharmacological blockage of HERG channels is not a therapeutically useful approach in SCLC cancer. A starting point for considering a potential therapeutic strategy in SCLC cancer could be the inhibition of cell proliferation by siRNA. Indeed, in vivo experiments have recently shown that injection of siRNA against HERG1 into SH-SY5Y tumors which had been inoculated subcutaneously in nude mice inhibited tumor growth [57]. Another yet relatively unexplored field is the use of HERG channel proteins as specific tumor markers [16].
Acknowledgment
We thank Renate Gehrcke, Annett Hasse, and Telse Kock for their continuous support in the lab and Annika Lange for her help in measuring cell proliferation. Supported by the Deutsche Forschungsgemeinschaft (Schw292/14-1).
Disclosure statement
The authors of this manuscript have nothing to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Afrasiabi E, Hietamaki M, Viitanen T, Sukumaran P, Bergelin N, Tornquist K. Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. Cell Signal. 2010;22:57–64. doi: 10.1016/j.cellsig.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, Schneider IC, Munch RC, Petznek H, Kontermann RE, Koehl U, Johnston IC, Keinanen K, Muller UC, Hohenadl C, Monyer H, Cichutek K, Buchholz CJ. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods. 2010;7:929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- 3.Arcangeli A, Becchetti A. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 2006;16:631–639. doi: 10.1016/j.tcb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. A novel inward-rectifying K+current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 6.Bauer CK, Meyerhof W, Schwarz JR. An inward-rectifying K+ current in clonal rat pituitary cells and its modulation by thyrotrophin-releasing hormone. J Physiol. 1990;429:169–189. doi: 10.1113/jphysiol.1990.sp018250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer CK, Schafer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the Erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Mol Cell Endocrinol. 1999;148:37–45. doi: 10.1016/S0303-7207(98)00241-X. [DOI] [PubMed] [Google Scholar]
- 8.Bauer CK, Schwarz JR. Physiology of EAG K+ channels. J Membr Biol. 2001;182:1–15. doi: 10.1007/s00232-001-0031-3. [DOI] [PubMed] [Google Scholar]
- 9.Bauer CK, Wulfsen I, Schafer R, Glassmeier G, Wimmers S, Flitsch J, Ludecke DK, Schwarz JR. HERG K(+) currents in human prolactin-secreting adenoma cells. Pflugers Arch. 2003;445:589–600. doi: 10.1007/s00424-002-0980-0. [DOI] [PubMed] [Google Scholar]
- 10.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. HERG encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- 12.Cayabyab FS, Schlichter LC. Regulation of an ERG K+ current by Src tyrosine kinase. J Biol Chem. 2002;277:13673–13681. doi: 10.1074/jbc.M108211200. [DOI] [PubMed] [Google Scholar]
- 13.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, Wymore RS, Arcangeli A. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem. 2003;278:2947–2955. doi: 10.1074/jbc.M210789200. [DOI] [PubMed] [Google Scholar]
- 15.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 16.Dolderer JH, Schuldes H, Bockhorn H, Altmannsberger M, Lambers C, von Zabern D, Jonas D, Schwegler H, Linke R, Schroder UH. HERG1 gene expression as a specific tumor marker in colorectal tissues. Eur J Surg Oncol. 2009;36:72–77. doi: 10.1016/j.ejso.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Hardman RM, Forsythe ID. Ether-a-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. J Physiol. 2009;587:2487–2497. doi: 10.1113/jphysiol.2009.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc Natl Acad Sci U S A. 2006;103:2886–2891. doi: 10.1073/pnas.0505909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirdes W, Napp N, Wulfsen I, Schweizer M, Schwarz JR, Bauer CK. ERG K+ currents modulate excitability in mouse mitral/tufted neurons. Pflugers Arch. 2009;459:55–70. doi: 10.1007/s00424-009-0709-4. [DOI] [PubMed] [Google Scholar]
- 20.Hirdes W, Schweizer M, Schuricht KS, Guddat SS, Wulfsen I, Bauer CK, Schwarz JR. Fast ERG K+ currents in rat embryonic serotonergic neurons. J Physiol. 2005;564:33–49. doi: 10.1113/jphysiol.2004.082123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, Lastraioli E, Polvani S, Bartolozzi B, Solazzo V, Gragnani L, Defilippi P, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG K + channels activation during β1 integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3 integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem. 2001;276:4923–4931. doi: 10.1074/jbc.M005682200. [DOI] [PubMed] [Google Scholar]
- 22.Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J. 2002;21:1889–1898. doi: 10.1093/emboj/21.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12:1096–1104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 24.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, Bencini L, Calistri M, Messerini L, Scatizzi M, Moretti R, Wanke E, Olivotto M, Mugnai G, Arcangeli A. HERG1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.CAN-03-2360. [DOI] [PubMed] [Google Scholar]
- 25.Masi A, Becchetti A, Restano-Cassulini R, Polvani S, Hofmann G, Buccoliero AM, Paglierani M, Pollo B, Taddei GL, Gallina P, Di Lorenzo N, Franceschetti S, Wanke E, Arcangeli A. HERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer. 2005;93:781–792. doi: 10.1038/sj.bjc.6602775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pancrazio JJ, Tabbara IA, Kim YI. Voltage-activated K+ conductance and cell proliferation in small-cell lung cancer. Anticancer Res. 1993;13:1231–1234. [PubMed] [Google Scholar]
- 27.Pancrazio JJ, Viglione MP, Tabbara IA, Kim YI. Voltage-dependent ion channels in small-cell lung cancer cells. Cancer Res. 1989;49:5901–5906. [PubMed] [Google Scholar]
- 28.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- 29.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205:115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 30.Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, Stuhmer W. Oncogenic potential of EAG K+ channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, Bartolozzi B, Becchetti A, Wanke E, Bernabei PA, Olivotto M, Pegoraro L, Arcangeli A. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–1798. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- 32.Pond AL, Nerbonne JM. ERG proteins and functional cardiac IKr channels in rat, mouse, and human heart. Trends Cardiovasc Med. 2001;11:286–294. doi: 10.1016/S1050-1738(01)00127-X. [DOI] [PubMed] [Google Scholar]
- 33.Pond AL, Scheve BK, Benedict AT, Petrecca K, Van Wagoner DR, Shrier A, Nerbonne JM. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional IKr channels. J Biol Chem. 2000;275:5997–6006. doi: 10.1074/jbc.275.8.5997. [DOI] [PubMed] [Google Scholar]
- 34.Raschi E, Vasina V, Poluzzi E, De Ponti F. The HERG K+ channel: target and antitarget strategies in drug development. Pharmacol Res. 2008;57:181–195. doi: 10.1016/j.phrs.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Ridley JM, Milnes JT, Zhang YH, Witchel HJ, Hancox JC. Inhibition of HERG K+ current and prolongation of the guinea-pig ventricular action potential by 4-aminopyridine. J Physiol. 2003;549:667–672. doi: 10.1113/jphysiol.2003.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacco T, Bruno A, Wanke E, Tempia F. Functional roles of an ERG current isolated in cerebellar Purkinje neurons. J Neurophysiol. 2003;90:1817–1828. doi: 10.1152/jn.00104.2003. [DOI] [PubMed] [Google Scholar]
- 37.Sanguinetti MC, Tristani-Firouzi M. HERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 38.Schafer R, Wulfsen I, Behrens S, Weinsberg F, Bauer CK, Schwarz JR. The ERG-like potassium current in rat lactotrophs. J Physiol. 1999;518(Pt 2):401–416. doi: 10.1111/j.1469-7793.1999.0401p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008;7:45–50. doi: 10.4161/cbt.7.1.5126. [DOI] [PubMed] [Google Scholar]
- 40.Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther. 2005;4:295–301. doi: 10.4161/cbt.4.3.1500. [DOI] [PubMed] [Google Scholar]
- 41.Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skryma RN, Prevarskaya NB, Dufy-Barbe L, Odessa MF, Audin J, Dufy B. Potassium conductance in the androgen-sensitive prostate cancer cell line, LNCaP: involvement in cell proliferation. Prostate. 1997;33:112–122. doi: 10.1002/(SICI)1097-0045(19971001)33:2<112::AID-PROS5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528–18534. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- 44.Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res. 1996;78:499–503. doi: 10.1161/01.res.78.3.499. [DOI] [PubMed] [Google Scholar]
- 45.Sturm P, Wimmers S, Schwarz JR, Bauer CK. Extracellular potassium effects are conserved within the rat ERG K+ channel family. J Physiol. 2005;564:329–345. doi: 10.1113/jphysiol.2004.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sui G, Soohoo C, el Affar B, Gay F, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Myers CD, Robertson GA. Dynamic control of deactivation gating by a soluble amino-terminal domain in HERG K+ channels. J Gen Physiol. 2000;115:749–758. doi: 10.1085/jgp.115.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- 50.Warmke JW, Ganetzky B. A family of potassium channel genes related to EAG in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webster R, Allan G, Anto-Awuakye K, Harrison A, Kidd T, Leishman D, Phipps J, Walker D. Pharmacokinetic/pharmacodynamic assessment of the effects of E4031, cisapride, terfenadine and terodiline on monophasic action potential duration in dog. Xenobiotica. 2001;31:633–650. doi: 10.1080/00498250110054632. [DOI] [PubMed] [Google Scholar]
- 52.Weinsberg F, Bauer CK, Schwarz JR. The class III antiarrhythmic agent E-4031 selectively blocks the inactivating inward-rectifying potassium current in rat anterior pituitary tumor cells (GH3/B6 cells) Pflugers Arch. 1997;434:1–10. doi: 10.1007/s004240050356. [DOI] [PubMed] [Google Scholar]
- 53.Wimmers S, Wulfsen I, Bauer CK, Schwarz JR. ERG1, ERG2 and ERG3 K channel subunits are able to form heteromultimers. Pflugers Arch. 2001;441:450–455. doi: 10.1007/s004240000467. [DOI] [PubMed] [Google Scholar]
- 54.Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- 55.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wulfsen I, Hauber HP, Schiemann D, Bauer CK, Schwarz JR. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J Neuroendocrinol. 2000;12:263–272. doi: 10.1046/j.1365-2826.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Wei XL, Jia YS, Zheng JQ. Silencing of HERG gene by shRNA inhibits SH-SY5Y cell growth in vitro and in vivo. Eur J Pharmacol. 2008;579:50–57. doi: 10.1016/j.ejphar.2007.10.008. [DOI] [PubMed] [Google Scholar]