Abstract
Summary
Advancing maternal age has been related to increased risk of fetal death and morbidity, as well as higher fracture risk during childhood, in the offspring. In the present study, we demonstrate that advancing maternal age is independently associated with reduced bone mass in the young adult male offspring.
Introduction
In Sweden the maternal age in both primi- and multipara mothers has steadily increased during the last three decades. It has been previously reported that advancing maternal age increases the risk of fetal death, but also of morbidity in the offspring, such as chromosome abnormalities, leukemia, diabetes mellitus type 1, and schizophrenia. Whether or not maternal age influences peak bone mass has not been reported. The aim of the present study was to investigate whether a high maternal age was associated with lower peak bone mass, as measured using DXA in a large cohort of male offspring [the Gothenburg Osteoporosis and Obesity Determinants study (GOOD)].
Methods
Through the Swedish multi-generation register, we identified the mothers of 1,009 GOOD study subjects. From the Swedish medical birth register detailed information about the medical circumstances at the time of child birth were obtained, including maternal and offspring anthropometrics (birth height and weight), maternal age, and smoking habits, parity and length of pregnancy.
Results
Maternal age was inversely correlated to areal BMD (aBMD) at the total body (r =−0.07, p = 0.03) and the lumbar spine (r =−0.09, p < 0.01). Using a linear regression model (with covariates including current physical activity, smoking, calcium intake, weight, present height and birth height, total body lean and fat mass in the offspring, and length of pregnancy), we found that maternal age negatively independently predicted lumbar spine aBMD (β =−0.08, p < 0.01) in the male offspring.
Conclusions
In conclusion, our results suggest that advancing maternal age could negatively affect bone mass in young adult men.
Keywords: Bone mineral density, Dual X-ray absorptiometry, Maternal age, Men
Introduction
In Sweden, the maternal age in both primi- and multipara mothers has steadily increased during the last three decades. In this period, the mean age of mothers giving birth, both primi- and multipara included, increased from 26.0 to 30.3 years of age. For primiparous women only, the age has increased from 23.8 to 28.4 years of age during the same period. In urban areas in Sweden, the age of mothers giving birth to their first born increased even more, from 24.8 years in 1973 to 30.1 years in 2005 [1]. It has been previously reported that advancing maternal age increases the risk of fetal death [2, 3], but also of other morbidities in the offspring, such as chromosome abnormalities and childhood cancers like leukemia and retinoblastoma [4, 5]. The maternal age has also been associated with the development of diabetes mellitus type 1 and schizophrenia in the offspring, but these associations were also found to be dependent on paternal age [6, 7]. A recently published prospective study in a Brazilian cohort has shown a higher incidence of fracture between birth and the age of 11 in children of older mothers [8]. Whether maternal age influences bone mass in the offspring has, however, not been reported.
Peak bone mass (PBM) has been shown to be mainly attained before the end of the second decade in life and has been demonstrated to account for up to half of the variation in BMD at age 65, indicating an important role of the level of PBM on the risk of developing osteoporosis [9–11]. PBM has been shown to be influenced mostly by genetic factors, but also environmental factors such as calcium and vitamin D intake and physical activity [12, 13]. Given the trend of increasing maternal age in industrialized countries and the previously reported studies revealing high maternal age as a risk factor for several diseases and fracture in the offspring, we wished to test the hypothesis if high maternal age was also associated with the skeletal phenotype in the offspring. In the present study, we examined whether high maternal age was associated with lower adult bone mass as measured using dual-X-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT) in a large cohort of male offspring at the age of PBM [11].
Materials and methods
The Gothenburg Osteoporosis and Obesity Determinants (GOOD) study was initiated with the aim to determine both environmental and genetic factors involved in the regulation of bone and fat mass. Through national population registers, study subjects were randomly identified, and by telephone, were asked to participate in the study. As the only exclusion criteria, subjects had to be between 18 and 20 years of age and willing to participate in the study. A total of 1,068 young men with the mean age of 18.9 ± 0.6 years were included, corresponding to 48.6% of the initially contacted study subjects. A standardized questionnaire was used to collect information about present amount of physical activity (hours/week, duration in years), smoking (yes or no), and calcium intake estimated from daily dairy product intake.
The GOOD study was approved by the local ethics committee at Gothenburg University. Written and oral informed consent was obtained from all the study participants.
Anthropometric measurements
Height and weight were measured using standardized equipment. The coefficient of variation (CV) values were less than 1% for these measurements.
Dual X-ray absorptiometry (DXA)
Total body lean mass, total body fat mass and areal bone mineral density (aBMD) (grams per square centimeter), bone mineral content (grams), and bone area (square centimeter) of the whole body, femoral neck and lumbar spine were assessed using the Lunar Prodigy DXA (GE Lunar Corp,. Madison, WI, USA). The CVs for total body lean mass and total body fat mass were 1.8% and 3.4%, respectively, and the CVs for the aBMD measurements were ranging from 0.5 to 3%.
Peripheral quantitative computed tomography (pQCT)
pQCT scans were performed using a single energy X-ray pQCT device (XCT-2000; Stratec Medizintechnik, GmbH, Pforzheim, Germany). Scans were made on the non-dominant arm through the diaphysis of the radius (at 25% of the bone length in the proximal direction of the distal end of the bone) to obtain cortical volumetric bone mineral density (vBMD; mg/cm3), cortical cross-sectional area (CSA; mm2), endosteal and periosteal circumference (mm). Trabecular vBMD was measured using a scan through the metaphysis of the radius (at 4% of the bone length in the proximal direction of the distal end of the bone). The CVs were less than 1% for all pQCT analyses.
Data on the mothers
Through the Swedish Multi-Generation Register, we identified the mothers of 1,009 GOOD study subjects. Maternal parameters were then obtained from the Swedish Medical Birth Register, which contains detailed information about the medical circumstances at the time of child birth, including maternal and offspring anthropometrics (height and weight), maternal age and smoking habits, parity and length of pregnancy. All mothers were de-identified by the administrative authority Statistics Sweden. Hence, the authors could not distinguish any mother by name, social security number, or by any other means.
Socioeconomic status
Information about the social position of the parents in 1985 (GOOD subjects born between 1983 and 1985) were obtained from Statistics Sweden as socioeconomic index (SEI), which is a well-recognized classification based on the expected level of education that comes with a certain occupation. Each study subject obtained a household SEI, which is determined by an order of dominance were the household received the highest SEI of the two parents [14]. By using the abovementioned order of dominance, the subjects were then divided into three major socioeconomic groups where group 1 corresponded to skilled and unskilled manual workers and non-manual workers on lower level. Group 2 corresponded to non-manual workers on midrange level and group 3 corresponded to non-manual workers on higher level.
Statistical analysis
Bivariate correlations were assessed using Pearson's correlation. Independent predictors of bone measurements were calculated using a stepwise linear regression model. In the first step variables correlated to aBMD of the lumbar spine were included and in the second step also variables correlated to maternal age were included. Multiple regression using spline functions was applied to estimate the relationship between maternal age and aBMD. The regression function was comprised by linear pieces at the ends and quadratic functions in the intermediate intervals, and the knots were chosen at the percentiles of maternal age, 10th percentile = 24 years (age), 50th = 29 years, and 90th = 36 years.
The comparison of bone measurements of subjects with mothers in the 90th percentile of age with all other mothers was assessed using independent samples T-test. Bone parameters adjusted for covariates were calculated using linear regression equations. A p value less than 0.05 was considered significant. All statistical analyses were performed using SPSS (version 16.0; SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the GOOD cohort
The characteristics of the young men, including current anthropometric data, as well as at the time of birth, calcium intake, smoking (yes or no), current level of physical activity (hours/week), total body adipose tissue, and lean mass are given in Table 1. Parental characteristics, including maternal and paternal age, maternal anthropometrics, maternal smoking in early pregnancy, maternal parity, length of pregnancy, vaginal delivery, or caesarian section and socioeconomic index of the household in 1985, are also presented in Table 1. Bone parameters, including aBMD, BMC and area of the total body, lumbar spine, femoral neck and the non-dominant radius, and cortical and trabecular vBMD, cortical cross-sectional area, periosteal and endosteal circumference of the non-dominant radius are given in Table 2.
Table 1.
Anthropometric characteristics, environmental factors, and circumstances at the time of birth of men in the GOOD cohort as well as parental characteristics
| Variables | No. | Mean ± SD |
|---|---|---|
| GOOD cohort | ||
| Age (year) | 1,009 | 18.9 ± 0.6 |
| Height (cm) | 1,009 | 181.7 ± 6.6 |
| Weight (kg) | 1,009 | 74.0 ± 11.9 |
| Calcium intake (mg/day) | 1,009 | 1,108 ± 727 |
| Smoking (%) | 1,009 | 9.0 |
| Physical activity (hours/week) | 1,009 | 4.3 ± 5.2 |
| Total body adipose tissue (kg) | 1,009 | 13.4 ± 8.0 |
| Total body lean mass (kg) | 1,009 | 57.6 ± 6.1 |
| Birth height (cm) | 998 | 50.8 ± 2.1 |
| Birth weight (g) | 977 | 3,580 ± 547 |
| Parental variables at the time of childbirth | ||
| Maternal age (year) | 1,009 | 29.5 ± 4.8 |
| Paternal age (year) | 1,002 | 32.6 ± 5.5 |
| Maternal height (cm) | 832 | 167.1 ± 5.8 |
| Maternal weight before pregnancy (kg) | 885 | 60.5 ± 8.2 |
| Maternal smoking in early pregnancy (%) | 967 | 25.7 |
| Maternal parity (n) | 1,009 | 1.65 ± 0.83 |
| Vaginal delivery (%) | 1,008 | 86.0 |
| Caesarean section (%) | 1,008 | 14.0 |
| Length of pregnancy (day) | 1,009 | 278 ± 12 |
| Socioeconomic index of the household 1985a | 960 | 2.04 ± 0.77 |
Table 1. Mean values and standard deviations
aBMD areal bone mineral density; BMC bone mineral content
aSocioeconomic index given from 1 to 3, where 1 is lower social position and 3 is higher
Table 2.
Bone parameters and their correlation and association with maternal age
| Bone variables | Mean ± SDa | r valuea | β-coefficientsb | β-coefficientsc | β-coefficientsd | |
|---|---|---|---|---|---|---|
| DXA | Total body aBMD (g/cm2) | 1.25 ± 0.10 | −0.070* | −0.036 | −0.032 | −0.031 |
| Lumbar spine aBMD (g/cm2) | 1.24 ± 0.15 | −0.092** | −0.076** | −0.076** | −0.091** | |
| Femoral neck aBMD (g/cm2) | 1.17 ± 0.16 | −0.021 | −0.006 | 0.001 | 0.007 | |
| Radius non-dominant aBMD (g/cm2) | 0.58 ± 0.06 | −0.062* | −0.035 | −0.005 | −0.004 | |
| Total body BMC (g) | 3,209 ± 447 | −0.055 | −0.040* | −0.038 | −0.033 | |
| Lumbar spine BMC (g) | 61.5 ± 10.9 | −0.081* | −0.078** | −0.084** | −0.090** | |
| Femoral neck BMC (g) | 6.45 ± 1.07 | −0.029 | −0.013 | −0.013 | −0.003 | |
| Radius non-dominant BMC (g) | 10.1 ± 1.5 | −0.077* | −0.075*** | −0.071** | −0.069** | |
| Total body area (cm2) | 2,561 ± 198 | −0.026 | −0.034 | −0.036 | −0.031 | |
| Lumbar spine area (cm2) | 49.5 ± 4.8 | −0.030 | −0.056* | −0.065** | −0.061* | |
| Femoral neck area (cm2) | 5.52 ± 0.39 | −0.022 | −0.034 | −0.028 | −0.015 | |
| Radius non-dominant area (cm2) | 17.4 ± 1.9 | −0.053 | −0.076** | −0.094*** | −0.091** | |
| pQCT | Radius cortical vBMD (mg/cm3) | 1,164 ± 23 | −0.009 | −0.010 | −0.025 | 0.005 |
| Radius cortical CSA (mm2) | 96.1 ± 11.7 | −0.073* | −0.068** | −0.064* | −0.046 | |
| Radius periosteal circumference (mm) | 42.1 ± 2.9 | −0.098** | −0.093*** | −0.079** | −0.158*** | |
| Radius endosteal circumference (mm) | 23.8 ± 3.1 | −0.093** | −0.093** | −0.144*** | −0.185*** | |
| Radius trabecular vBMD (mg/cm3) | 219 ± 41 | −0.014 | 0,010 | 0.019 | −0.007 | |
Table 2 Mean values and standard deviations. Bivariate correlation with maternal age was assessed using Pearson's correlation. r values are presented. Standardized β-coefficients were assessed using a stepwise linear regression model
a n = 1,009
b n = 997, adjusted for calcium intake, current level of physical activity, adult height and weight, birth height, total body adipose tissue and lean mass, length of pregnancy, and present smoking in the offspring
c n = 907, adjusted for calcium intake, current level of physical activity, adult height and weight, birth height, total body adipose tissue and lean mass, length of pregnancy, present smoking in the offspring, SEI-index, maternal parity, maternal smoking, and paternal age
d n = 705, adjusted for calcium intake, current level of physical activity, adult height and weight, birth height, total body adipose tissue and lean mass, length of pregnancy, present smoking in the offspring, SEI-index, maternal parity, maternal smoking, paternal age, maternal weight prior to pregnancy, and maternal height
*p < 0.05, **p < 0.01, ***p < 0.001
Bivariate correlations between maternal age and characteristics of the young men and other parental characteristics
Maternal age was directly correlated to socioeconomic status in 1985, parity and paternal age while it was inversely correlated to the current level of physical activity in the offspring, length of pregnancy, and smoking in early pregnancy (Table 3).
Table 3.
Associations between maternal age, anthropometrics and parental variables, and other related variables
| Variables | Maternal age (years) | |
|---|---|---|
| Offspring characteristics | r value | p value |
| Age (year) | −0.044 | 0.166 |
| Height (cm) | 0.060 | 0.056 |
| Weight (kg) | −0.056 | 0.075 |
| Calcium intake (mg/day) | −0.019 | 0.545 |
| Smoking (yes/no) | −0.061 | 0.051 |
| Physical activity (hours/week) | −0.063 | 0.047 |
| Total body adipose tissue (kg) | −0.059 | 0.061 |
| Total body lean mass (kg) | −0.012 | 0.693 |
| Birth height (cm) | 0.045 | 0.154 |
| Birth weight (g) | 0.054 | 0.093 |
| Parental characteristics | ||
| Socioeconomic index 1985 | 0.341 | <0.001 |
| Maternal characteristics | ||
| Length of pregnancy (day) | −0.087 | 0.006 |
| Parity (n) | 0.392 | <0.001 |
| Weight before pregnancy (kg) | 0.027 | 0.415 |
| Height (cm) | 0.008 | 0.810 |
| Smoking in early pregnancy (yes/no) | −0.106 | 0.001 |
| Caesarian section (yes/no) | 0.058 | 0.067 |
| Paternal characteristics | ||
| Age (year) | 0.670 | <0.001 |
Table 3 Pearson's correlation were used. r and p values are shown
Bivariate correlations between maternal age and bone parameters in the offspring
Maternal age was inversely correlated to aBMD at the total body and to aBMD and BMC at the lumbar spine and the non-dominant radius. There were also an inverse relationship found between maternal age and cortical cross-sectional area and periosteal and endosteal circumference of the non-dominant radius (Table 2).
Correlations between aBMD at the lumbar spine, parental characteristics and other characteristics of the GOOD cohort
In addition to maternal age, aBMD at the lumbar spine was also inversely correlated with present smoking (r = −0.093, p = 0.003) in the offspring and directly correlated to calcium intake (r = 0.138, p = <0.001), current level of physical activity (r = 0.286, p = <0.001), adult height (r = 0.145, p = <0.001) and weight (r = 0.347, p = <0.001), birth height (r = 0.065, p = 0.041), total body adipose tissue (r = 0.122, p = <0.001), and lean mass (r = 0.440, p = <0.001) and length of pregnancy (r = 0.078, p = 0.013).
No correlation was seen with aBMD at the lumbar spine and the other variables correlated to maternal age, i.e., socioeconomic status of the household in 1985 (r = −0.043, p = 0.180), parity of the mothers (r = 0.014, p = 0.645), maternal smoking in early pregnancy (r = 0.013, p = 0.688), and paternal age (r = −0.042, p = 0.179). Nor was lumbar spine aBMD correlated to caesarean section (r = 0.015, p = 0.629), birth weight (r = 0.040, p = 0.212) or age of the GOOD subjects (r = 0.017, p = 0.591).
Maternal age as an independent predictor of aBMD
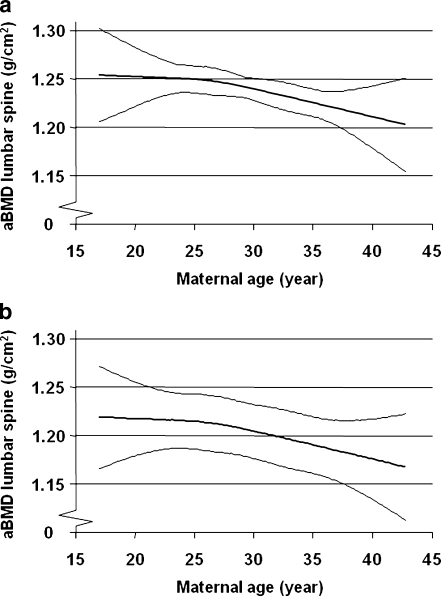
To determine the independent predictors of aBMD at the lumbar spine a stepwise linear regression model was used. In this model, parameters correlated with aBMD at the lumbar spine were included as covariates, i.e., maternal age, calcium intake, current level of physical activity, adult height and weight, birth height, total body adipose tissue and lean mass, length of pregnancy, and present smoking. We found that the current level of physical activity (β = 0.154, p = <0.001) and total body lean mass in the offspring (β = 0.451, p = <0.001) were positive independent predictors, while maternal age (β = −0.076, p = 0.007), present smoking (β = −0.061, p = 0.030), and adult height in the offspring (β = −0.100, p = 0.003) were negative independent predictors of aBMD at the lumbar spine. Using the same covariates in a linear regression analysis with the other bone parameters (as dependent variable), including both DXA and pQCT-derived measurements, we demonstrated that maternal age was also a negative independent predictor of lumbar spine BMC, lumbar spine area, total body BMC, radius BMC, radius area, radius cortical cross-sectional area (CSA), radius periosteal, and endosteal circumference (Table 2). According to this regression analysis, every year increase in maternal age was associated with a 0.00233 g/cm2 (unstandardized B) decrease in lumbar spine aBMD. The inverse relationship between maternal age and aBMD at the lumbar spine, adjusted for physical activity, total body lean mass, and adult height was observed for both smoking and non-smoking offspring (Fig. 1a and b).
Fig. 1.
a. Advancing maternal age is associated with reduced lumbar spine aBMD in the non-smoking male offspring after adjustment for current physical activity, adult height, and total body lean mass in the offspring. Means and 95% confidence intervals are shown. b. Advancing maternal age is associated with reduced lumbar spine aBMD in the smoking male offspring after adjustment for current physical activity, adult height, and total body lean mass in the offspring. Means and 95% confidence intervals are shown
In order to further investigate the independent relationship between maternal age and aBMD at the lumbar spine, we also included other possible confounders, variables correlated with maternal age in the regression analysis, i.e., socioeconomic status of the parental household in 1985, maternal parity, paternal age, and maternal smoking in early pregnancy. In this model, in which also all previously used offspring confounders and length of pregnancy were included (variables correlated to aBMD of the lumbar spine), maternal age remained an independent predictor of aBMD, BMC and area of the lumbar spine, BMC, area, cortical CSA, periosteal and endosteal circumference of the non-dominant radius, but not of BMC of the total body (Table 2).
The role of maternal anthropometrics—subsample analysis
In a subsample of the mothers, we were able to extract weight (n = 885) and height prior to pregnancy (n = 832). Maternal weight was positively correlated to adult and birth weight in the offspring (r = 0.340, p = <0.001 and r = 0.199, p = <0.001, respectively) and aBMD of the lumbar spine (r = 0.083, p = 0.013). Maternal height was positively correlated to adult and birth height in the offspring (r = 0.496, p = <0.001 and r = 0.195, p = <0.001, respectively) but not to aBMD of the lumbar spine in the offspring (r = 0.039, p = 0.258). When including these variables in the regression analysis (n = 705) with all other previously used variables, maternal age remained an independent predictor of aBMD, BMC, and area of the lumbar spine, BMC, area, periosteal and endosteal circumference of the non-dominant radius, but not of cortical CSA of the radius (Table 2).
Mothers >36 years (90th percentile) had sons with lower aBMD at several sites than sons of mothers ≤36 years
The mothers were divided into two groups, of which the first consisted of the oldest mothers (>36 years), corresponding to the 90th percentile of age, and the second of the remaining mothers, 36 years or younger (n = 920), allowing the comparison of anthropometrics and bone variables in sons of the oldest mothers with all other mothers. Bone measurements were adjusted for covariates correlated to aBMD at the lumbar spine (total body lean mass, total body fat mass, current smoking, calcium intake, current physical activity, adult height, adult weight, birth height, and length of pregnancy). We compared the two groups by assessing independent samples T-test. There were no significant differences in height or weight, neither at birth nor in young adulthood. Sons of mothers older than 36 years had significantly lower aBMD at the total body (1.6%), lumbar spine (2.6%), and femoral neck (2.8%), as well as lower BMC at the total body (2.7%), lumbar spine (3.2%), femoral neck (4.0%), and non-dominant radius (2.7%) than sons of mothers 36 years or younger (Table 4). A slight reduction was also observed for bone area of the total body (1.0%) but not of the lumbar spine, femoral neck, or the non-dominant radius. Of the pQCT-measurements, only cortical CSA of the radius (2.0%) was significantly lower in sons of mothers older than 36 years of age than in sons of younger mothers (Table 4).
Table 4.
Anthropometrics and adjusted areal BMD, BMC, and bone area in the male offspring divided by maternal age, corresponding to the 90th percentile (older than 36 years)
| Variables | Mothers ≤ 36 mean ± SD | Mothers >36 (90th percentile) mean ± SD | p value | |
|---|---|---|---|---|
| Height (cm) | 181.7 ± 6.6a | 182.3 ± 6.9d | 0.393 | |
| Weight (kg) | 74.1 ± 12.0a | 72.8 ± 11.6d | 0.314 | |
| Birth height (cm) | 50.8 ± 2.1b | 50.8 ± 2.1e | 0.942 | |
| Birth weight (kg) | 3,576 ± 549c | 3,622 ± 526f | 0.443 | |
| DXA | Total body aBMD (g/cm2) | 1.251 ± 0.075b | 1.231 ± 0.061e | 0.005 |
| Lumbar spine aBMD (g/cm2) | 1.239 ± 0.128b | 1.207 ± 0.126e | 0.024 | |
| Femoral neck aBMD (g/cm2) | 1.170 ± 0.135b | 1.137 ± 0.112e | 0.012 | |
| Radius non-dominant aBMD (g/cm2) | 0.582 ± 0.049b | 0.573 ± 0.047e | 0.077 | |
| Total body BMC (g) | 3,219 ± 278b | 3,131 ± 215e | <0.001 | |
| Lumbar spine BMC (g) | 61.66 ± 8.46b | 59.70 ± 7.31e | 0.020 | |
| Femoral neck BMC (g) | 6.479 ± 0.827b | 6.223 ± 0.617e | <0.001 | |
| Radius non-dominant BMC (g) | 10.13 ± 1.08b | 9.86 ± 1.00e | 0.018 | |
| Total body area (cm2) | 2,564 ± 114b | 2,538 ± 90e | 0.013 | |
| Lumbar spine area (cm2) | 49.56 ± 3.56b | 49.36 ± 3.13e | 0.569 | |
| Femoral neck area (cm2) | 5.531 ± 0.334b | 5.475 ± 0.324e | 0.123 | |
| Radius non-dominant (cm2) | 17.40 ± 1.40b | 17.20 ± 1.23e | 0.157 | |
| pQCT | Radius cortical vBMD (mg/cm3) | 1,165 ± 23b | 1,162 ± 22e | 0.302 |
| Radius cortical CSA (mm2) | 96.30 ± 9.26b | 94.40 ± 8.48e | 0.049 | |
| Radius periosteal circumference (mm) | 42.16 ± 2.33b | 41.72 ± 2.23e | 0.084 | |
| Radius endosteal circumference (mm) | 23.80 ± 2.76b | 23.54 ± 2.63e | 0.379 | |
| Radius trabecular vBMD (mg/cm3) | 218.8 ± 39.0b | 219.7 ± 35.1e | 0.810 | |
Table 4 Differences between groups were investigated using independent samples t-test
Bone measurements were adjusted for total body lean mass, total body fat mass, current smoking, calcium intake, current physical activity, adult height, adult weight, birth height, and length of pregnancy
a n = 920, b n = 910, c n = 892, d n = 89, e n = 88, f n = 85
Discussion
In the present study, we have demonstrated that advancing maternal age was associated with reduced aBMD and BMC of the lumbar spine at the age of PBM in the male offspring, independently of the possible confounders that are known to affect bone mass in late adolescence. The observed association was small but obvious when viewing the sons of the oldest mothers (90th percentile, >36 years), which had lower bone mass, visible at several bone sites, including also both aBMD and BMC at the femoral neck and total body, than the sons of the younger mothers. Based upon our results, we hypothesize that a critical point between 30 and 35 years of age exists, where the negative influence of advancing maternal age on bone mass is more pronounced. Our results are in line with the previous finding of a higher fracture risk among children of mothers giving birth at advancing age in a Brazilian cohort [8].
We also found that increasing maternal age was associated with reduced bone area of the lumbar spine in the offspring, but this association was only found after adjusting for several covariates, including offspring anthropometrics. This indicates that the association found between maternal age and aBMD could, at least partly, be due to bone size. When evaluating the results from the appendicular skeleton, here represented by the radius, we found that aBMD of the radius was inversely correlated to maternal age, but when adjusting for covariates, no association was found. There was, however, as in the lumbar spine, an inverse association found between both the area and BMC of the non-dominant radius and maternal age. Aiming to discriminate whether the association between maternal age and DXA-derived BMC was mainly due to volumetric BMD or bone size, we used pQCT measurements of the non-dominant radius. We found that maternal age most strongly predicted the parameters of bone size, i.e., cortical CSA and especially periosteal and endosteal circumferences, but not volumetric BMD. As indicated by these data, it seems plausible that the association between BMC and maternal age could be explained by bone size. There was, however, no differences found in anthropometrics, neither at birth nor in young adulthood, when comparing the sons of the oldest mothers to the sons of the younger mothers. Comparing these two groups, we found that only cortical CSA of the pQCT parameters was significantly reduced in the sons of the oldest mothers, while the associations found in the linear models (Table 2) for periosteal and endosteal circumferences could not be repeated, indicating that the latter associations had linear relationships. Possibly, these associations could not be detected with reduced statistical power as in the dichotomized comparison of the sons with the oldest mothers and the sons of the remaining mothers.
PBM has been shown to be of great importance as a predictor of the risk of developing osteoporosis later in life [15]. Given the trend of advancing maternal age during the last three decades in Sweden, the question rises whether this will influence the incidence rates of osteoporosis in Sweden in the future. Looking back on Swedish population statistics, the maternal age was also high in 1930 (mean age 29.4 years, primi- and multipara), which theoretically, according to our results, might have played a role in the increased rates of osteoporosis and fracture we are looking at today [1, 16]. In the present study, we show that every year increase in maternal age was associated with a 0.00233 g/cm2 (unstandardized B) decrease in adjusted lumbar spine aBMD, corresponding to about 1.6% of 1 SD (1 SD equaling 0.147 g/cm2) in the offspring. Assuming a linear relationship, e.g., a 15-year difference in maternal age would correspond to a difference in about 24% of a standard deviation in lumbar spine aBMD. The possible effect is hardly of any clinical significance on the individual level, but if maternal age continues to rise, a shift in the distribution of BMD in the offspring population could be the result, which could lead to an increased incidence of osteoporosis in the future.
In the present study, we found an association between advancing maternal age and reduced bone mass in the offspring, even though we included a large number of known confounders. Social position is a parental characteristic that has previously been shown to be positively related to bone mass acquisition in 10-year old children as a consequence of enhanced gain in height [17]. In our material, the adult height of the GOOD subjects was positively correlated to bone density and bone mineral content, while the socioeconomic index (SEI) was not, and maternal age remained an independent predictor of bone mass in the offspring also after adjusting for SEI. Adult height, however, was shown to be a negative independent predictor when including all variables correlated to aBMD at the lumbar spine in the linear regression analysis.
Maternal height has been shown to predict hip fracture risk in a Finnish study [18]. Inclusion of maternal height did not either alter the obtained results in the present study. This was however only possible to test for in a large subsample of the subjects (n = 705). Furthermore, inclusion of several known predictors, such as physical activity, calcium intake, and height and body composition parameters did not explain the association between bone parameters in the offspring and maternal age.
A Canadian study has shown an association between delayed childbearing and low birth weight [19], which in turn has been shown to be a predictor of bone mass later in life, mediated largely by bone size [20]. In our cohort, there was, however, no correlation seen between maternal age and birth size, i.e., birth weight and height. Length of pregnancy showed a weak negative correlation with maternal age but was of no importance when included in the regression analysis.
Other possible explanations, which we have not been able to adjust for, may be found in placental function, partly reflected though in birth anthropometrics, or other aspects potentially affected by maternal aging such as the environment in utero. One might also speculate in epigenetic causes. With aging and exposure to environmental factors in general, recent research has shown that DNA methylation and histone modification alters the genetic phenotype and may increase the susceptibility of several diseases, which may also be inherited by offspring via germ line cells [21]. The degree of modified DNA would be expected to be higher in older mothers and subsequently imply an increased susceptibility to morbidity in the offspring, possibly including also bone quality. In order to establish and confirm our findings concerning the association between maternal age and bone mass in the offspring, further studies on the topic are required.
There are some limitations in the present study. Firstly, there were some deficits in the medical birth register concerning maternal anthropometrics resulting in a markedly reduced number of subjects when adjusting for all possible confounders. This reduced the statistical power of the analysis. Secondly, the association between maternal age and bone mass in male offspring is rather small and probably of limited clinical significance in itself. Since our reported results were derived from a cross-sectional association study, we are not able to delineate whether the found association between increasing maternal age and decreased aBMD in the offspring is possibly due to intra-uterine or from environmentally affected extra-uterine factors.
In conclusion, we demonstrate that advancing maternal age is associated with reduced bone mass in a large cohort of young adult male offspring, but additional studies are required to elucidate whether a high maternal age could increase the susceptibility of developing low bone mass and osteoporosis.
Acknowledgments
This work was supported by the Swedish Research Council, the Lundberg Foundation, and ALF/LUA grants from the Sahlgrenska University Hospital.
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Statistics Sweden . Tables on the population in Sweden 2006. Stockholm: Statistics Sweden; 2007. [Google Scholar]
- 2.Fretts RC, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333(15):953–957. doi: 10.1056/NEJM199510123331501. [DOI] [PubMed] [Google Scholar]
- 3.Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod. 2007;22(5):1264–1272. doi: 10.1093/humrep/del522. [DOI] [PubMed] [Google Scholar]
- 4.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58(3):282–285. [PubMed] [Google Scholar]
- 5.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35(6):1495–1503. doi: 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 6.Ekeus C, Olausson PO, Hjern A. Psychiatric morbidity is related to parental age: a national cohort study. Psychol Med. 2006;36(2):269–276. doi: 10.1017/S0033291705006549. [DOI] [PubMed] [Google Scholar]
- 7.Harjutsalo V, Podar T, Tuomilehto J. Cumulative incidence of type 1 diabetes in 10, 168 siblings of Finnish young-onset type 1 diabetic patients. Diabetes. 2005;54(2):563–569. doi: 10.2337/diabetes.54.2.563. [DOI] [PubMed] [Google Scholar]
- 8.Hallal PC, et al. The role of early life variables on the risk of fractures from birth to early adolescence: a prospective birth cohort study. Osteoporos Int. 2009;20(11):1873–1879. doi: 10.1007/s00198-009-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui SL, Slemenda CW, Johnston CC., Jr The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int. 1990;1(1):30–34. doi: 10.1007/BF01880413. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PJ, et al. Genetic influences on bone turnover, bone density and fracture. Eur J Endocrinol. 1995;133(3):265–271. doi: 10.1530/eje.0.1330265. [DOI] [PubMed] [Google Scholar]
- 11.Lorentzon M, Mellstrom D, Ohlsson C. Age of attainment of peak bone mass is site specific in Swedish men—a GOOD study. J Bone Miner Res. 2005;20(7):1223–1227. doi: 10.1359/JBMR.050306. [DOI] [PubMed] [Google Scholar]
- 12.Poole KE, Compston JE. Osteoporosis and its management. BMJ. 2006;333(7581):1251–1256. doi: 10.1136/bmj.39050.597350.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzoli R, Bonjour JP. Determinants of peak bone mass and mechanisms of bone loss. Osteoporos Int. 1999;9(Suppl 2):S17–S23. doi: 10.1007/PL00004155. [DOI] [PubMed] [Google Scholar]
- 14.Statistics Sweden, Socioeconomic Classification (SEI). 1982, Statistics Sweden: Stockholm.
- 15.Seeman E, et al. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320(9):554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- 16.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 17.Clark EM, Ness A, Tobias JH. Social position affects bone mass in childhood through opposing actions on height and weight. J Bone Miner Res. 2005;20(12):2082–2089. doi: 10.1359/JBMR.050808. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C, et al. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12(8):623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 19.Tough SC, et al. Delayed childbearing and its impact on population rate changes in lower birth weight, multiple birth, and preterm delivery. Pediatrics. 2002;109(3):399–403. doi: 10.1542/peds.109.3.399. [DOI] [PubMed] [Google Scholar]
- 20.Antoniades L, et al. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatol Oxf. 2003;42(6):791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 21.Junien C, Nathanielsz P. Report on the IASO Stock Conference 2006: early and lifelong environmental epigenomic programming of metabolic syndrome, obesity and type II diabetes. Obes Rev. 2007;8(6):487–502. doi: 10.1111/j.1467-789X.2007.00371.x. [DOI] [PubMed] [Google Scholar]