Abstract
Peripheral nerve development involves multiple classes of glia that cooperate to form overlapping glial layers paired with the deposition of a surrounding extracellular matrix (ECM). The formation of this tubular structure protects the ensheathed axons from physical and pathogenic damage and from changes in the ionic environment. Integrins, a major family of ECM receptors, play a number of roles in the development of myelinating Schwann cells, one class of glia ensheathing the peripheral nerves of vertebrates. However, the identity and the role of the integrin complexes utilized by the other classes of peripheral nerve glia have not been determined in any animal. Here, we show that, in the peripheral nerves of Drosophila melanogaster, two integrin complexes (αPS2βPS and αPS3βPS) are expressed in the different glial layers and form adhesion complexes with integrin-linked kinase and Talin. Knockdown of the common beta subunit (βPS) using inducible RNAi in all glial cells results in lethality and glial defects. Analysis of integrin complex function in specific glial layers showed that loss of βPS in the outermost layer (the perineurial glia) results in a failure to wrap the nerve, a phenotype similar to that of Matrix metalloproteinase 2-mediated degradation of the ECM. Knockdown of βPS integrin in the innermost wrapping glia causes a loss of glial processes around axons. Together, our data suggest that integrins are employed in different glial layers to mediate the development and maintenance of the protective glial sheath in Drosophila peripheral nerves.
Keywords: Glia, Integrins, Peripheral nervous system development, Drosophila
INTRODUCTION
The development of the glial sheath in peripheral nerves is vital to provide structural support, insulate and protect axons from physical damage and pathogens. The architecture of peripheral nerves consists of multiple glial layers and a surrounding basal lamina. Centermost in vertebrate nerves are the myelinating or non-myelinating Schwann cells that wrap individual axons or bundles of axons, respectively. Groups of myelinated or non-myelinated nerve fibers are initially encased by a basal lamina, later becoming embedded in a collagenous connective tissue, called the endoneurium, to form fascicles. Each fascicle is sheathed by the perineurium, a protective barrier of overlapping squamous-like perineurial cells (Olsson, 1990). Similarly, the Drosophila larval peripheral nerve is made up of several distinct layers (Stork et al., 2008). The innermost wrapping glia (WG) separate and ensheath axons in a manner similar to vertebrate non-myelinating Schwann cells. The WG are next surrounded by subperineurial glia (SPG), which form intercellular septate junctions and create the blood-nerve barrier (Stork et al., 2008). The outermost cell layer is a monolayer of squamous-like perineurial glia (PG) (Lavery et al., 2007). The final layer is the neural lamella (NL), a dense basal lamina encasing each peripheral nerve. Drosophila and vertebrate peripheral glia express many of the same proteins, such as NCAM and L1 (Freeman and Doherty, 2006) and homologs of the paranodal junction proteins (Bhat, 2003), and use many of the same developmental programs, such as Erb/neuregulin signaling (reviewed by Parker and Auld, 2006; Newbern and Birchmeier, 2010).
In vertebrates, the basal lamina contains extracellular matrix (ECM) components known to be important for glial development. For example, laminins, a major ECM component, are essential for Schwann cell differentiation, axon sorting and myelination (Chen and Strickland, 2003; Wallquist et al., 2005; Yang et al., 2005; Yu et al., 2005). Similarly, non-myelinating Schwann cells lacking laminins fail to differentiate and the associated C-fibers are lost (Yu et al., 2009). These ECM signals are transduced by specific receptors, which include Dystroglycan, Glypican and integrins (Feltri and Wrabetz, 2005). Integrins are the best-characterized ECM receptors in Schwann cells and consist of one alpha and one beta subunit. Loss of β1 integrin or integrin-linked kinase (Ilk) in myelinating Schwann cells results in defects in radial sorting and myelination (Feltri et al., 2002; Fernandez-Valle et al., 1994; Pereira et al., 2009). Although there is evidence for a role of integrin adhesion and signaling in myelinating Schwann cells, the role of integrins in the development of the non-myelinating Schwann cells or of the perineurium is not understood.
Complicating the investigation of integrins in peripheral nerve development is the complexity of integrin heterodimer expression in vertebrates (Feltri and Wrabetz, 2005). By contrast, Drosophila melanogaster has a relatively simple family of integrin subunits consisting of five alpha and two beta subunits (Brown et al., 2000). Therefore, we used Drosophila to investigate the role of integrins and ECM interactions during the development of the glial layers of the peripheral nerve. Here, we show that specific integrin heterodimers play a role in the development and maintenance of the glial layers. Downregulation of integrin expression results in wrapping defects in both the PG and WG. Furthermore, the basal lamina is essential for the proper maintenance of the perineurial wrap. Collectively, our results demonstrate that Drosophila integrins and the basal lamina are essential for proper glial sheath development and maintenance in the peripheral nerve.
MATERIALS AND METHODS
Fly strains and genetics
The following fly strains were used: repo-GAL4 (Sepp et al., 2001); SPG-GAL4 (Schwabe et al., 2005); nrv2-GAL4 (Sun et al., 1999); Gli-GAL4 (Sepp and Auld, 1999); 46F-GAL4 (a gift from Dr Yong Rao, McGill University); UAS-mCD8-GFP (Lee and Luo, 1999); UAS-mCD8-RFP (a gift from Dr Elizabeth Gavis, Princeton University); UAS-Mmp2 (Page-McCaw et al., 2003); UAS-Dicer2 (Dietzl et al., 2007); tubP-GAL80ts (McGuire et al., 2003); mys1 (Bunch et al., 1992); FRT19A,tubP-Gal80,hsFLP,w* (Lee and Luo, 1999); repo-FLP (Stork et al., 2008); and UAS-nls-GFP (a gift from Dr Douglas Allan, University of British Columbia). The following GFP protein-trap insertions were used: perlecan-GFP; Jupiter-GFP; nrv2-GFP; Ilk-GFP; talin-GFP (Kelso et al., 2004; Morin et al., 2001). UAS-RNAi strains were obtained from the VDRC (Austria) (Dietzl et al., 2007), NIG (Japan) and TRiP (Harvard). RNAi experiments were carried out at 25°C and with UAS-Dicer2 in both control and experimental crosses unless specified. Control and βPS MARCM clones were generated using female FRT19A; +/+; repo-GAL4, UAS-CD8-GFP/TM6B, Tb or mys1, FRT19A/FM7; +/+; repo-GAL4, UAS-CD8-GFP/TM6B, Tb with male FRT19A, tubP-Gal80, hsFLP, w*/Y; +/+; repo-FLP.
Immunohistochemistry and imaging analysis
The following primary antibodies were obtained from the Developmental Studies Hybridoma bank (NICHD, University of Iowa): mouse anti-βPS (CF.6G11) (Brower et al., 1984) used at 1:10; mouse anti-αPS2 (CF.2C7) (Brower et al., 1984) at 1:5; and mouse anti-Repo (8D12) (Alfonso and Jones, 2002) at 1:50. Other primary antibodies were: rabbit anti-αPS3 (Wada et al., 2007) used at 1:200; rabbit anti-HRP (horseradish peroxidase; Jackson ImmunoResearch) at 1:500; and rabbit anti-Drosophila γ-laminin (LanB2) (Abcam) at 1:100. All secondary antibodies were used at 1:200: goat anti-mouse Alexa 568 and Alexa 647; goat anti-rabbit Alexa 568 and Alexa 647 (Molecular Probes/Invitrogen).
Dissection and fixation for immunofluorescence were performed according to standard procedures (Sepp et al., 2000). Unless specified otherwise, images were obtained with a DeltaVision Spectris (Applied Precision) using a 60× objective (NA 1.4) with 0.2 μm z-sections. Image stacks were deconvolved and rotated with SoftWorx (Applied Precision) based on measured point spread functions of 0.2 μm fluorescent beads (Molecular Probes) mounted in Vectashield (Vector Laboratories). Images were exported to Photoshop and Illustrator CS4 (Adobe) for compilation. For lower magnification images, images were captured using a 20× objective (NA 0.4, DeltaVision) or a 10× objective (NA 0.3, Axioskop 2, Zeiss).
PG numbers were counted in nerves from abdominal segment 8 (A8) from late third instar larvae. The mean and the standard deviation were calculated in Excel 2010 (Microsoft).
RESULTS
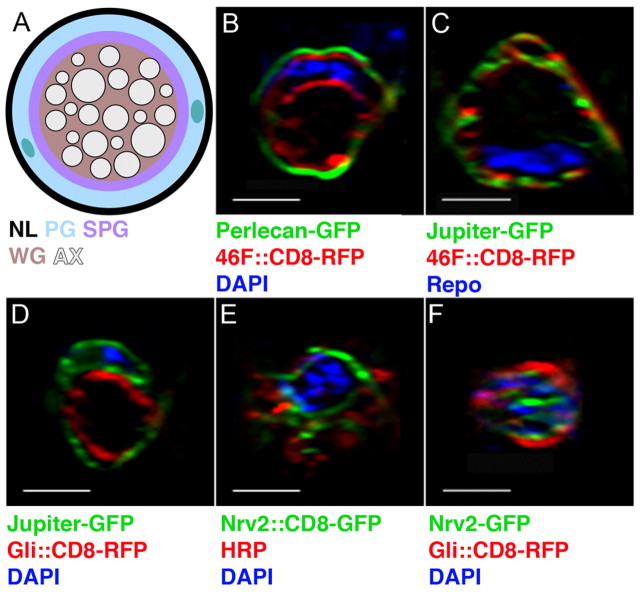
In the larval peripheral nerve, axons are wrapped by three consecutive layers of glia: the outermost perineurial glia (PG), intermediate subperineurial glia (SPG) and inner wrapping glia (WG) (Fig. 1A) (Stork et al., 2008). The dense basal lamina (the neural lamella, NL) that surrounds the nerve can be visualized with the proteoglycan Perlecan endogenously tagged with GFP (Fig. 1B). The PG are located just below the NL and specifically express the enhancer-trap line 46F-GAL4 as well as Jupiter-GFP, a microtubule-associated protein (Karpova et al., 2006) endogenously tagged with GFP (Fig. 1C,D) (Table 1). Directly below the perineurial layer are the SPG, which express either SPG-GAL4 (Stork et al., 2008) or Gliotactin (Gli)-GAL4 (Fig. 1D, Table 1). In the center of the peripheral nerve are the WG that ensheath individual axons and axonal bundles. WG express Nervana 2 (Nrv2)-GAL4 (Fig. 1E) and the Nrv2 protein tagged with GFP (Nrv2-GFP) (Fig. 1F, Table 1).
Fig. 1.
Axons are surrounded by three glial layers in the Drosophila larval peripheral nerve. (A) Transverse section of a third instar larval peripheral nerve. From external to internal are the neural lamella (NL), perineurial glia (PG), subperineurial glia (SPG), wrapping glia (WG) and axons (AX). Two PG nuclei are shown (green). (B-F) Orthogonal sections from third instar nerves illustrate the GFP-tagged proteins and GAL4 drivers used to label the ECM and the different cellular glial layers. Panels have been digitally expanded. The NL was labeled using Perlecan-GFP (B, green). PG were labeled using 46F-GAL4::CD8-RFP (B and C, red) and Jupiter-GFP (C and D, green). SPG were labeled using Gli-GAL4::CD8-RFP (D and F, red). WG were labeled using Nrv2-GAL4::CD8-GFP (E, green) and Nrv2-GFP (F, green). Axons were immunolabeled using an anti-HRP antibody (E, red; F, blue). Glial nuclei are shown by DAPI labeling (blue), except in C where the SPG nucleus is immunolabeled with a Repo antibody (blue). Scale bars: 5 μm.
Table 1.
Summary of markers for ECM and glial layers in the peripheral nerve
Specific integrins are located in the glial cells of the larval peripheral nerve
We began our investigation of the role of integrins in peripheral nerve development by examining which integrins are expressed by the glia. We were unable to detect integrin expression in embryonic glia by genetic or immunofluorescence analysis (data not shown). Thus, our analysis concentrated on larval peripheral glia and on those integrin subunits with known vertebrate homologs (βPS, αPS1, αPS2, αPS3) (Brown et al., 2000). All three classes (PG, SPG and WG) expressed integrin complexes when assayed with an antibody to the beta subunit, βPS (Mys) (Fig. 2). In addition, we observed immunolabeling with antibodies to two alpha subunits, αPS2 and αPS3 (Fig. 3), but not αPS1 (data not shown). The integrin subunits were observed in discrete puncta or stripes and were associated with Ilk-GFP (Fig. 2A, Fig. 3A, see Fig. S1 in the supplementary material) and Talin-GFP (see Fig. S2A-C in the supplementary material). The GFP fusion proteins only partially overlapped with βPS integrin; compare the puncta (arrowheads) between the two focal planes Z=31 (see Fig. S1B in the supplementary material) and Z=34 (see Fig. S1C in the supplementary material), which do overlap in an orthogonal section of the nerve (arrows in Fig. S1D in the supplementary material). This was not unexpected as Ilk and Talin form adhesion complexes with integrins through binding to the intracellular domain of beta integrin (Delon and Brown, 2007) and the integrin antibodies are thought to bind the extracellular domain of these transmembrane receptors (Brower et al., 1984). The colocalization of the integrin subunits with Ilk and Talin in the larval nerve suggests that adhesion complexes are found throughout the glial layers of the peripheral nerve.
Fig. 2.
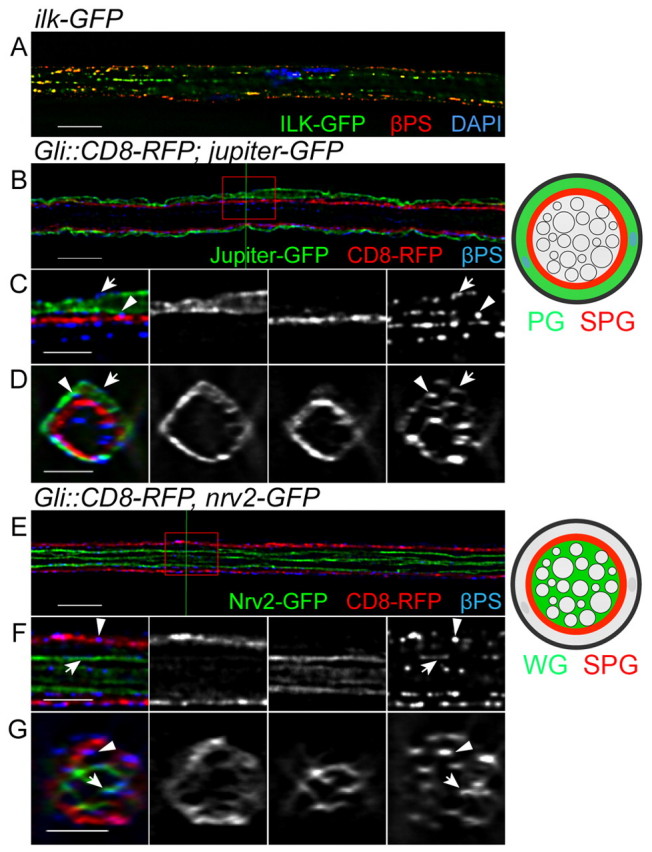
βPS integrin is expressed in the peripheral glia layers. Immunolabeling for the βPS integrin subunit in Drosophila third instar nerves expressing either Ilk-GFP or different glial markers in single 0.2 μm z-sections. (A) βPS (red), Ilk-GFP (green) and DAPI (blue) labeling. Both βPS and Ilk form puncta and are associated with each other in the peripheral nerve. (B-G) βPS labeling in the different glial subtypes: PG using Jupiter-GFP (B-D, green), SPG using Gli-GAL4::CD8-RFP (B-G, red) and WG using Nrv2-GFP (E-G, green). The labeled glial layers are also illustrated to the right. The red boxes in B and E were digitally expanded as shown in C and F, respectively. The green lines in B and E indicate the positions of the orthogonal sections in D and G, respectively. βPS integrin was found in the PG outer membrane (C and D, arrow), the PG-SPG boundary (C and D, arrowhead), the SPG inner membrane (F and G, arrowhead) and the WG membrane (F and G, arrows). Scale bars: 10 μm in A,B,E; 5 μm in C,D,F,G.
Fig. 3.
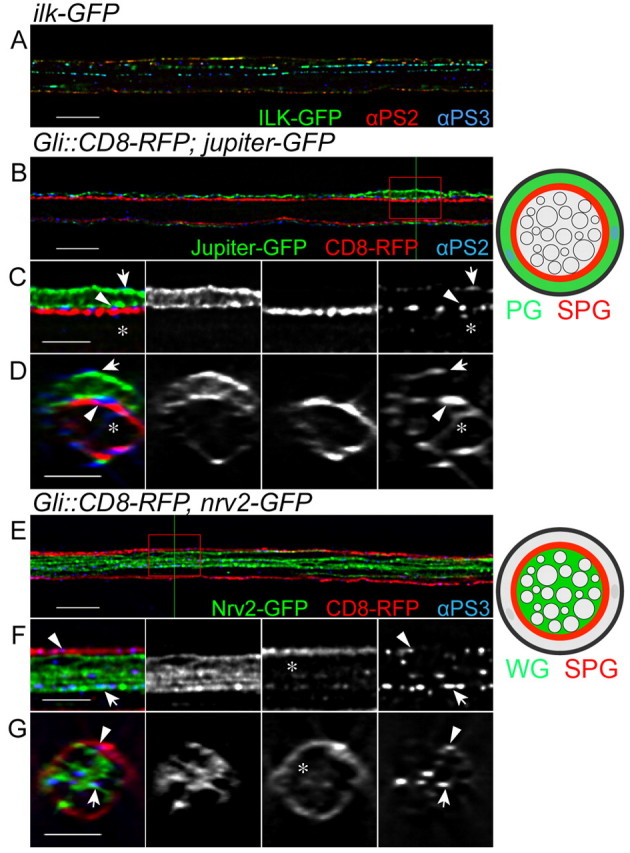
αPS2 and αPS3 integrins are expressed in different glial layers of the larval peripheral nerve. Drosophila third instar larval nerves immunolabeled with antibodies to αPS2 (A, red; B-D, blue) and αPS3 (A,E-G, blue) integrins in single 0.2 μm z-sections. (A) Ilk-GFP (green) and integrin alpha subunit αPS2 (red) and αPS3 (blue) immunolabeling. Both alpha subunits form puncta and are associated with Ilk in the peripheral nerve. (B-G) Specific glial layers were labeled with different markers: PG using Jupiter-GFP (B-D, green), SPG using Gli-GAL4::CD8-RFP (B-G, red) and WG using Nrv2-GFP (E-G, green). The labeled glial layers are also illustrated to the right. The red boxes in B and E were digitally magnified as shown in C and F, respectively. D and G are orthogonal sections of z-stacks at the positions indicated by green lines in B and E, respectively. (B-D) αPS2 integrin labeling is mostly found in the outer glial cells, the PG outer membrane (arrows), the PG-SPG boundary (arrowheads) and in the SPG inner membrane (asterisks). (E-G) αPS3 integrin labeling is mostly found in the internal glial layers, in the SPG inner membrane (arrowheads) and in the WG membrane (arrows). αPS3 integrin labeling was often associated with the SPG membrane protrusions (asterisks). Scale bars: 10 μm in A,B,E; 5 μm in C,D,F,G.
The beta integrin subunit is expressed in all glial cell layers
To address which glial layers express different integrin complexes, we used a combination of proteins endogenously tagged with GFP and GAL4 drivers to label the individual glial layers (Fig. 1; Table 1). First, we examined the distribution of the common βPS subunit, which is able to form heterodimers with all alpha subunits. PG were labeled with Jupiter endogenously tagged with GFP in conjunction with SPG labeled using Gli-GAL4 driving the expression of UAS-CD8-RFP (Gli::CD8-RFP). Immunolabeling of βPS integrin was observed on the PG outer membrane (Fig. 2C,D, arrows) suggesting an interaction of the integrin complex with the external ECM/NL (Stork et al., 2008). βPS integrin immunolabeling was also found between Jupiter-GFP and Gli::CD8-RFP (Fig. 2C,D, arrowheads), suggesting that these integrin proteins also localize to the PG and SPG boundary.
The βPS was also observed in the nerve, internal to the CD8-RFP-labeled SPG (Fig. 2C,D), in the areas occupied by WG and axons. To study whether the internal βPS integrin is located at the WG membrane, Nervana 2 endogenously tagged with GFP (Nrv2-GFP) (Morin et al., 2001) was used to label the WG (Fig. 2E-G). At the same time, Gli::CD8-RFP was used to label the SPG membrane. βPS immunolabeling was consistently found to associate with both the Gli::CD8-RFP-labeled SPG (Fig. 2F,G, arrowheads) and with Nrv2-GFP. βPS was often observed between Nrv2-GFP and protrusions of CD8-RFP (Fig. 2F,G, arrows) and thus might mediate interactions between the SPG and WG.
Importantly, all of the βPS integrin observed in the peripheral nerve appears to be expressed by glial cells and not neurons. We confirmed this by knockdown of βPS expression using βPS-RNAi specifically in the glia cells (see below). Our data indicate that βPS integrin is expressed by the glial cells and forms adhesion complexes in the glial membranes in the larval peripheral nerve.
Differential glial expression of integrin alpha subunits
Both the αPS2 and αPS3 integrin subunits were located to adhesion complexes with Ilk-GFP and Talin-GFP in the larval peripheral nerve and were expressed with different intensities in the different glial layers (see Fig. S1E-G in the supplementary material). The specific glial layer markers outlined above were used to further characterize the discrete localization of the alpha subunits (Fig. 3). αPS2 integrin immunolabeling was found on the external surface of the nerve, outside of the Jupiter-GFP-labeled PG, similar to βPS (Fig. 3B-D, arrows). This further supports a role for the integrin complex in mediating adhesion between the PG and the ECM. αPS2 was also found between Jupiter-GFP and Gli::CD8-RFP (Fig. 3C,D, arrowheads), indicating that αPS2 is also localized at the PG and SPG boundary. A few αPS2-positive puncta were also found in the center of the nerve, internal to Gli::CD8-RFP (Fig. 3C,D, asterisks), suggesting that αPS2 might be expressed at low levels by the internal WG or by extensions of the SPG into the center of the nerve.
Conversely, most of the αPS3 integrin immunolabeling was found in the center of the nerve internal to the Gli::CD8-RFP (Fig. 3F,G, arrows and arrowheads). αPS3 was located between the RFP-labeled SPG processes and Nrv2-GFP-labeled processes. Moreover, αPS3 was associated with CD8-RFP-labeled membranes seen protruding between the internal Nrv2-GFP-labeled members of the PG (Fig. 3F,G, asterisks), suggesting that αPS3 might be involved in an interaction between SPG and WG. However, we cannot rule out the possibility that αPS3 expression might indicate an interaction between the WG and their associated axons. In general, our data suggest that the αPS2 and αPS3 integrin subunits are both expressed by the different glial layers and might be important for glia-ECM and glia-glia interactions.
Integrin function is necessary in larval peripheral glia
The question then arises, what functions do integrins have in the peripheral glia? Null mutants of βPS (mys) are embryonic lethal and we were unable to detect βPS immunolabeling in embryonic glia (data not shown). In mys mutant embryos, we were unable to detect any defects in glia cell migration (data not shown). This, paired with a lack of peripheral axon defasciculation, which is a hallmark of disruption in glial ensheathment, suggests that integrin function occurs at later developmental stages. Therefore, we took two approaches to remove βPS function from peripheral glia during larval development: MARCM analysis and RNA interference (RNAi). To generate mosaic clones for a mys null in peripheral glia we used the MARCM technique and assayed glial development in third instar larvae. CD8-GFP-labeled mys clones were seen in the peripheral (Fig. 4A, arrows) and central (Fig. 4A, arrowhead) nervous systems. Using this approach, the majority of glial clones were in the PG (14/14 in control larvae and 14/16 in mutant larvae). In control wild-type clones, the CD8-GFP-labeled PG formed a thin layer on the outside of the HRP-labeled axons (Fig. 4B), resulting in an intact circle around the nerve (Fig. 4D). In controls, βPS immunolabeling was consistently found in the perineurial membrane (Fig. 4C,D, arrows) and internal glial layers. In the mys null clones, βPS labeling was absent from the GFP-labeled perineurial membrane (Fig. 4F,G, arrowheads) but still present in the internal wild-type SPG and WG layers. Loss of βPS integrin resulted in PG that failed to form a uniform sheath, such that CD8-GFP-labeled membranes were observed only on one side of the nerve (Fig. 4E) and failed to encircle the nerve (Fig. 4G). It is important to note that the CD8-GFP-labeled membranes of the βPS mutant PG still covered a significant distance along the length of some nerves suggesting that, without βPS integrin, PG were able to send out processes along the nerve. Although MARCM analysis revealed the importance of βPS integrin in larval PG development, this approach had two disadvantages. First, the majority of glial clones were in the PG. SPG (n=0) and WG (n=2) clones were rare, as these glial subtypes do not actively divide at later stages. Second, in each nerve with PG clones, only a small population of PG were βPS mutants, making it difficult to evaluate the overall impact.
Fig. 4.
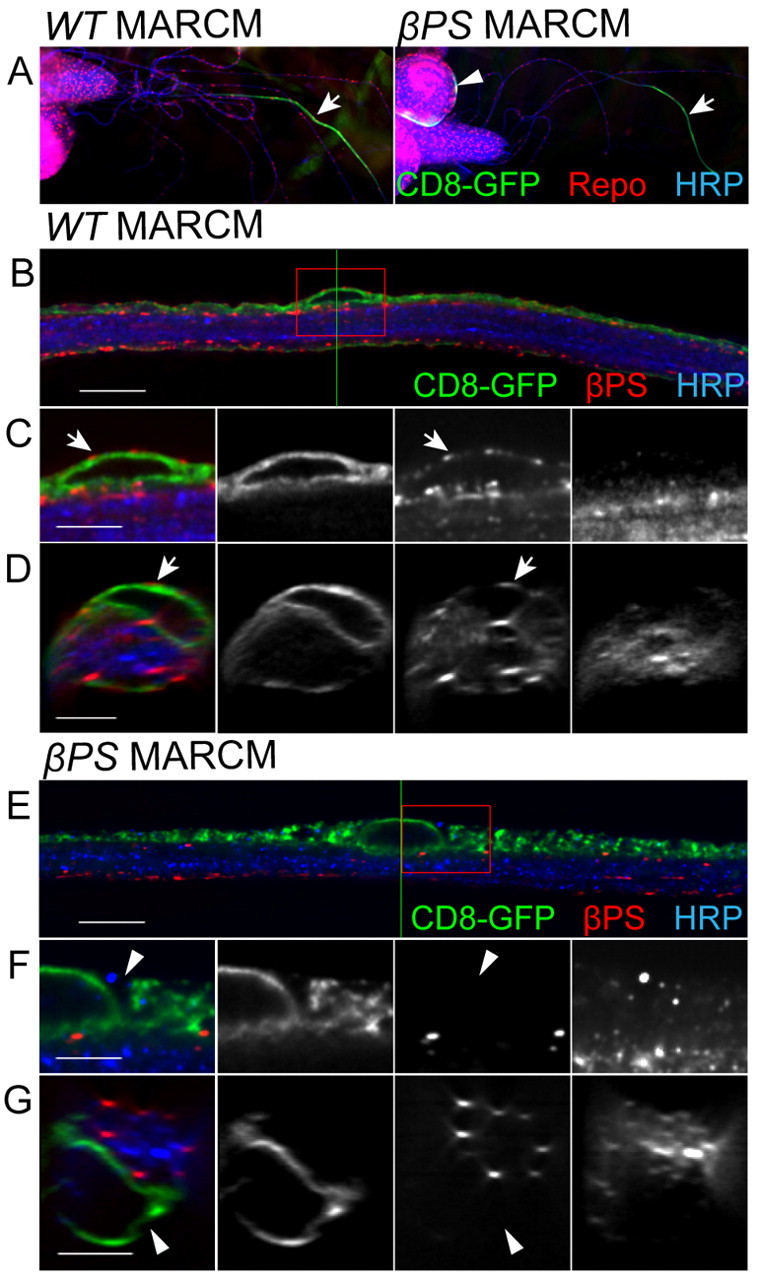
MARCM analysis of a βPS mutant in the larval peripheral nerve. MARCM using flipase expression in glia was used to generate wild-type or homozygous βPS (mys) clones labeled with CD8-GFP. (A) Control and mys glial clones were identified with CD8-GFP and immunolabeled for Repo (red) and HRP (blue). At low magnification (10× objective), clones were seen in both peripheral nerves (arrows) and the CNS (arrowheads). (B-G) High-magnification images of nerves with control and mutant PG clones shown in single 0.2 μm z-sections (B,E) and orthogonal sections of z-stacks (D,G). Green lines indicate the positions of orthogonal sections. Boxed regions were digitally expanded as shown in C and F. (B-D) In control clones, CD8-GFP labeling wraps around the circumference of the nerve. Arrows indicate βPS labeling (red) in the PG outer membrane, as compared with the core of HRP-labeled axons (blue). (E-G) In mys clones, CD8-GFP labeling is found only on one side of the HRP labeling in the z-section. The CD8-GFP-labeled membrane does not wrap around the nerve in the orthogonal section. βPS labeling is not found in the PG outer membrane (arrowheads), but is still seen in the inner layers of glia within the nerve. Scale bars: 10 μm in B,E; 5 μm in C,D,F,G.
To overcome these limitations, we used RNAi (Tavernarakis et al., 2000) to knock down integrins in all or individual glial subtypes. RNAi lines known to target specific integrin subunits (Perkins et al., 2010) were obtained; these target at least two independent regions of each integrin subunit. We tested the ability to knock down specific integrin subunits in glia using the repo-GAL4 driver. Repo is a transcription factor that is exclusively expressed in all glia except the midline glia (Xiong et al., 1994). When repo-GAL4 was used to drive βPS-RNAi, immunolabeling by the βPS antibody was decreased (Fig. 5B) as compared with control nerves (Fig. 5A). The degree of βPS integrin immunolabeling in the underlying body wall muscles was used as an internal control. This suggests that βPS-RNAi is able to knock down βPS expression specifically in glia. In addition, the loss of βPS labeling throughout the nerve in the repo::βPS-RNAi larvae suggests that βPS integrin is expressed only by glial cells and not by the neurons.
Fig. 5.
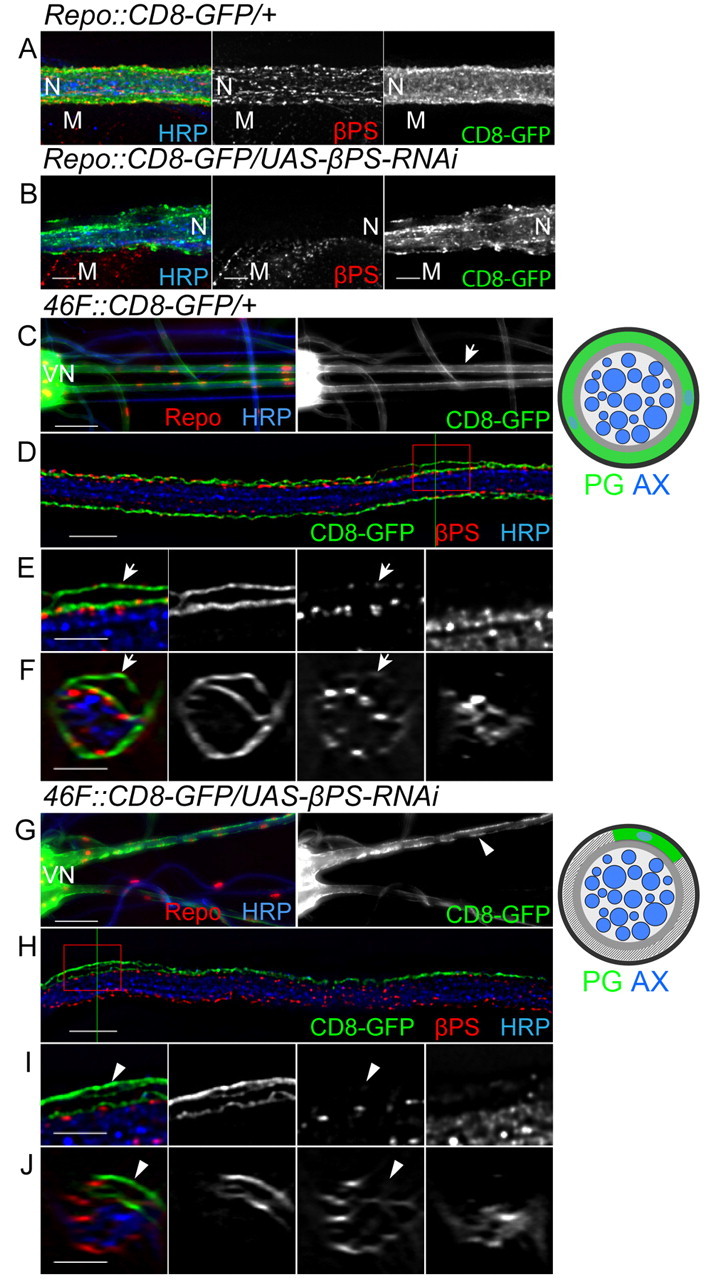
Expression of βPS-RNAi in peripheral glia. (A,B) repo-GAL4 was used to express CD8-GFP (green) and βPS-RNAi in all peripheral glia. All panels are projections of the entire z-stack. βPS labeling (red) was greatly decreased in the RNAi nerve (B) when compared with control nerve (A). As an internal control, βPS labeling was also observed in muscles and remained unchanged. CD8-GFP (green) was not evenly distributed along the RNAi nerve (B) compared with the control nerve (A). Axons were labeled with HRP (blue). (C-J) 46F-GAL4 was used to express CD8-GFP (green) and βPS-RNAi in the PG. (C,G) Lower magnification views (20× objective) of control (C) and mutant (G) nerves. Note the seamless coverage of GFP in control nerves (arrow) and the discontinuity of GFP in mutant nerves (arrowhead). (D-F,H-J) High-magnification images of control (D,F) and a mutant (H,J) nerves in single 0.2 μm z-sections (D,H) and orthogonal sections of z-stacks (F,J). Green lines indicate the positions of orthogonal sections. Boxed regions were digitally expanded as shown in E and I. (D-F) In the control nerve, CD8-GFP labeling wraps around the circumference of the nerve. Arrows indicate βPS labeling (red) in the PG outer membrane, as compared with the core of HRP-labeled axons (blue). (H-J) In the 46F::βPS-RNAi nerve, CD8-GFP labeling is found only on one side of the nerve (HRP-labeled axons, blue) and fails to wrap the entire circumference. Arrowheads point to PG outer membrane where no βPS labeling is found. The labeled glial layers and morphological changes are illustrated to the right. N, nerve; M, muscle; VN, ventral nerve cord. Scale bars: 50 μm in C,G; 10 μm in D,H; 5 μm in A,B,E,F,I,J.
αPS2-RNAi and αPS3-RNAi lines were used to knock down expression of the corresponding alpha subunits. In repo::αPS2-RNAi nerves, αPS2 labeling was removed but the αPS3 labeling appeared normal (see Fig. S3B in the supplementary material). Consistently, αPS3 labeling, but not αPS2 labeling, was greatly reduced in repo::αPS3-RNAi nerves (see Fig. S3C in the supplementary material), indicating that the αPS2-RNAi and αPS3-RNAi constructs were subunit specific. Labeling of muscles and of trachea were used as internal staining controls for αPS2 and αPS3, respectively.
The knockdown of the integrin complex in glial cells caused glial defects and decreased viability. For example, repo::βPS-RNAi animals died in late third instar and pupal stages. When UAS-CD8-GFP was co-expressed to label the glial membrane, we observed morphological changes paired with reduced intensity and changes in the distribution of CD8-GFP (Fig. 5B). This suggests that βPS integrin is necessary in glial cells in the larval peripheral nerve. However, repo-GAL4 is expressed in all three glial layers, which are functionally and morphologically distinct. Therefore, to test for integrin function in the individual glial layers, different GAL4 drivers were used to express integrin RNAi in specific glial subtypes.
Integrin function is necessary for perineurial glia wrapping
To study how loss of integrin affects the outer PG layer, 46F-GAL4 was used to express the integrin RNAi constructs. In control third instar larvae, 46F-GAL4-driven CD8-GFP formed a seamless sheath around the exterior of the peripheral nerves (Fig. 5C,F). The coverage of 46F::CD8-GFP throughout the entire surface of HRP labeling (see Movie 1 in the supplementary material) suggests that the membranes from different perineurial cells attach to each other and wrap around the nerve collaboratively. In third instar larvae with 46F-GAL4 driving the expression of βPS-RNAi (46F::βPS-RNAi), βPS antibody labeling was decreased in the PG (Fig. 5I,J, arrowheads) as compared with controls (Fig. 5E,F, arrows). The βPS labeling appeared normal in the internal glia, indicating that βPS integrin expression was knocked down specifically in the PG.
βPS RNAi caused similar morphological changes to the PG as observed in the βPS (mys) null MARCM clones. The CD8-GFP-labeled PG membrane was observed only on one side of the nerve (Fig. 5H) and failed to encircle the nerve (Fig. 5J). Moreover, individual PG cells became distinct (Fig. 5G) as the PG became detached from each other (see Movie 2 in the supplementary material).
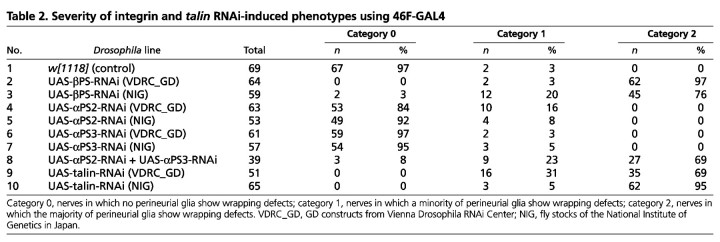
Given that αPS2 integrin labeling was mostly observed on both sides of perineurial cells, we asked whether loss of αPS2 integrin would phenocopy the βPS-RNAi. 46F-GAL4 was used to express UAS-αPS2-RNAi and similar PG wrapping phenotypes were observed (data not shown). However, the penetrance of the phenotypes was much lower in the 46F::αPS2-RNAi larvae than in the 46F::βPS-RNAi larvae (Table 2). The severity of the defects was also different. In 46F::αPS2-RNAi larvae, all defective nerves fell into category 1, in which a minority of PG failed to wrap, whereas in 46F::βPS-RNAi most of the defective nerves fell into category 2, in which the majority of perineurial cells displayed a wrapping failure. Given the efficiency of UAS-αPS2-RNAi (see Fig. S3B in the supplementary material), the difference between 46F::αPS2-RNAi and 46F::βPS-RNAi might be due to compensation by another alpha subunit. As the αPS3 integrin was also found in the peripheral nerve, UAS-αPS3-RNAi was co-expressed with UAS-αPS2-RNAi using 46F-GAL4. As expected, more nerves (92%) had PG that failed to wrap and most of them (69%) were scored as category 2 (Table 2). This suggests that αPS2 and αPS3 integrins are functionally redundant in the PG, similar to observations made concerning midgut formation (Martin-Bermudo et al., 1999).
Table 2.
Severity of integrin and talin RNAi-induced phenotypes using 46F-GAL4
To determine whether PG wrapping requires the integrin adhesion complex, we specifically knocked down Talin using RNAi driven by 46F-GAL4 and observed wrapping defects similar to those caused by integrin RNAi (Table 2, see Fig. S2D-F in the supplementary material). Our data suggested that PG wrapping of the peripheral nerve requires an integrin adhesion complex that contains αPS2βPS or αPS3βPS integrin plus Talin.
To check whether the PG wrapping defect is due to an insufficient number of PG to match the growing nerve surface, we quantified the PG number in A8 nerves by co-expressing a nuclear-localized GFP (UAS-nls-GFP) using 46F-GAL4. Control nerves had 40±6 PG on average (n=12). Knockdown of βPS resulted in either a reduction or an increase in PG nuclei depending on the RNAi line used [13±3 (n=11) for βPS-RNAi (VDRC_GD) and 57±7 (n=14) for βPS-RNAi (NIG)]. However, knockdown of Talin also showed an increase in the number of PG [50±7 (n=12) for Talin-RNAi (NIG) and 55±8 (n=14) for Talin-RNAi (VDRC_GD)]. As all these RNAi lines generate the glial wrapping phenotype, these changes in glial number are unlikely to be responsible for the failure of the PG to wrap the growing nerve.
The neural lamella is required by the perineurial glia
Most integrins function through binding to the ECM, including αPS2βPS integrin in Drosophila (Bokel and Brown, 2002). Integrins are in turn important for ECM deposition, such as in epithelial morphogenesis (Narasimha and Brown, 2004). However, when βPS integrin was knocked down in all glial layers using repo-GAL4, Perlecan-GFP and γ-Laminin labeling appeared normal in RNAi nerves as compared with control nerves (see Fig. S4 in the supplementary material). This suggests that glial integrins are not required for NL formation.
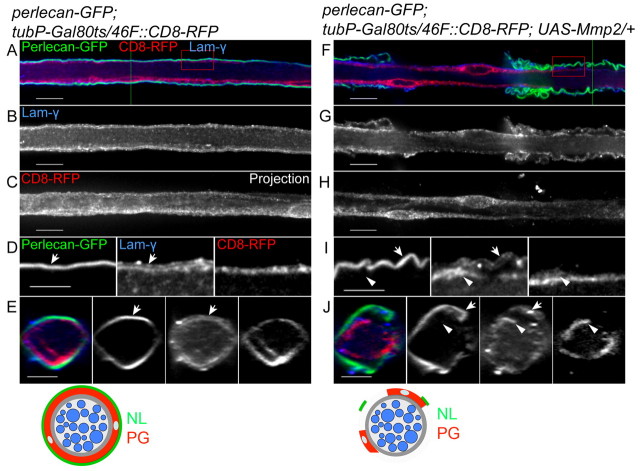
The question is, then, whether integrin function in the PG relies on the NL. The major components of the NL, such as Collagen IV, Perlecan and laminins, are deposited by migrating hemocytes during embryogenesis (Olofsson and Page, 2005; Stork et al., 2008). Consistently, expression of perlecan (trol) and collagen IV (viking) RNAi in glia did not affect or remove the NL (data not shown). Instead, we chose to remove the entire ECM layer by expressing a matrix metalloproteinase, Matrix metalloproteinase 2 (Mmp2), in glial cells. Mmp2 is one of two Mmp family members in Drosophila (Page-McCaw et al., 2003) and is attached to the extracellular membrane through a glycophosphatidylinositol (GPI) anchor (Llano et al., 2002). Constitutive expression of Mmp2 by repo-GAL4 or 46F-GAL4 caused lethality at embryonic and larval first instar stages. Therefore, to study the function of the NL in later stages, a temperature-sensitive inhibitor of GAL4 (GAL80ts) was used. Embryos and larvae were raised at the permissive temperature (18°C) and then, at the early third instar stage, Mmp2 expression was activated by transferring the larvae to the restrictive temperature (29°C) for one day prior to dissection. The ECM was labeled using Perlecan-GFP (or Collagen IV-GFP, data not shown) and Laminin immunolabeling to determine the effectiveness of the Mmp2 in disrupting the NL. In control nerves, Perlecan-GFP and γ-Laminin were both found in the NL surrounding the PG (Fig. 6A-E). When Mmp2 was expressed by the PG, Perlecan-GFP and Laminin were greatly reduced and became detached from the PG (Fig. 6F and arrows in 6I,J), suggesting that overexpression of Mmp2 in PG was sufficient to remove the NL. Surprisingly, high levels of Laminin immunolabeling were observed within the PG (Fig. 6I,J, arrowheads), suggesting that glia might upregulate Laminin to compensate for the loss of the NL.
Fig. 6.
Overexpression of Mmp2 degrades the neural lamella and causes a perineurial glial wrapping defect. 46F-GAL4 was used to express CD8-RFP (red) and UAS-Mmp2 in the PG. Expression was regulated by a temperature-sensitive GAL80 under the control of a ubiquitous tubulin promoter (tubP-GAL80ts). The NL was labeled by Perlecan-GFP (green) and γ-Laminin (blue). The labeling and changes to the NL and PG are also illustrated beneath. (A,B,F,G) Single 0.2 μm z-sections of the nerves; (C,H) projections of the entire z-stack. Boxed regions were digitally expanded as shown in D and I. (E,J) Orthogonal sections of z-stacks from the positions indicated by the green lines. (A-E) In a control nerve, Perlecan-GFP (green) and γ-Laminin (blue) labeled the outermost NL (arrows), which surrounds the CD8-RFP-labeled PG (red). Diffuse, low-level Laminin staining was apparent within the nerve. (F-J) In a 46F::Mmp2 nerve, both Perlecan-GFP (green) and γ-Laminin immunolabeling (blue) indicate that the remaining NL is partially detached from the surface of the nerve (arrows). γ-Laminin immunolabeling appeared to be increased within the CD8-RFP-labeled (red) PG (arrowheads). The PG no longer wrapped around the entire circumference of the nerve and borders of distinct, individual PG were identified. Scale bars: 10 μm in A-C,F-H; 5 μm in D,E,I,J.
The PG (46F::CD8-RFP) expressing Mmp2 did not form a complete circle around the nerve (Fig. 5H,J). Thus, disruption of the ECM by Mmp2 generated a wrapping defect in the PG similar to that of the βPS knockdown in the 46F::βPS-RNAi nerve. Remarkably, the short-term expression of Mmp2 in the PG resulted in a strong CNS phenotype in which the ventral nerve cord became thin and elongated compared with that of controls (see Fig. S5 in the supplementary material). This suggests a role of the ECM and PG in maintaining nervous system morphology by constricting the ventral nerve cord.
Integrin function is necessary for axon ensheathment by wrapping glia
In the larval nerve, βPS integrin is not only found in the outer glial cells, but is also observed in the central regions where the WG ensheath axons (Fig. 2E-G). To study integrin function in the WG, the Nrv2-GAL4 driver was used to knock down βPS in GFP-labeled WG (Fig. 7). In wild-type nerves, the WG (n=72) labeled with CD8-GFP formed complex processes along the nerve (Fig. 7B; see Movie 3 in the supplementary material). The CD8-GFP labeling filled in the space between the HRP-labeled axons (Fig. 7A-C), as each WG produces extensive processes that wrap around axons. In the Nrv2::βPS-RNAi larvae, CD8-GFP still formed processes in between the HRP labeling and spread along the nerve. However, the complexity of the processes was greatly decreased. For example, in the βPS-RNAi line (VDRC_GD29619), all WG (90/90) had severe defects in which CD8-GFP labeling was observed in a single longitudinal process (Fig. 7D-F; see Movie 4 in the supplementary material). Importantly, this phenotype was repeated in the two βPS (mys) null MARCM clones that we were able to obtain in the WG (data not shown). This suggests that the WG are unable to form or maintain complex processes when βPS integrin is reduced.
Fig. 7.
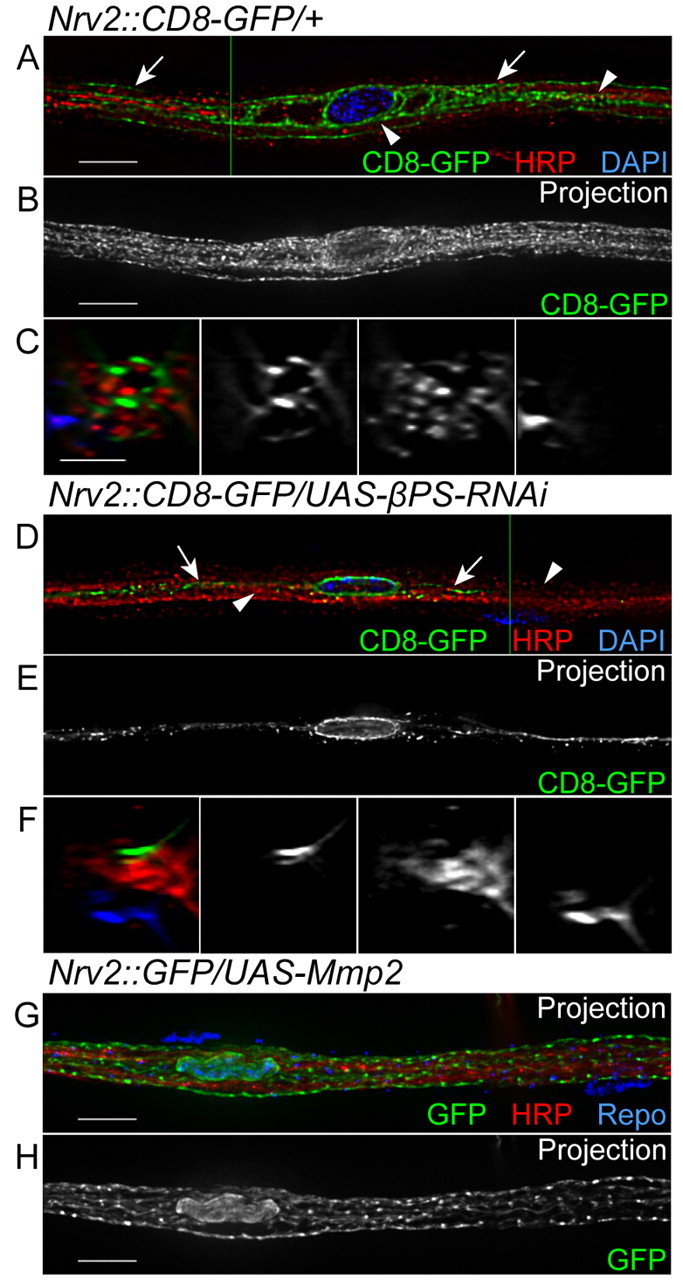
Expression of βPS-RNAi in the wrapping glia. Nrv2-GAL4 was used to express CD8-GFP (green) and βPS-RNAi in the WG. Anti-HRP and DAPI labeling were used for axons (red) and nuclei (blue), respectively. The labeling and phenotypes observed in the WG are illustrated to the right. (A,D) Single 0.2 μm z-sections; (B,E,G,H) projections of the entire z-stack; (C,F) orthogonal sections at the positions indicated by the green lines. (A-C) In a control nerve, CD8-GFP surrounded the DAPI-labeled glia nucleus (blue) and extended along the nerve to fill complex processes (arrows) in between the HRP labeling (red, arrowheads). (D-F) In an Nrv2::βPS-RNAi nerve, CD8-GFP was found only in a single process that extended along the labeled axons (red, arrowheads). Arrows point to small projections and puncta of CD8-GFP. (G,H) In an Nrv2::Mmp2 nerve, the CD8-GFP-labeled processes of the WG appeared normal and spread throughout the labeled axons, similar to the control nerve. Glial nuclei were identified by Repo immunolabeling (blue in G). Scale bars: 10 μm in A,B,D,E,G,H; 5 μm in C,F.
Although βPS had a clear function in the WG, we were unable to detect the presence of ECM components in the internal regions of the peripheral nerve. For example, Perlecan-GFP and Viking-GFP (Collagen IV) were observed exclusively in the NL with no internal labeling (Fig. 6A,F; data not shown). We also assayed the distribution of Laminins. Drosophila has two alpha genes but only one beta (LanB1) and one gamma (LanB2) gene. Strong beta and gamma laminin expression was detected in the NL, but only diffusely in internal regions of the nerve (Fig. 6B,D,E). However, we cannot rule out the possibility that low levels of ECM might be deposited internally given the diffuse laminin labeling we observed. Therefore, to test whether an internal ECM is necessary for the ensheathment of peripheral axons, Mmp2 was overexpressed in the WG using Nrv2-GAL4. Unlike repo-GAL4 or 46F-GAL4, constitutive expression of Mmp2 with Nrv2-GAL4 did not cause lethality or a decrease in the WG processes in the peripheral nerve (Fig. 7G,H). This suggests that the internal βPS integrin might function in the WG by binding to an unknown ligand that is not an ECM component.
DISCUSSION
The integrin complex in peripheral glia
Peripheral nerves in vertebrates and Drosophila are organized in similar ways, with central axons wrapped by an inner class of glia that are surrounded in turn by layers of external glia. The glia and the surrounding ECM establish a tubular sheath to protect axons from physical damage and pathogens (Parmantier et al., 1999). We show that at least two integrin heterodimers are expressed in these different glial layers and are localized at focal adhesions with Ilk and Talin. We found that αPS2βPS integrin is prevalent in the PG and that the αPS3βPS integrin is more prevalent in the WG. Since αPS2 integrin is expressed mostly in the outermost PG and can bind ligands that contain the tripeptide RGD sequence (Bunch and Brower, 1992; Fogerty et al., 1994), it most likely functions by binding to ECM ligands in the NL. The majority of αPS3 integrin is expressed by the internal SPG and WG and αPS3 integrin has been shown to interact with laminins in Drosophila (Schock and Perrimon, 2003; Stark et al., 1997). However, it is possible that the integrin complex in the WG might have other ligands and might mediate direct cell-cell interactions as we failed to detect a pronounced basal lamina associated with the peripheral axons and Mmp2 expression had no effect in the internal regions of the peripheral nerves.
Integrin-ECM interactions are required for perineurial glia function
PG form the outermost glial layer in the Drosophila nervous system but their origin and function are not well understood, even though they were identified some time ago (Edwards et al., 1993; Schmid et al., 1999). Drosophila PG are structurally similar to their vertebrate counterparts and have been proposed to have similar roles (Lavery et al., 2007). Vertebrate perineurial cells and the associated collagen fibers provide important mechanical support to nerves and their development might rely on ECM-mediated signals, as β1 integrin is found in the perineurium (Feltri et al., 2002). However, little is known about the role of integrins in perineurial cells.
Our results show that Drosophila PG express αPS2 and βPS integrin subunits (and to a lesser extent αPS3), which colocalize with both Ilk and Talin. Knocking down integrins disrupts perineurial wrapping, but we were unable to distinguish whether the PG failed to initiate wrapping or failed to maintain their processes around the nerves to accommodate the growing nerve surface. However, degradation of the NL by Mmp2 overexpression generates a PG wrapping phenotype similar to that of βPS RNAi. Thus, binding of βPS integrin to ligands in the ECM mediates the radial spread of PG around the tubular structure of the nerve and suggests that PG retract their membrane when integrin-ECM interaction is interrupted. Moreover, knockdown of Talin in the PG produces a similar wrapping defect. This suggests that, in the PG, integrin adhesion complexes mediate the connection between the extracellular NL and the intracellular actin cytoskeleton and are required for the initiation or maintenance of glial ensheathment.
The function of the PG in the peripheral nerve is not well understood. The PG do not generate an impermeable barrier and it is the SPG layer that creates the blood-nerve barrier (Stork et al., 2008). Loss of PG ensheathment did not result in paralysis or lethality, which are signs of a disrupted blood-brain barrier (Baumgartner et al., 1996; Schwabe et al., 2005). Larger molecules (∼500 kDa) are blocked by the NL or the PG (Stork et al., 2008), perhaps mirroring the protective function of the vertebrate perineurium against pathogens (Parmantier et al., 1999). However, in Mmp2-overexpressing larvae, the affected nerves become thin and are difficult to retain intact during tissue preparation. This suggests that the NL and PG provide important mechanical support as is seen with the perineurium in mammalian nerves (Parmantier et al., 1999).
βPS integrin is required to maintain wrapping glia ensheathment of the peripheral axons
In the center of Drosophila peripheral nerves, the WG embed axons in bundles or individually within single membrane wraps, similar to the non-myelinating Schwann cells of vertebrate peripheral nerves (Corfas et al., 2004). Our results show that WG predominantly express αPS3 and βPS integrin subunits in complexes positive for Ilk and Talin along the WG membrane. When βPS expression is knocked down, the complexity of the glial processes between the associated axons is greatly reduced. Only a few long processes and small membrane protrusions are observed around the axons, suggesting that the WG might be retracting their processes in the absence of βPS integrin. The role of integrins appears to be conserved between WG and Schwann cells in vertebrates. For example, myelinating Schwann cells lacking β1 integrin or Ilk do not extend membrane processes around axons, resulting in impaired radial sorting (Feltri et al., 2002; Pereira et al., 2009). The role of integrins in non-myelinating Schwann cells is not known but our results suggest that integrins have similar functions in Drosophila and vertebrates in mediating the glial ensheathment of peripheral axons.
No clear ECM has been observed in the internal regions of Drosophila nerves by immunofluorescence analysis or transmission electron microscopy (Lavery et al., 2007; Stork et al., 2008). Therefore, the integrin complex could promote WG sheath formation by mediating direct cell-cell adhesion between the glial membrane and its associated axon, or between glial membranes. A potential candidate for an integrin-interacting protein expressed on axons or glia is Neuroglian, the Drosophila L1 (Nrcam) homolog. L1 is an Ig domain transmembrane protein that is known to bind RGD-dependent integrins (Ruppert et al., 1995; Montgomery et al., 1996). Loss of the integrin-binding domain of L1 results in wrapping defects in both myelinating and non-myelinating Schwann cells (Itoh et al., 2005). However, we cannot rule out the presence of low levels of ECM given the weak laminin immunolabeling that we observed. Even though Mmp2 expression in the WG had no effect, it is still possible that the integrin-mediated adhesion is Mmp2 resistant. To resolve this issue, further ultrastructural and genetic studies will be required.
Supplementary Material
Acknowledgments
We thank Dr Shigeo Hayashi for generously providing antibodies; Drs Guy Tanentzapf, Christian Klämbt, Doug Allan, the Bloomington and DGRC Stock Centers for fly stocks; the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks; Doug Allan, Patrick Cafferty, Mary Gilbert and Guy Tanentzapf for helpful discussions and comments on the manuscript. X.X. was supported by a scholarship from the Multiple Sclerosis Society of Canada. This study was supported by grants to V.J.A. from the Canadian Institutes of Health Research and the Natural Science and Engineering Research Council of Canada.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.064816/-/DC1
References
- Alfonso T. B., Jones B. W. (2002). gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev. Biol. 248, 369-383 [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Littleton J. T., Broadie K., Bhat M. A., Harbecke R., Lengyel J. A., Chiquet-Ehrismann R., Prokop A., Bellen H. J. (1996). A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87, 1059-1068 [DOI] [PubMed] [Google Scholar]
- Bhat M. (2003). Molecular organization of axo-glial junctions. Curr. Opin. Neurobiol. 13, 552-559 [DOI] [PubMed] [Google Scholar]
- Bokel C., Brown N. H. (2002). Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 3, 311-321 [DOI] [PubMed] [Google Scholar]
- Brower D. L., Wilcox M., Piovant M., Smith R. J., Reger L. A. (1984). Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA 81, 7485-7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Gregory S. L., Martin-Bermudo M. D. (2000). Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 223, 1-16 [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Brower D. L. (1992). Drosophila PS2 integrin mediates RGD-dependent cell-matrix interactions. Development 116, 239-247 [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Salatino R., Engelsgjerd M. C., Mukai L., West R. F., Brower D. L. (1992). Characterization of mutant alleles of myospheroid, the gene encoding the beta subunit of the Drosophila PS integrins. Genetics 132, 519-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Strickland S. (2003). Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163, 889-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G., Velardez M. O., Ko C. P., Ratner N., Peles E. (2004). Mechanisms and roles of axon-Schwann cell interactions. J. Neurosci. 24, 9250-9260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I., Brown N. H. (2007). Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 19, 43-50 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156 [DOI] [PubMed] [Google Scholar]
- Edwards J. S., Swales L. S., Bate M. (1993). The differentiation between neuroglia and connective tissue sheath in insect ganglia revisited: the neural lamella and perineurial sheath cells are absent in a mesodermless mutant of Drosophila. J. Comp. Neurol. 333, 301-308 [DOI] [PubMed] [Google Scholar]
- Feltri M. L., Wrabetz L. (2005). Laminins and their receptors in Schwann cells and hereditary neuropathies. J. Peripher. Nerv. Syst. 10, 128-143 [DOI] [PubMed] [Google Scholar]
- Feltri M. L., Graus Porta D., Previtali S. C., Nodari A., Migliavacca B., Cassetti A., Littlewood-Evans A., Reichardt L. F., Messing A., Quattrini A., et al. (2002). Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Valle C., Gwynn L., Wood P. M., Carbonetto S., Bunge M. B. (1994). Anti-beta 1 integrin antibody inhibits Schwann cell myelination. J. Neurobiol. 25, 1207-1226 [DOI] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., Nelson R. E., Brower D. L., Gullberg D., Fessler J. H. (1994). Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development 120, 1747-1758 [DOI] [PubMed] [Google Scholar]
- Freeman M. R., Doherty J. (2006). Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 29, 82-90 [DOI] [PubMed] [Google Scholar]
- Itoh K., Fushiki S., Kamiguchi H., Arnold B., Altevogt P., Lemmon V. (2005). Disrupted Schwann cell-axon interactions in peripheral nerves of mice with altered L1-integrin interactions. Mol. Cell. Neurosci. 30, 624-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova N., Bobinnec Y., Fouix S., Huitorel P., Debec A. (2006). Jupiter, a new Drosophila protein associated with microtubules. Cell Motil. Cytoskel. 63, 301-312 [DOI] [PubMed] [Google Scholar]
- Kelso R. J., Buszczak M., Quinones A. T., Castiblanco C., Mazzalupo S., Cooley L. (2004). Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 32, D418-D420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery W., Hall V., Yager J. C., Rottgers A., Wells M. C., Stern M. (2007). Phosphatidylinositol 3-kinase and Akt nonautonomously promote perineurial glial growth in Drosophila peripheral nerves. J. Neurosci. 27, 279-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461 [DOI] [PubMed] [Google Scholar]
- Llano E., Adam G., Pendas A. M., Quesada V., Sanchez L. M., Santamaria I., Noselli S., Lopez-Otin C. (2002). Structural and enzymatic characterization of Drosophila Dm2-MMP, a membrane-bound matrix metalloproteinase with tissue-specific expression. J. Biol. Chem. 277, 23321-23329 [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Alvarez-Garcia I., Brown N. H. (1999). Migration of the Drosophila primordial midgut cells requires coordination of diverse PS integrin functions. Development 126, 5161-5169 [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. (2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765-1768 [DOI] [PubMed] [Google Scholar]
- Montgomery A. M., Becker J. C., Siu C. H., Lemmon V. P., Cheresh D. A., Pancook J. D., Zhao X., Reisfeld R. A. (1996). Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin alpha v beta 3. J. Cell Biol. 132, 475-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. (2001). A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050-15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha M., Brown N. H. (2004). Novel functions for integrins in epithelial morphogenesis. Curr. Biol. 14, 381-385 [DOI] [PubMed] [Google Scholar]
- Newbern J., Birchmeier C. (2010). Nrg/ErbB sigaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 21, 922-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B., Page D. T. (2005). Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev. Biol. 279, 233-243 [DOI] [PubMed] [Google Scholar]
- Olsson Y. (1990). Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit. Rev. Neurobiol. 5, 265-311 [PubMed] [Google Scholar]
- Page-McCaw A., Serano J., Sante J. M., Rubin G. M. (2003). Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 4, 95-106 [DOI] [PubMed] [Google Scholar]
- Parker R. J., Auld V. J. (2006). Roles of glia in the Drosophila nervous system. Semin. Cell Dev. Biol. 17, 66-77 [DOI] [PubMed] [Google Scholar]
- Parmantier E., Lynn B., Lawson D., Turmaine M., Namini S. S., Chakrabarti L., McMahon A. P., Jessen K. R., Mirsky R. (1999). Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23, 713-724 [DOI] [PubMed] [Google Scholar]
- Pereira J. A., Benninger Y., Baumann R., Goncalves A. F., Ozcelik M., Thurnherr T., Tricaud N., Meijer D., Fassler R., Suter U., et al. (2009). Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 185, 147-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. D., Ellis S. J., Asghari P., Shamsian A., Moore E. D., Tanentzapf G. (2010). Integrin-mediated adhesion maintains sarcomeric integrity. Dev. Biol. 338, 15-27 [DOI] [PubMed] [Google Scholar]
- Ruppert M., Aigner S., Hubbe M., Yagita H., Altevogt P. (1995). The L1 adhesion molecule is a cellular ligand for VLA-5. J. Cell Biol. 131, 1881-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Chiba A., Doe C. Q. (1999). Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development 126, 4653-4689 [DOI] [PubMed] [Google Scholar]
- Schock F., Perrimon N. (2003). Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 17, 597-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T., Bainton R. J., Fetter R. D., Heberlein U., Gaul U. (2005). GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 123, 133-144 [DOI] [PubMed] [Google Scholar]
- Sepp K. J., Auld V. J. (1999). Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics 151, 1093-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp K. J., Schulte J., Auld V. J. (2000). Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia 30, 122-133 [DOI] [PubMed] [Google Scholar]
- Sepp K. J., Schulte J., Auld V. J. (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 238, 47-63 [DOI] [PubMed] [Google Scholar]
- Stark K. A., Yee G. H., Roote C. E., Williams E. L., Zusman S., Hynes R. O. (1997). A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development 124, 4583-4594 [DOI] [PubMed] [Google Scholar]
- Stork T., Engelen D., Krudewig A., Silies M., Bainton R. J., Klambt C. (2008). Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 28, 587-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Xu P., Salvaterra P. M. (1999). Dynamic visualization of nervous system in live Drosophila. Proc. Natl. Acad. Sci. USA 96, 10438-10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N., Wang S. L., Dorovkov M., Ryazanov A., Driscoll M. (2000). Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat. Genet. 24, 180-183 [DOI] [PubMed] [Google Scholar]
- Wada A., Kato K., Uwo M. F., Yonemura S., Hayashi S. (2007). Specialized extraembryonic cells connect embryonic and extraembryonic epidermis in response to Dpp during dorsal closure in Drosophila. Dev. Biol. 301, 340-349 [DOI] [PubMed] [Google Scholar]
- Wallquist W., Plantman S., Thams S., Thyboll J., Kortesmaa J., Lannergren J., Domogatskaya A., Ogren S. O., Risling M., Hammarberg H., et al. (2005). Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J. Neurosci. 25, 3692-3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. C., Okano H., Patel N. H., Blendy J. A., Montell C. (1994). repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 8, 981-994 [DOI] [PubMed] [Google Scholar]
- Yang D., Bierman J., Tarumi Y. S., Zhong Y. P., Rangwala R., Proctor T. M., Miyagoe-Suzuki Y., Takeda S., Miner J. H., Sherman L. S., et al. (2005). Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 168, 655-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. M., Feltri M. L., Wrabetz L., Strickland S., Chen Z. L. (2005). Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25, 4463-4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. M., Yu H., Chen Z. L., Strickland S. (2009). Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function. Glia 57, 850-859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.