Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults. GBM is comprised of highly proliferative and infiltrative neoplastic cells that frequently invade eloquent areas of the cerebral hemispheres,6 producing progressive neurological deficits and symptoms of raised intracranial pressure. When possible, surgical resection of GBMs after diagnosis is used to relieve mass effect, confirm the diagnosis pathologically and set the stage for multimodal adjunctive therapy.3,4,10,14
A long-standing point of contention among neurosurgeons is the capacity of surgical cytoreduction to prolong quality survival. A fine balance must be struck between the aggressive removal of malignant tissue and minimizing the risk of worsening or inducing new neurologic deficits that may negatively impact outcomes. Furthermore, residual microscopic disease, even in the most seemingly resectable lesions, invariably results in disease progression within months,12 which perhaps lends support for a more conservative approach particularly in elderly patients with poor functional status.
To develop an optimal balance between maximal surgical cytoreduction and minimization of operative risk, the neurosurgeon must assess patient preoperative prognosis and tumor location to determine the extent of resection (EOR) that provides maximal survival and functional benefit. For lesions in non-eloquent areas, the answer may be an aggressive gross total removal. For lesions near or involving eloquent or critical areas, the answer may be a subtotal resection or tissue biopsy only, due to the potential costs of surgically acquired deficits.
To date, the best data assessing the balance between EOR and survival benefit comes from large institutional retrospective analyses. Lacroix et al. reported on the outcomes of 416 patients with newly diagnosed and recurrent GBMs.5 Seventy-five percent of patients had a Karnofsky performance status (KPS) of 80 or more and 92% of the lesions were near or involved eloquent or deep brain structures. The authors found that an EOR of 98% or more was associated with improved survival. While overall survival benefit at this EOR or more was 4.2 months, complication rates and functional outcomes were not provided. Many have used this study to justify performing debulking surgery only when a greater than 98% EOR is thought to be achievable.
More recently, Sanai et al. reported the outcomes on 500 newly diagnosed GBM patients undergoing initial resection.9 Patients had a median KPS 80 and 69% of tumors involved eloquent areas. Using intraoperative functional mapping adjuncts where appropriate, significant survival benefits were seen with an EOR of as little as 78% but increased as the EOR approached 100%, at which point median overall survival exceeded 16 months (compared to 12.2 months for the entire cohort). With its relatively large and homogeneous patient sample, this series provides substantive evidence that the cytoreductive threshold for a meaningful survival benefit may be lower than previously thought.
Identifying an EOR threshold is only one aspect, however, of the glioma surgeon’s dilemma. In order to develop rational operative strategies, the relationship between EOR, tumor resectability and surgical risk must also be carefully elucidated. A gross total resection of a tumor located entirely within the anterior right temporal lobe entails different risks, and hence different implications for the goal of resection, compared to one abutting the motor cortex or infiltrating the thalamus.
Emerging evidence indicates that the consequences of surgically acquired neurologic deficits are significant. In a series of 306 consecutive patients with good performance status operated on for newly diagnosed GBM, McGirt et al. describe 15 patients (5%) who developed a new language deficit and 19 patients (6%) who developed a new motor deficit perioperatively.7 Both types of deficit were associated with a decreased median survival (reduced survival of 3.2 months and 3.8 months, respectively). This would suggest that, at least in the case of tumors near or involving motor and language cortex, patients risk losing virtually all survival gains from cytoreduction. Thus, it is imperative that these eloquent areas be identified and preserved meticulously.8,11,13
In their article, Gulati et al. contribute to our understanding of the impact of morbidity on outcomes in their series of 144 consecutive patients undergoing initial resection of a GBM. Similar to previous studies, patients were of good functional status preoperatively. Although intraoperative electrophysiological techniques were not used, functional neuronavigation employing fMRI and white matter tractography was used in lesions involving eloquent cortex. Surgically acquired cognitive, motor, language, coordination and visual deficits occurred at an overall rate of 15.3%. Rates of language and motor deficits were 4.9% and 5.6%, which are comparable to other studies. Similar to McGirt et al. and others, 1,2 the current study finds that patients with surgically acquired neurological deficits were at significantly increased odds of experiencing worsened functional outcome.
Gulati et al. go on to examine the functional impact of general perioperative complications, which occurred at an overall rate of 19.4%, and find that these also predicted poorer outcomes, although at the lower odds ratio of 4.1. The most common of these were new onset seizures, urinary tract infections and surgical site hematomas. Interestingly, both perioperative complications and acquired neurological deficits were associated with a decreased likelihood of receiving adjunctive radiotherapy and chemotherapy, both of which were independently predictive of improved 12-month rates of survival. This suggests that the ramifications of the adverse effects of surgical treatment may extend well beyond proximate physiologic sequelae. Rather, patients may be cast into prognostic categories postoperatively that dictate the course of all subsequent treatment.
Perhaps most interestingly, Gulati et al. find that the rates of perioperative complications and acquired neurological deficits were constant irrespective of extent of resection. They use this to voice a warranted word of caution against the role of subtotal resections in GBM, since in their series only gross total resections were associated with improved survival. However, it is important to recognize that the similar rates of surgically acquired neurological deficits in the subtotal, near total, and gross total resection categories also imply that tumors in these groups differed in their average inherent resectability.
Unfortunately, few published studies on the extent of resection in glioma rigorously control for tumor resectability. Studies may describe tumors by location or depth, or categorize them as eloquent or non-eloquent in a binary fashion. None, to our knowledge, have attempted to adjust for resectability as a continuous variable derived from functional volumetric data, which is what ideally would be required to define with precision the “therapeutic window” of surgical cytoreduction in any given patient. Additionally, more detailed data on resectability would allow valid comparisons to be made across different studies, which would in turn aid in improving the validity of systematic reviews and the development of practice guidelines. A 98% threshold of resection for a survival benefit identified in a series with a large number of non-eloquent tumors, for example, cannot be taken at face value to be corroborative of the same threshold identified in a series with a large number of eloquent or deep tumors.
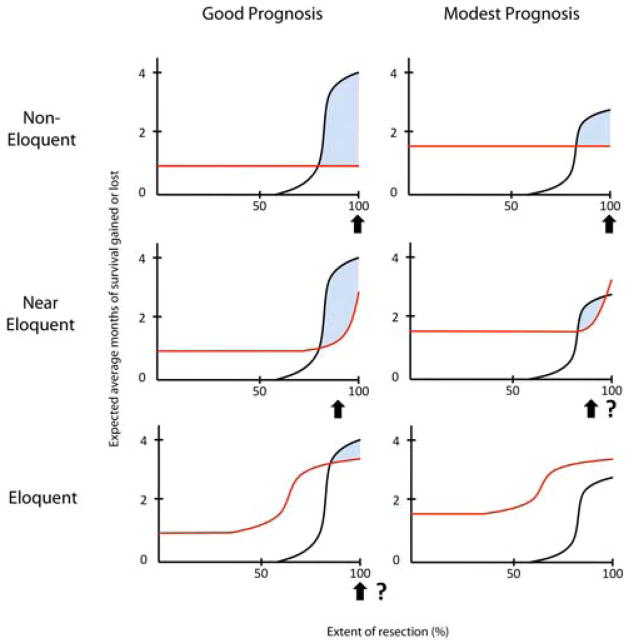
Synthesizing what is currently known about resection thresholds (78 to 100%), maximum survival benefit from surgery (approximately 4 months), and the rate of surgical complications and their potential impact on survival (perhaps a reduction of as much as 3 to 4 months), one might envision the existence of a complex relationship between EOR and net survival benefit that varies according to preoperative prognosis and degree of involvement of critical brain areas (Figure 1). In this paradigm, while the EOR threshold remains identical at 80% in every scenario, subtle changes in the expected magnitude of survival gains (maximum of the black curve) and the level of general perioperative risk (minimum of the red curve) can dramatically alter the area of the therapeutic window. The challenge for future studies will be to expose the nature of these risk-benefit relationships unambiguously so that neurosurgeons have available an evidence-based therapeutic blueprint that can readily be applied to diverse individual patients.
Figure 1.
Conceptual framework for the relationship between risk and benefit for cytoreductive glioblastoma surgery according to tumor resectability and preoperative prognosis. Black curves indicate expected average months of survival gained from tumor cytoreduction if the extent of resection (EOR) threshold is assumed to be approximately 80%. Red curves indicate expected average months of survival lost due to general perioperative complications and surgically acquired neurological deficits. Shaded area indicates the range of EOR within which a net survival benefit exists. Arrows indicate EOR at which benefit is maximal for each scenario. Question marks indicate scenarios where projected net benefit may not be clinically meaningful.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, Gupta L, Tang-Wai DF, Arusell RM, Clark MM, Buckner JC. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76:283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 2.Chaichana KL, Halthore AN, Parker SL, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa A. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604–612. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciric I, Ammirati M, Vick N, Mikhael M. Supratentorial gliomas: surgical considerations and immediate postoperative results. Gross total resection versus partial resection. Neurosurgery. 1987;21:21–26. doi: 10.1227/00006123-198707000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Fadul C, Wood J, Thaler H, Galicich J, Patterson RH, Jr, Posner JB. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374–1379. doi: 10.1212/wnl.38.9.1374. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Cavenee WK, Ohgaki H, Wiestler OD. WHO classification of tumours of the central nervous system. World Health Organization; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. discussion 469-470. [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 9.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011 doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 10.Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. discussion 1055-1046. [DOI] [PubMed] [Google Scholar]
- 11.Signorelli F, Ruggeri F, Iofrida G, Isnard J, Chirchiglia D, Lavano A, Volpentesta G, Signorelli CD, Guyotat J. Indications and limits of intraoperative cortico-subcortical mapping in brain tumor surgery: an analysis of 101 consecutive cases. J Neurosurg Sci. 2007;51:113–127. [PubMed] [Google Scholar]
- 12.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 13.Wu JS, Zhou LF, Tang WJ, Mao Y, Hu J, Song YY, Hong XN, Du GH. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61:935–948. doi: 10.1227/01.neu.0000303189.80049.ab. discussion 948-939. [DOI] [PubMed] [Google Scholar]
- 14.Yasargil MG, Kadri PA, Yasargil DC. Microsurgery for malignant gliomas. J Neurooncol. 2004;69:67–81. doi: 10.1023/b:neon.0000041872.78927.d5. [DOI] [PubMed] [Google Scholar]