Abstract
Poorly differentiated neuroendocrine carcinomas (NEC) of the pancreas are rare malignant neoplasms with a poor prognosis. The aim of this study was to determine the clinicopathologic and genetic features of poorly differentiated NECs and compare them to other types of pancreatic neoplasms. We investigated alterations of KRAS, CDKN2A/p16, TP53, SMAD4/DPC4, DAXX, ATRX, PTEN, Bcl2 and RB1 by immunohistochemistry and/or targeted exomic sequencing in surgically resected specimens of nine small cell NEC, 10 large cell NECs and 11 well-differentiated neuroendocrine tumors (PanNETs) of the pancreas. Abnormal immunolabeling patterns of p53 and Rb were frequent (p53, 18 of 19, 95%; Rb, 14 of 19, 74%) in both small cell and large cell NEC, whereas Smad4/Dpc4, DAXX and ATRX labeling were intact in virtually all of these same carcinomas. Abnormal immunolabeling of p53 and Rb proteins correlated with intragenic mutations in the TP53 and RB1 genes. By contrast, DAXX and ATRX was lost in 45% of PanNETs whereas p53 and Rb immunolabeling was intact in these same cases. Overexpression of Bcl-2 protein was observed in all nine small cell NECs (100%) and in five of 10 (50%) large cell NECs compared to only two of 11 (18%) PanNETs. Bcl-2 overexpression was significantly correlated with higher mitotic rate and Ki-67 labeling index in neoplasms in which it was present. Small cell NECs are genetically similar to large cell NECs, and these genetic changes are distinct from those reported in PanNETs. The finding of Bcl-2 overexpression in poorly differentiated NECs, particularly small cell NEC, suggests that Bcl-2 antagonists/inhibitors may be a viable treatment option for these patients.
INTRODUCTION
Neuroendocrine neoplasms of the pancreas are uncommon and represent 1–2% of all clinically apparent pancreatic neoplasms (4). Various proposals regarding the classification and nomenclature of neuroendocrine neoplasms have been put forth, and often differ in the use of specific terminology and criteria for grading and staging (27) (28). In the 2010 WHO classification neuroendocrine neoplasms are classified into well-differentiated (low- to intermediate-grade) neuroendocrine tumors (PanNETs) and poorly differentiated (high-grade) neuroendocrine carcinomas (NECs) based solely on tumor proliferative rate (4). Well-differentiated PanNETs are relatively indolent whereas poorly differentiated NECs are highly aggressive; therapy also differs significantly between these two neoplasm categories (4). The clinicopathologic and genetic features of NECs are, however, largely unknown leading to inconsistency in their clinical management (19). Moreover, since small cell NEC of the pancreas is very rare as compared with large cell NEC (9), knowledge of this specific disease entity is mostly derived from case reports.
The origin of poorly differentiated NECs of the pancreas is uncertain. Hypotheses are that they derive from ductal precursors, from well-differentiated neuroendocrine neoplasms, or de novo (4) (54). The derivation from pancreatic ductal adenocarcinomas (PDAC) with neuroendocrine differentiation has been suggested and supported by cases with composite conventional ductal adenocarcinoma and high grade NEC (35) (17). However, many genetic alterations that characterize PDACs (KRAS, CDKN2A/p16, and SMAD4/DPC4) are not found with any significant frequency in poorly differentiated NECs (4). De-differentiation of well-differentiated PanNET has also been proposed (35, 17), although until recently the genetic features of well-differentiated PanNET were not characterized well enough to allow direct comparisons. However, recent whole exome sequencing identified several frequently mutated genes in well differentiated PanNETs, including MEN1, mutated in 44.1%; DAXX (death-domain associated protein) in 25%; ATRX (alpha thalassemia/mental retardation syndrome X-linked) in17.6%; TSC2 in 8.8% and PTEN in 7.3% (21). These recent discoveries provide an opportunity to compare the genetic changes in small cell and large cells NECs to these known genetic changes in well-differentiated PanNETs.
Here we characterized the clinicopathologic features and molecular genetic alterations of surgically resected small cell and large cell NECs of the pancreas and compared them to those of well-differentiated PanNETs. We now show that small and large cell NECs are genetically related entities and that the genetic changes in these neoplasms are distinct from those reported in well-differentiated PanNET.
PATIENTS AND METHODS
Patients
To identify poorly differentiated NECs for study, we performed a search of the Johns Hopkins Pathology Archives using the term “small cell carcinoma”, “large cell carcinoma” or “neuroendocrine carcinoma” and “whipple” or “distal pancreatectomy” spanning January 1, 1988 to July 1, 2010. After the carcinomas derived from the duodenum and common bile ducts were excluded, this search identified nine patients diagnosed with a primary small cell NEC or large cell NEC of the pancreas. None of the patients had radiographic evidence of a lung primary by preoperative radiological examination, or direct invasion from a contiguous site, particularly the ampulla of Vater, by gross examination of the resected surgical specimens. An additional ten cases of small cell or large cell NEC provided from the files of the Memorial Sloan-Kettering Cancer Center. In all cases the diagnosis was confirmed by positive immunoreactivity for synaptophysin and/or chromogranin A, as well as for CD56 expression. None of the nine small cell NECs labeled with antibodies to CD99, a marker for primitive neuroectodermal tumors (data not shown) (36). To compare and contrast the molecular features of poorly differentiated NECs with those of non-functioning well-differentiated PanNETs, paraffin embedded samples of PanNET from 11 patients who had their tumors surgically resected at the Johns Hopkins Hospital or Memorial Sloan-Kettering Cancer Center were also obtained. Approval was obtained by the Institutional Review Boards of both Johns Hopkins and MSKCC for study of all samples used.
The histologic features of all PanNETs and poorly differentiated NECs were reviewed, and each case was also assessed for mitotic indices and Ki67 labeling index. PanNETs were divided into two categories: G1, < 2 mitoses per 10 high power fields (HPF) and/or ≤ 2% Ki67 index; G2, 2–20 mitoses per 10 HPF and/or 3–20% Ki67 labeling index (4). NECs were defined by the presence of > 20 mitoses per 10 HPF and/or > 20% Ki67 labeling index. In cases in which the grade differed for mitotic count compared with Ki67 labeling index, the higher grade was assumed (4). After review, two neoplasms originally diagnosed as large cell NEC of the pancreas in the original pathological reports at operation based on abnormal cytologic features and necrosis (patient no. 20 and 21 in Table 1), were reclassified as well-differentiated PanNET, grade 2 due to their mitotic rates of 2 and 3 per 10 HPF and their Ki67 labeling indices of <20%. Poorly differentiated NEC were divided into small cell NEC and large cell NEC, according to the WHO 2010 classification (4). Specifically, carcinomas with a diffuse, infiltrative growth pattern, small to medium-sized cells with minimal cytoplasm, and fusiform nuclei with finely granular chromatin pattern, inconspicuous nucleoli, and nuclear molding by adjacent nuclei were categorized as small cell NEC (Fig. 1A). By contrast, carcinomas with a more pronounced nesting pattern, abundant necrosis, and containing neoplastic cells with a moderate amount of amphophilic cytoplasm, large nuclei with coarsely clumped chromatin and prominent nucleoli were categorized as large cell NEC (Fig. 1B).
Table 1.
Clinicopathological features of pancreatic neuroendocrine neoplasms
| Case | Age at surgery |
Gender | Tumor location |
Tumor size (cm) |
Lymph node metastasis |
Venous vessel invasion |
Perineural invasion |
Treatment after surgery |
Overall survival (months) |
Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Small Cell Neuroendocrine Carcinoma (Small Cell NEC) | ||||||||||
| 1 | 48 | M | head | NA | NA | + | NA | NA | NA | NA |
| 2 | 47 | F | head | 3 | 13/22 | + | − | NA | 25 | DOD |
| 3 | 53 | F | tail | 4.5 | 2/17 | + | + | chemotherapy (unknown regimen) | 13 | DOD |
| 4 | 55 | F | NA | NA | NA | NA | NA | NA | 18 | DOD |
| 5 | 60 | F | head | 3 | 10/23 | + | + | EP | 13 | DOD |
| 6 | 46 | F | tail | 8.9 | 2/22 | + | + | IP | 9 | NED |
| 7 | 56 | F | head | 5.2 | 4/17 | + | + | IP → EP | 15 | DOD |
| 8 | 71 | M | tail | 6 | 13/22 | + | + | NA | 17 | DOD |
| 9 | 74 | M | head | 7.5 | NA | + | + | no treatment | 1 | DOD |
| Mean± SD (Median) | 56.6±10.1 (55) | 5.4±2.2 (5.2) | 13.9±7.0 (14) | |||||||
| Large Cell Neuroendocrine Carcinoma (Large Cell NEC) | ||||||||||
| 10 | 81 | F | head | 3 | 2/16 | + | + | RT | 6 | DOD |
| 11 | 84 | M | head | 5 | 1/23 | + | − | declined | 47 | DOD |
| 12 | 30 | M | tail | NA | 0/3 | + | NA | STZ/ADM → EP → GEM/CPT-11 | 104 | DOD |
| 13 | 37 | M | body | 5.2 | 0/9 | + | + | GEM/L-OHP/erlotinib | 4 | DOD |
| 14 | 42 | M | tail | 4.5 | 6/26 | + | + | sorafenib/capecitabine → GEM/CPT-11 | 12 | AWD |
| 15 | 79 | F | head | 2.5 | 1/9 | + | − | no treatment | 8 | DOD |
| 16 | 71 | M | body | NA | 13/22 | + | + | NA | 18 | DOD |
| 17 | 59 | M | head | 3.1 | 1/5 | + | + | IP → etoposide → sunitinib | 24 | DOD |
| 18 | 73 | F | head | NA | 1/5 | − | − | NA | 1 | DOD |
| 19 | 40 | F | body | 2 | 5/13 | + | − | RT/5-FU | 6 | DOD |
| Mean± SD (Median) | 55.8±20.6 (65) | 4.5±1.3 (3.1) | 17.9±31.5 (10) | |||||||
| Well-differentiated Neuroendocrine Tumor (NET, G2, intermediate-grade) | ||||||||||
| 20 | 88 | F | head | 1.5 | 3/44 | + | + | |||
| 21 | 50 | F | head | 4 | 7/18 | + | + | |||
| 22 | 73 | M | tail | 2.5 | 2/19 | + | + | |||
| 23 | 58 | M | tail | 13 | 0/9 | − | − | |||
| 24 | 48 | M | head | 5 | 8/30 | + | + | |||
| Well-differentiated Neuroendocrine Tumor (NET, G1, low-grade) | ||||||||||
| 25 | 41 | F | tail | 6 | 0/unknown | − | − | |||
| 26 | 41 | M | tail | 3.5 | 0/1 | − | − | |||
| 27 | 42 | M | body | 18 | 0/19 | + | − | |||
| 28 | 61 | F | tail | 5 | 0/17 | − | − | |||
| 29 | 52 | F | head | 9 | 0/19 | − | − | |||
| 30 | 53 | F | body | 5 | 0/43 | − | − | |||
| Mean± SD (Median) | 55.2±14.5 (52) | 6.6±4.9 (5) | ||||||||
| P Value (Poor vs Well Diff NEC) | 0.46 | 0.29 | 0.20 | 0.06 | 0.008 | 0.005 | 0.08 | − | − | |
| P Value (SCNEC vs LCNEC) | 0.35 | 0.19 | 0.29 | 0.04 | 0.375 | 0.56 | 0.20 | − | 0.21 | |
NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor PD, pancreaticoduodenectomy; TP, total pancreatectomy; Bx, biopsy; PH, partial hepatectomy; DP, distal pancreatectomy; PP, partial pancreatectomy; NA, not available; EP, etoposide/cisplatin; IP, irinotecan/cisplatin; RT, radiotherapy; STZ, streptozotocin; ADM, adriamycin; GEM, gemcitabine; CPT-11, irinotecan; L-OHP, oxaliplatin; 5-FU, fluorouracil; DOD, died of disease; NED, no evidence of disease; AWD, alive with disease.
Figure 1. Histologic features of neuroendocrine neoplasms of the pancreas.
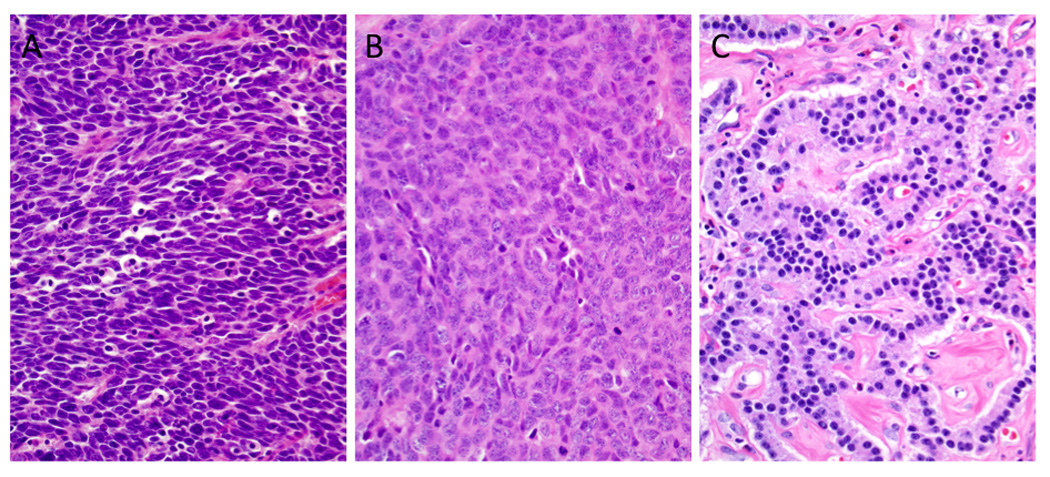
(A) Small cell neuroendocrine carcinoma (small cell NEC). The neoplasm is composed of small cells with minimal cytoplasm, fusiform nuclei, inapparent nucleoli, and a high mitotic rate. Apoptotic bodies are also frequent. (B) Compared to the carcinoma shown in A, this neoplasm contains large cells with abundant cytoplasm. The nuclei have an open chromatin pattern with prominent nucleoli. Abundant mitoses are also seen. (C) Well-differentiated neuroendocrine tumor. Note the small uniform nuclei with delicate chromatin. The neoplastic cells show a trabecular growth pattern in a background of hyalinized and vascular stroma (hematoxylin and eosin stains, original magnifications x200).
Immunohistochemistry
Paraffin blocks were cut into sections 4 µm thick for immunolabeling. Antibodies used for immunohistochemical labeling are summarized in Supplemental Digital Content 1. Reactive small lymphocytes in each case were regarded as internal positive controls for Ki67 and Bcl-2 labeling. Islet cells, stromal cells and endothelial cells in each case were regarded as internal positive controls for Rb, p16, DAXX, ATRX, Pten, Smad4/Dpc4, synaptophysin, chromogranin A, CD56, Pdx1 and PAX8 labeling. For p53 immunolabeling, scattered acinar cells with nuclear labeling were typically present in the adjacent normal tissue. Therefore, p53 immunolabeling was considered to be “abnormal” when either the neoplastic cells showed a virtual absence of immunolabeling compared to adjacent normal tissue (immunolabeling in <5% of neoplastic cells) suggesting the presence of an intragenic deletion, nonsense or frameshift mutation (Fig. 2A) (32) (50) (40), or showed robust nuclear accumulation of immunolabeled protein in the ≥ 30% of the neoplastic cells compared to adjacent normal cells (Fig. 2B)(2). Negative controls for each of the antibodies were performed using nonimmune serum instead of the primary antibody.
Figure 2. Immunohistochemical labeling patterns for DAXX and ATRX.
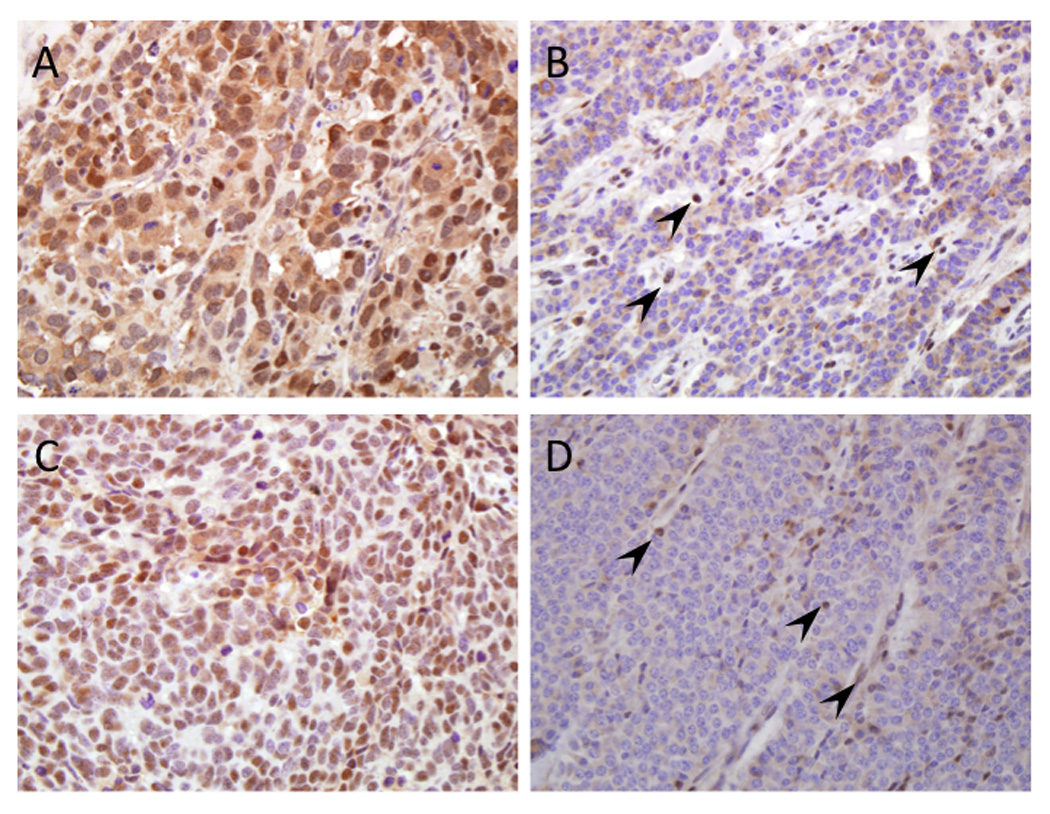
(A) Example of positive nuclear labeling for DAXX in a large cell neuroendocrine carcinoma. (B) Loss of nuclear labeling of DAXX in a well-differentiated neuroendocrine tumor. Endothelial cells and lymphocytes within the stroma are positive. (C) Positive nuclear labeling for ATRX in a small cell neuroendocrine carcinoma. (D) Loss of nuclear labeling of ATRX in a well-differentiated neuroendocrine tumor. Similar to that for DAXX shown in panel B, endothelial cells and lymphocytes within the stroma are positive (original magnifications x200).
Sequencing
Seventeen snap frozen specimens with matched normal tissue corresponding to the Johns Hopkins subset of paraffin embedded neuroendocrine neoplasms used for immunolabeling (4 small cell NECs, 3 large cell NECs, 11 PanNETs) were obtained from the Johns Hopkins Tumor Bank. The frozen tissue samples were embedded in OCT compound (Sakura Finetek, Tokyo, Japan), sectioned by a cyrostat and stained by hematoxylin and eosin. Tumor tissues were dissected macroscopically or microscopically using a PALM MicroLaser System (Carl Zeiss MicroImaging, Oberkochen, Germany). Genomic DNA from dissected tissues was extracted using phenol-chloroform or QIAmp DNA Micro Kit (Qiagen, Valencia, CA). Genomic DNA from microdissected tissues was quantified by calculating long interspersed nuclear elements (LINE) by real-time PCR as described previously (53). Whole genome amplification (WGA) was performed using 10 ng total template gDNA. PCR amplification was performed using 20 ng of gDNA (macrodissected samples) or WGA products (microdissected samples) for KRAS exons 1 and 2, TP53 exons 5–9, CDKN2A/p16 exons 1 and 2, and RB1 exons 1–25 using intragenic primers flanking these exons (see Supplemental Digital Content 2). PCR products were sequenced by use of a M13F primer (5’-GTAAAACGACGGCCAGT-3’) or M13R primer (5’-CAGGAAACAGCTATGACC-3’) that were incorporated into the forward and reverse primer of each primer pair, respectively (Beckman Coulter Genomics, Danvers, MA). Sequencing data were analyzed with sequencher 4.10 software (Gene Codes, Ann Arbor, MI).
Statistics
Frequency distributions were compared by χ2 test, or a Fisher exact test for sample sizes <5. Continuous variables were compared using an unpaired Student’s t-test. P values ≤0.05 were considered statistically significant.
RESULTS
Clinicopathologic Analysis
A total of 19 poorly differentiated NECs were studied, representing nine small cell NECs and 10 large cell NECs. The clinicopathologic features of these carcinomas are shown in Figure 1 and Table 1, compared with those of 11 well-differentiated PanNETs. There was no difference in the mean age, gender distribution, or tumor location within the pancreas among patients with poorly differentiated NEC versus PanNET, nor was there a difference in these characteristics between patients with small cell versus large cell NEC. While there was a trend towards larger mean tumor diameter for PanNETs compared to all poorly differentiated NECs, among the poorly differentiated NECs specifically small cell NECs had a significantly larger mean diameter than large cell NECs (5.4±2.2 versus 4.5±1.3 cm, p=0.04). Poorly differentiated NECs were significantly more likely to have lymph node metastases (p=0.008) and vessel invasion (p=0.005) at diagnosis compared to PanNETs. The median post-operative survival for small cell NECs was 14 months (range 1–25 months), and for large cell NECs was 10 months (range 1–104 months). Information regarding adjuvant treatment was available for 14 of 19 patients with poorly differentiated NEC, indicating that a subset received either etoposide/cisplatin or irinotecan/cisplatin, the standard therapy for patients with small cell lung carcinoma (39, 30).
Immunohistochemical Analyses
The results of immunohistochemical labeling are summarized in Table 2. Poorly differentiated NECs exhibited a high proliferative rate as assessed by Ki67 labeling, with mean labeling index of 51.4%. Among poorly differentiated NEC, small cell NECs showed a significantly higher mean labeling index than large cell NEC (60.4% versus 43.4%, p=0.02). Mitotic counts generally correlated with Ki-67 labeling indices, however there was no difference in the mean mitotic counts among these two groups (40.9±13.6 versus 34.3±19.5).
Table 2.
Immunohistochemical Features of Pancreatic Neuroendocrine Neoplasms
| Marker | Proliferation | Markers of PanNET (ref 20) | Markers of Neuroendocrine Differentiation |
Markers of Cell Cycle Regulation |
PDAC Marker |
Therapeutic Target |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67 (%) | Mitotic Rate |
DAXX | ATRX | Pten | CD56 | Pdx1 | PAX8 | p53 | Rb | p16 | Smad4 | Bcl-2 | |
| Small Cell Neuroendocrine Carcinoma (Small Cell NEC)a | |||||||||||||
| 1 | 58.1 | 50 | + | + | − | + | + | + | + | − | + | + | + |
| 2 | 36.9 | 42 | + | + | + | + | − | − | + | − | + | + | + |
| 3 | 55.9 | 36 | + | + | + | + | − | − | + | − | + | + | + |
| 4 | 57.1 | 22 | + | + | + | + | − | + | + | + | − | + | + |
| 5 | 59.7 | 56 | + | + | + | + | − | + | + | − | + | + | + |
| 6 | 77.6 | 33 | + | + | + | + | NAb | − | + | − | + | + | + |
| 7 | 55.1 | 49 | + | + | + | + | − | − | + | − | + | + | + |
| 8 | 57.4 | 22 | + | + | + | + | − | − | + | − | + | + | + |
| 9 | 85.8 | 58 | + | + | + | + | + | + | + | − | + | + | + |
| Large Cell Neuroendocrine Carcinoma (Large Cell NEC)a | |||||||||||||
| 10 | 55.2 | 53 | + | + | + | + | − | − | + | − | + | + | + |
| 11 | 68.4 | 31 | + | + | + | + | + | + | + | − | + | + | − |
| 12 | 56.1 | 74 | + | + | + | − | − | − | + | − | − | + | + |
| 13 | 67.1 | 48 | + | + | + | − | + | − | + | − | + | + | − |
| 14 | 39.5 | 29 | + | + | + | + | + | + | + | + | − | + | + |
| 15 | 38.7 | 28 | + | + | + | + | − | + | + | − | + | + | + |
| 16 | 37.2 | 25 | + | + | + | + | − | − | + | − | + | + | + |
| 17 | 22.0 | 31 | + | + | + | + | − | + | + | + | − | − | − |
| 18 | 30.1 | 4 | + | + | + | − | − | − | + | + | − | + | − |
| 19 | 20.0 | 20 | + | + | + | + | + | + | − | + | − | + | − |
| Well-differentiated Neuroendocrine Tumor (NET, G2, intermediate-grade) | |||||||||||||
| 20 | 9.2 | 3 | + | + | + | + | NAb | − | − | + | + | + | + |
| 21 | 8.9 | 2 | + | + | + | − | + | + | − | + | + | + | + |
| 22 | 3.3 | 2 | + | − | + | + | + | + | − | + | + | + | − |
| 23 | 5.7 | 1 | + | + | + | − | + | − | − | + | + | + | − |
| 24 | 2.3 | 2 | + | + | + | + | + | − | − | + | + | + | − |
| Well-differentiated Neuroendocrine Tumor (NET, G1, low-grade) | |||||||||||||
| 25 | 0.4 | 0 | + | + | + | + | NAb | + | − | + | + | + | − |
| 26 | 1.3 | 0 | + | − | + | NAb | + | + | − | + | + | + | − |
| 27 | 1.7 | 0 | + | + | + | − | + | + | − | + | + | + | − |
| 28 | 0.8 | 0 | + | −c | + | + | + | + | − | + | + | + | − |
| 29 | 0.8 | 0 | + | + | + | + | + | − | − | + | + | + | − |
| 30 | 0.2 | 1 | −c | −c | + | + | − | − | − | + | + | + | − |
p=0.02, mean Ki67 labeling index of Small Cell NEC versus Large Cell NEC.
NA, not analyzed or technical failure.
Areas of both positive and negative immunolabeling present in the same section (heterogeneity).
The diagnosis of neuroendocrine differentiation was confirmed in these cases by positive immunoreactivity for synaptophysin, chromogranin A and CD56 (data not shown), and there was no significant difference in the frequency or labeling patterns between small cell NEC, large cell NEC and well-differentiated PanNET for these markers. We next performed immunolabeling for DAXX, ATRX and Pten, three of the five genes that undergo somatic alteration in well-differentiated PanNETs (21). In that study, loss of immunolabeling for each protein correlated with an intragenic mutation or deletion of the respective gene (21) (56). All nine small cell NECs and all 10 large cell NECs immunolabeled for nuclear DAXX and ATRX (Figure 2A, 2C), whereas 1 (9.1%) and 4 (36.4%) of the 11 well-differentiated PanNETs had loss of nuclear DAXX and ATRX immunolabeling, respectively (Figure 2B, 2D). Loss of DAXX and ATRX labeling was mutually exclusive, and thus a total of 5 of 11 (45%) of PanNETs showed loss of this cellular pathway similar to that reported by Jiao et al. (21). Pten expression was lost in one of nine (11%) small cell NECs and in none of 10 large cell NECs or 11 well-differentiated PanNETs.
Pdx1 (pancreatic duodenal homeobox 1) is a homeodomain transcription factor with critical regulatory roles in early pancreas development (42) and is used as a pancreatic lineage marker (13, 45). Two of 8 (25%) available sections of small cell NEC and 4 of 10 (40%) large cell NEC immunolabeled for Pdx1, whereas Pdx1 expression was seen in eight of nine (88.9%) well-differentiated PanNETs. PAX (paired box) 8, also a transcription factor recently reported to be frequently expressed in well-differentiated PanNETs (48) (31), was expressed in four of 9 (44%) small cell NEC, five of 10 (50%) large cell NEC, and 6 of 11 (54.5%) well-differentiated PanNETs.
Abnormal immunolabeling for p53 was detected in all nine (100%) small cell NECs and in nine of 10 (90%) large cell NECs (Figure 3A and B), whereas none of 11 well-differentiated PanNETs showed abnormal labeling for p53. Similarly, Rb protein expression was lost in 8 of 9 (88.9%) small cell NECs (Figure 3C) and in six of 10 (60%) large cell NECs. Of interest, all five NEC (one small cell NEC and four large cell NEC) with retention of Rb protein expression showed loss of p16 labeling (Figure 4), indicating Rb and p16 loss is mutually exclusive in poorly differentiated NECs and that virtually all poorly differentiated NECs have disruption of the Rb/p16 cell cycle pathway. By contrast, positive immunolabeling for Rb and p16 was observed in all well-differentiated PanNETs analyzed. Abnormal immunolabeling for p53 and/or Rb/p16 was robustly associated with a mitotic rate and Ki-67 labeling index of >20% (p=0.000001). Finally, we immunolabeled all neuroendocrine neoplasms for Smad4/Dpc4 that serves as a robust marker of SMAD4/DPC4 genetic status and that is lost in 55% of pancreatic ductal adenocarcinomas. Loss of Smad4/Dpc4 labeling was seen in none of nine small cell NEC, one of 10 (10%) large cell NEC and in none of 11 well-differentiated PanNETs, indicating inactivation of the SMAD4/DPC4 gene is a rare event in neuroendocrine neoplasms.
Figure 3. Immunohistochemical labeling for p53, Rb1 and Bcl-2 in small cell neuroendocrine carcinomas.
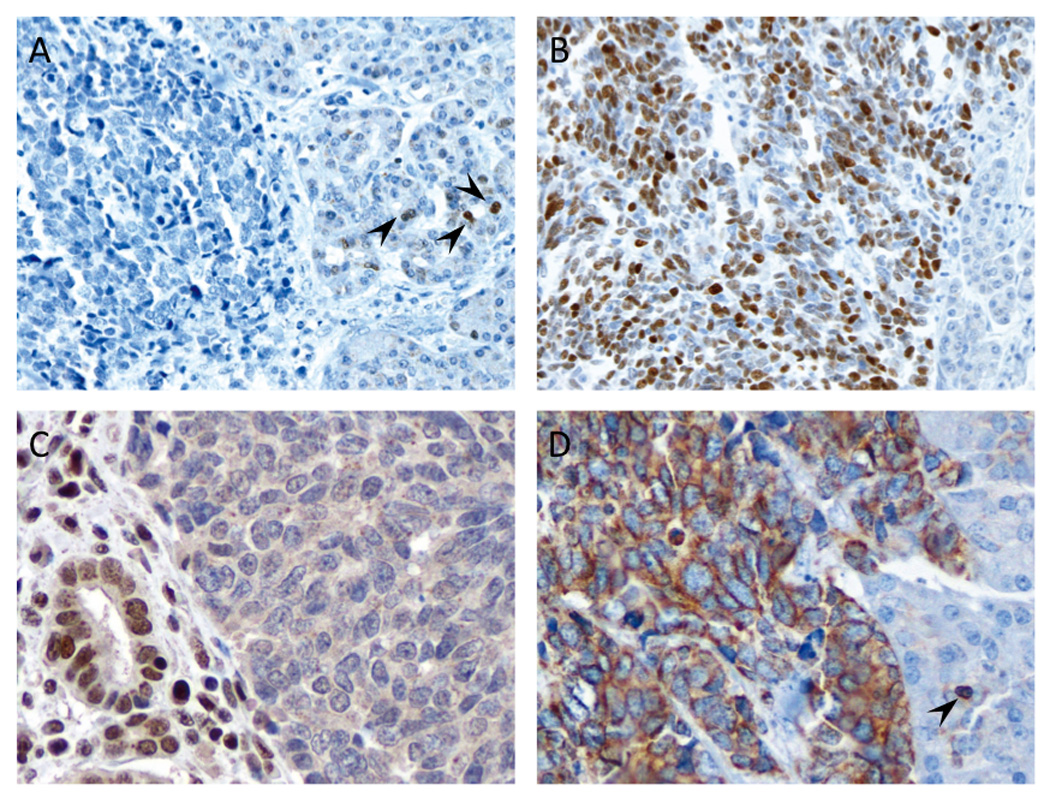
(A) Example of loss of nuclear labeling for p53. Scattered reactive acinar cells with positive nuclear labeling are present in the adjacent normal tissue (arrowheads). Sequencing analysis for TP53 in this patient revealed a nonsense mutation (p.E307X). (B) Example of diffusely positive nuclear labeling for p53. (C) Example of loss of nuclear labeling for retinoblastoma (Rb1) protein. Adjacent nonneoplastic cells show positive nuclear labeling (left side). (D) Diffuse cytoplasmic positivity for Bcl-2 protein. Note the reactive lymphocyte regarded as an internal positive control for this protein (arrowhead) (original magnifications x200).
Figure 4. Immunohistochemical labeling for Rb1 and p16 in a large cell neuroendocrine carcinoma.
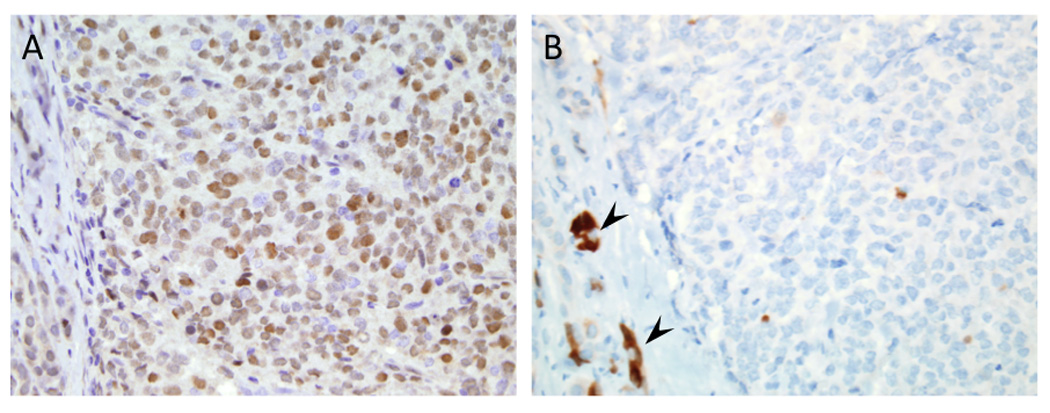
(A) Positive nuclear immunolabeling for Rb1. (B) Loss of p16 labeling in a serial section of the same large cell neuroendocrine carcinoma. Reactive acinar cells (arrowheads) serve as positive internal controls for labeling (original magnifications x200).
Overexpression of Bcl-2 protein was observed in nine of nine (100%) small cell NECs (Fig. 3D), five of 10 (50%) large cell NECs and 2 of 5 (40%) intermediate-grade well-differentiated PanNETs (G2) immunolabeled for Bcl-2. Of note, these two intermediate-grade well-differentiated PanNETs (G2) showing overexpression of Bcl-2 protein were originally diagnosed as large cell NEC of the pancreas based on morphologic features despite their relatively lower Ki67 labeling index and mitotic rates (Table 2). By contrast, none of six low-grade well-differentiated PanNETs (G1) immunolabeled for Bcl-2. Among all neoplasms studied, positive labeling for Bcl-2 was significantly associated with both a higher mitotic rate (p=0.0002) and Ki-67 labeling index (p=0.0001), and this relationship persisted when analyzed specifically among poor differentiated NEC and well differentiated PanNETs (Table 3).
Table 3.
Relationship of Bcl-2 Overexpression to Proliferation
| Bcl-2 Overexpression | |||
|---|---|---|---|
| No (n=14) | Yes (n=16) | P Value | |
| All Tumors (n=30) | |||
| Mitotic Rate | 10.0±15.8 | 36.4±19.8 | 0.0002 |
| Ki-67 Labeling Index | 16.0±23.9 | 49.3±20.6 | 0.0001 |
| No (n=5) | Yes (n=11) | ||
| Poorly Differentiated NEC (n=19) | |||
| Mitotic Rate | 26.8±16.2 | 41.2±15.9 | 0.05 |
| Ki-67 Labeling Index | 41.5±24.2 | 48.3±14.3 | 0.07 |
| No (n=9) | Yes (n=2) | ||
| Well Differentiated PanNET (n=11) | |||
| Mitotic Rate | 0.7±0.9 | 2.5±0.7 | 0.01 |
| Ki-67 Labeling Index | 1.8±1.7 | 9.1±0.2 | 0.0002 |
Genetic Analysis
To determine if genetic alterations of the TP53, RB1 or CDKN2A/p16 genes underlie the observed immunolabeling patterns seen in poorly differentiated NECs, we Sanger sequenced the TP53, RB1 and CDKN2A/p16 genes in snap frozen samples of four small cell NECs, three large cell NECs and 11 well-differentiated PanNETs (G2, 4 cases and G1, 7 cases). Activating mutations in KRAS, present in virtually all pancreatic ductal adenocarcinomas, were also assessed.
The results of sequencing efforts are summarized in Table 4. An activating KRAS gene mutation was identified in two of seven poorly differentiated NECs, corresponding to one of four small cell NEC and one of three large cell NEC. One of these KRAS mutations was a rare homozygous dinucleotide point mutation identified at codon 12 (Glycine to Leucine, GGT to CTT) (11). Inactivating mutations in the TP53 gene were identified in four of seven poorly differentiated NEC, corresponding to two small cell NECs and two large cell NECs. Two of these mutations were nonsense mutations (p.E307X and p.R196X) predicted to result in loss of expression, and by immunolabeling both of these carcinomas showed a complete absence of p53 labeling. By contrast, the remaining two mutations were missense mutations (p.S127T and p.V216L) (Figure 5) and by immunolabeling both of these carcinomas showed a robust nuclear accumulation of p53 protein. None of the 11 well-differentiated PanNETs harbored a mutation in TP53 consistent with both normal immunolabeling patterns and exomic sequencing of these lesions (21). Inactivating mutations in the RB1 gene were identified in five poorly differentiated NECs corresponding to three of four (75%) small cell NECs and two of three (66.7%) large cell NECs; of these, two were splice site mutations (c.861-2T>A and c.1219-2A>G) (Figure 5) and three were frameshift mutations (c.803_804del, c.868_869insA and c.2237_2251del). All five NECs with a RB1 mutation showed complete absence of immunolabeling. Again, none of the 11 well-differentiated PanNETs of the pancreas showed a mutation in RB1 consistent with immunolabeling data and previous exomic sequencing (21). No CDKN2A/p16 mutations were observed in the four small cell NECs or three large cell NECs, nor were they seen in 11 well-differentiated PanNETs.
Table 4.
Genetic features of pancreatic neuroendocrine neoplasms
| Case no. in Tables 1 and 2 |
KRAS sequencing |
TP53 | RB1 | CDKN2A/p16 | |||
|---|---|---|---|---|---|---|---|
| TP53 sequencing | p53 IHC | RB1 sequencing | Rb IHC |
CDKN2A/p16 sequencing |
p16 IHC | ||
| Small cell neuroendocrine carcinoma (Small cell NEC) | |||||||
| 2 | WT | Nonsense Mutation (p.E307X) | abnormal | Frameshift Mutation (c.803_804del) | negative | WT | positive |
| 3 | WT | WT | abnormal | Splice Site Mutation (c.861-2T>A) | negative | WT | positive |
| 4 | WT | WT | abnormal | WT | negative | WT | negative |
| 5 | Missense Mutation (p.G12L) | Missense Mutation (p.S127T) | abnormal | Splice Site Mutation (c.1219-2A>G) | negative | WT | positive |
| Large cell neuroendocrine carcinoma (Large cell NEC) | |||||||
| 10 | Missense Mutation (p.G12D) | Nonsense Mutation (p.R196X) | abnormal | Frameshift Mutation (c.2237_2251del) | negative | WT | positive |
| 11 | WT | Missense Mutation (p.V216L) | abnormal | Frameshift Mutation (c.868_869insA) | negative | WT | positive |
| 12 | WT | WT | abnormal | WT | negative | WT | negative |
| Well-differentiated neuroendocrine tumor (NET, G2, intermediate-grade) | |||||||
| 20 | WT | WT | normal | WT | positive | WT | positive |
| 21 | WT | WT | normal | WT | positive | WT | positive |
| 22 | WT | WT | normal | WT | positive | WT | positive |
| 23 | WT | WT | normal | WT | positive | WT | positive |
| 24 | WT | WT | normal | WT | positive | WT | positive |
| Well-differentiated neuroendocrine tumor (NET, G1, low-grade) | |||||||
| 25 | WT | WT | normal | WT | positive | WT | positive |
| 26 | WT | WT | normal | WT | positive | WT | positive |
| 27 | WT | WT | normal | WT | positive | WT | positive |
| 28 | WT | WT | normal | WT | positive | WT | positive |
| 29 | WT | WT | normal | WT | positive | WT | positive |
| 30 | WT | WT | normal | WT | positive | WT | positive |
IHC, immunohistochemistry; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor; WT, wild-type gene.
Figure 5. Sequencing analysis for TP53 and RB1 in small cell neuroendocrine carcinoma.
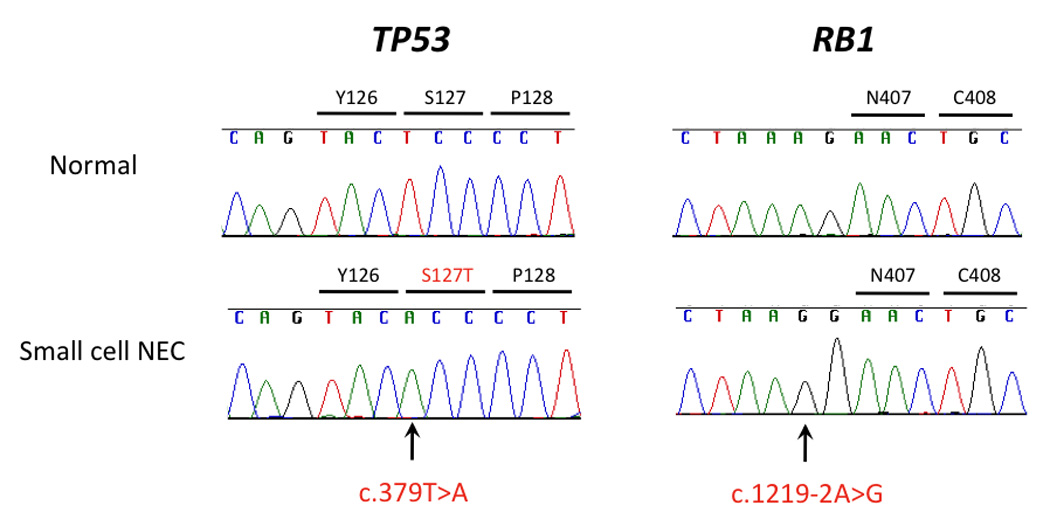
This carcinoma (Case #5) contained a homozygous missense mutation (c.379T>A, p.S127T) in exon 5 of TP53, as well as a homozygous mutation of the intron 12 acceptor splice site (c.1219-2A>G) of RB1.
DISCUSSION
Poorly differentiated NECs are uncommon neoplasms; among these small cell NEC of the pancreas is extremely rare. We therefore examined the clinicopathologic, immunohistochemical and genetic features of a series of small cell and large cell NEC and compared them to that of well-differentiated PanNETs. Consistent with previous reports, poorly differentiated NEC most often occurred in the head of pancreas (47). Moreover, the poorly differentiated NECs in the current study had aggressive biological behaviors, featuring vascular invasion (94%), perineural invasion (69%) and lymph node metastases (88%). However, 11 of 18 (61%) patients with available information survived over 12 months (median survival 13 months), conceivably because this study focused on a group of highly selected patients with limited disease at diagnosis and who were able to undergo surgical resection. By contrast, most cases reported in the literature were diagnosed at an advanced stage of disease and their overall survival varied between 1–12 months (25, 7).
Our data suggests that small cell and large cell NEC are genetically related entities. Abnormal immunolabeling for p53 and the Rb/p16 pathways was detected in virtually all small cell and large cell NECs, but not in any PanNETs, indicating that co-inactivation of these two pathways is a fundamental genetic feature of poorly differentiated NEC. Moreover, loss of Rb protein was mutually exclusive with loss of p16 protein in keeping with the close relationship of these protein products in cell cycle regulation (41). Sequencing analyses of poorly differentiated NECs indicated that intragenic mutation is the major mechanism underlying loss of p53 and Rb protein expression. By contrast, genetic inactivation of RB1 is reported to be an uncommon event in both PDAC and PanNETs (3) (14) (8). Related to this finding, inactivating mutations in DAXX and ATRX were exclusively found in PanNET but not small cell or large cell NECs. While this finding may support the notion that NEC is a genetically distinct entity from well-differentiated PanNET, it does not fully rule out the possibility that some well-differentiated PanNETs may transform genetically into poorly differentiated NECs (17). This hypothesis is only indirectly supported by their shared expression of generic neuroendocrine cell markers, and by the presence in both tumor cell types of small dense-core granules of neuroendocrine caliber. However, Ordonez et al. reported that untrastructual features may serve to separate NECs of the pancreas from well-differentiated PanNET in which the granules are distinctly larger and are distributed throughout the cytoplasm while dendritic processes are not usually seen (43). Moreover, Jiao et al. reported that patients with well-differentiated PanNETs that did not have DAXX/ATRX mutations survived significantly shorter than those patients with these mutations, suggesting that PanNETs without these mutations might identify a biologically specific subgroup with the potential to progress to neuroendocrine carcinoma (21).
In addition to the nature of poorly differentiated NECs and their potential relationship to well-differentiated PanNET, there has also been much speculation on the relationship of poorly differentiated NECs to PDAC (17). One of the genetic changes frequently seen in ductal adenocarcinomas, loss of SMAD4/DPC4, was infrequently observed in poorly differentiated NECs in this study (1 of 19, 5%). KRAS mutations, an almost uniform feature of PDAC (16), was observed in only two of seven poorly differentiated NECs (28%), suggesting that most poorly differentiated NECs do not arise from preexisting ductal lesions. Again, this does not entirely rule out the possibility that some PDACs may have give rise to coexistent poorly differentiated NECs (35) (17), although this may be a relatively uncommon occurrence.
Of interest, the loss of Rb protein is frequently observed in other poorly differentiated NECs of the gallbladder (46) and small cell carcinomas of the ampulla of Vater (38). These results suggest that inactivation of Rb protein is a characteristic molecular event in high-grade neuroendocrine carcinomas in the gastrointestinal tract. Interestingly, in mouse models of human small cell lung carcinoma, Cre-mediated deletion of RB1 and TP53 conditional alleles in the lungs of adult mice results in the development of tumors that share many characteristics with human small cell lung carcinoma, including their histopathology, the expression of neuroendocrine markers, and their ability to metastasize (33). Another experimental study demonstrated that conditional deletion of RB1 alone in mouse airway cells induced the proliferation of neuroendocrine cells (52). Thus, combined loss of p53 and Rb function appears to be a necessary step for the development of this tumor and that alterations of the RB1 gene may play an important role in terms of manifestation of neuroendocrine features (6) (24) (26).
Interestingly, overexpression of Bcl-2 protein was observed in all of nine small cell NECs and in 50% of large cell NECs, but in only 18% of well differentiated PanNETS in the current study. Moreover, our data indicates that Bcl-2 overexpression may account for the relatively higher proliferation rate seen in the small cell NEC compared to the large cell NEC, and in some G2 well-differentiated PanNETs compared to G1 tumors. Bcl-2 is a central apoptotic inhibitor and overexpression of Bcl-2 protein is a characteristic finding in small cell lung carcinomas (20). Chemotherapeutic agents, including platinum, have been shown to exert their cytotoxic effects by inducing apoptosis via the mitochondrial (or intrinsic) pathway regulated by Bcl-2 (23). Bcl-2 overexpression increases resistance to chemotherapy in both in vitro and in vivo models (12) (49). Furthermore, high levels of Bcl-2 protein have been associated with neuroendocrine differentiation (20) (1) and a more aggressive malignant phenotype (22). It has been of great interest to assess the potential therapeutic synergy between Bcl-2 antagonists/inhibitors and standard chemotherapies, and several Bcl-2 antagonists/inhibitors, including obatoclax mesylate (GX15-070), are currently entering clinical trial in small cell lung carcinomas (44). Thus, the finding of Bcl-2 protein overexpression in poorly differentiated NECs of the pancreas, irrespective of small or large cell morphology, suggests that Bcl-2 antagonists/inhibitors might be a viable treatment option to test for these patients.
The molecular abnormalities and median survival of small cell NEC, large cell NEC, well-differentiated PanNET, PDAC and small cell lung carcinoma are summarized in Table 5, based on the current study and previously published reports that include our own work (55) (9) (4) (29) (15) (37) (34) (51) (10, 18). There is no consensus on the treatment of poorly differentiated NECs, specifically small cell NECs, of the pancreas at this time (19). In our series, six of eight (75%) patients with small cell NEC and five of ten (50%) with large cell NEC and available information survived at least 12 months, suggesting a potential role for surgery for limited disease. Surgery alone, however, is unlikely to be sufficient for the treatment of poorly differentiated NEC because most patients in our series eventually died of recurrence or metastasis. Prospective randomized trials of therapy for this disease are unlikely in the future because of the rarity of the disease. Systemic therapies, including pre- or postoperative chemotherapy with platinum agents, may be promising for the improvement of the prognosis in patients with poorly differentiated NEC of the pancreas, even if the size of the primary cancer is small and the disease is limited. In support of this notion, among the nine patients with poorly differentiated NEC in the current study with known treatment history, five received a cisplatin-based regimen as first or second line treatment, and the median survival of these patients was 15 months. By contrast, the four patients who received alternative treatments had a median survival of 6 months. Thus, as the genetic alterations of small cell and large cell NEC of the pancreas are highly similar to those of small cell lung carcinoma, it may be reasonable to manage poorly differentiated NECs of the pancreas along broadly similar lines to other carcinomas with small cell morphology, as reported in high-grade neuroendocrine carcinomas of other organs (5).
Table 5.
Molecular abnormalities and median survival in small cell neuroendocrine carcinoma (small cell NEC), large cell neuroendocrine carcinoma (large cell NEC), well-differentiated neuroendocrine tumor (NET) of the pancreas, pancreatic ductal adenocarcinoma (PDAC) and small cell lung carcinoma.
| Characteristic | Small cell NEC (present study) |
Large cell NEC (present study) |
Well- differentiated NET |
PDAC | Small cell lung carcinoma |
|---|---|---|---|---|---|
| KRAS | 25% | 33% | 0% (present study) |
> 90% | 0–10% |
| p16 | 11% | 50% | 0% (present study) |
80–95% | 0–10% |
| p53 | 100% | 90% | 0% (present study) |
75% | 80–90% |
| Smad4/Dpc4 | 0% | 10% | 0% (present study) |
55% | 0% |
| Rb | 89% | 60% | 0% (present study) |
13% (present study) |
80–100% |
| Bcl-2 | 100% | 50% | 18% (present study) |
20% | 75–95% |
| Median survival | 14 months | 10 months | 99 months | 6 months | 9–12 months |
NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor; PDAC, pancreatic ductal adenocarcinoma.
In conclusion, poorly differentiated NECs, represented by small cell and large cell NECs, are highly aggressive neoplasms with a dismal prognosis. We now show that small cell and large cell NECs are genetically similar to each other, and distinct from well-differentiated PanNET. Management of small and large cell NECs of the pancreas may benefit from therapeutic approaches broadly similar to that of small cell carcinomas arising in other organs.
Supplementary Material
Acknowledgments
Financial Support: Supported by National Institutes of Health grants CA140599 and P50 CA62924, The Uehara Memorial Foundation, The George Rubis Endowment for Pancreatic Cancer Research, The Michael Rolfe Pancreatic Cancer Foundation, Sigma Beta Sorority, The Joseph C. Monastra Foundation, The Alfredo Scatena Memorial, and The Patty Boshell Pancreas Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflicts of interest related to this work.
REFERENCES
- 1.Atasoy P, Bozdogan O, Ozturk S, et al. Bcl2 expression and its correlation with neuroendocrine differentiation in colon carcinomas. Tumori. 2004;90:233–238. doi: 10.1177/030089160409000213. [DOI] [PubMed] [Google Scholar]
- 2.Baas IO, Mulder JW, Offerhaus GJ, et al. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. The Journal of pathology. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 3.Barton CM, McKie AB, Hogg A, et al. Abnormalities of the RB1 and DCC tumor suppressor genes: uncommon in human pancreatic adenocarcinoma. Molecular carcinogenesis. 1995;13:61–69. doi: 10.1002/mc.2940130202. [DOI] [PubMed] [Google Scholar]
- 4.Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 5.Brennan SM, Gregory DL, Stillie A, et al. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116:888–895. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]
- 6.Cagle PT, el-Naggar AK, Xu HJ, et al. Differential retinoblastoma protein expression in neuroendocrine tumors of the lung. Potential diagnostic implications. Am J Pathol. 1997;150:393–400. [PMC free article] [PubMed] [Google Scholar]
- 7.Chung MS, Ha TK, Lee KG, et al. A case of long survival in poorly differentiated small cell carcinoma of the pancreas. World J Gastroenterol. 2008;14:4964–4967. doi: 10.3748/wjg.14.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delle Fave G, Corleto VD. Oncogenes, growth factors, receptor expression and proliferation markers in digestive neuroendocrine tumours. A critical reappraisal. Ann Oncol. 2001;12 Suppl 2:S13–S17. [PubMed] [Google Scholar]
- 9.Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–7803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 10.El Maalouf G, Rodier JM, Faivre S, et al. Could we expect to improve survival in small cell lung cancer? Lung Cancer. 2007;57 Suppl 2:S30–S34. doi: 10.1016/S0169-5002(07)70425-7. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein SD, Przygodzki R, Pricolo VE, et al. K-ras-2 topographic genotyping of pancreatic adenocarcinoma. Arch Surg. 1994;129:367–372. doi: 10.1001/archsurg.1994.01420280037005. discussion 372-363. [DOI] [PubMed] [Google Scholar]
- 12.Fisher TC, Milner AE, Gregory CD, et al. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993;53:3321–3326. [PubMed] [Google Scholar]
- 13.Gao N, LeLay J, Vatamaniuk MZ, et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes B, Ramaswamy A, Ziegler A, et al. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51–59. doi: 10.1097/00000658-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 16.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 17.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 18.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40:313–318. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- 20.Jiang SX, Kameya T, Sato Y, et al. Bcl-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol. 1996;148:837–846. [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann SH, Gores GJ. Apoptosis in cancer: cause and cure. Bioessays. 2000;22:1007–1017. doi: 10.1002/1521-1878(200011)22:11<1007::AID-BIES7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaye FJ. RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene. 2002;21:6908–6914. doi: 10.1038/sj.onc.1205834. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita K, Minami T, Ohmori Y, et al. Curative resection of a small cell carcinoma of the pancreas: report of a case of long survival without chemotherapy. J Gastroenterol Hepatol. 2004;19:1087–1091. doi: 10.1111/j.1440-1746.2004.02910.x. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura H, Yazawa T, Sato H, et al. Small cell lung cancer: significance of RB alterations and TTF-1 expression in its carcinogenesis, phenotype, and biology. Endocr Pathol. 2009;20:101–107. doi: 10.1007/s12022-009-9072-4. [DOI] [PubMed] [Google Scholar]
- 27.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 28.Kloppel G, Rindi G, Perren A, et al. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456:595–597. doi: 10.1007/s00428-010-0924-6. [DOI] [PubMed] [Google Scholar]
- 29.Kouvaraki MA, Solorzano CC, Shapiro SE, et al. Surgical treatment of non-functioning pancreatic islet cell tumors. J Surg Oncol. 2005;89:170–185. doi: 10.1002/jso.20178. [DOI] [PubMed] [Google Scholar]
- 30.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long KB, Srivastava A, Hirsch MS, et al. PAX8 Expression in well-differentiated pancreatic endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol. 2010;34:723–729. doi: 10.1097/PAS.0b013e3181da0a20. [DOI] [PubMed] [Google Scholar]
- 32.Melhem MF, Law JC, el-Ashmawy L, et al. Assessment of sensitivity and specificity of immunohistochemical staining of p53 in lung and head and neck cancers. Am J Pathol. 1995;146:1170–1177. [PMC free article] [PubMed] [Google Scholar]
- 33.Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 34.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 35.Motojima K, Furui J, Terada M, et al. Small cell carcinoma of the pancreas and biliary tract. J Surg Oncol. 1990;45:164–168. doi: 10.1002/jso.2930450306. [DOI] [PubMed] [Google Scholar]
- 36.Movahedi-Lankarani S, Hruban RH, Westra WH, et al. Primitive neuroectodermal tumors of the pancreas: a report of seven cases of a rare neoplasm. Am J Surg Pathol. 2002;26:1040–1047. doi: 10.1097/00000478-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Nagatake M, Takagi Y, Osada H, et al. Somatic in vivo alterations of the DPC4 gene at 18q21 in human lung cancers. Cancer Res. 1996;56:2718–2720. [PubMed] [Google Scholar]
- 38.Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005;29:588–594. doi: 10.1097/01.pas.0000157974.05397.4f. [DOI] [PubMed] [Google Scholar]
- 39.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 40.Obata A, Eura M, Sasaki J, et al. Clinical significance of p53 functional loss in squamous cell carcinoma of the oropharynx. Int J Cancer. 2000;89:187–193. doi: 10.1002/(sici)1097-0215(20000320)89:2<187::aid-ijc14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Ohtani N, Yamakoshi K, Takahashi A, et al. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- 42.Oliver-Krasinski JM, Kasner MT, Yang J, et al. The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest. 2009;119:1888–1898. doi: 10.1172/JCI37028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordonez NG, Cleary KR, Mackay B. Small cell undifferentiated carcinoma of the pancreas. Ultrastruct Pathol. 1997;21:467–474. doi: 10.3109/01913129709021947. [DOI] [PubMed] [Google Scholar]
- 44.Paik PK, Rudin CM, Brown A, et al. A phase I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in solid tumor malignancies. Cancer Chemother Pharmacol. 2010;66:1079–1085. doi: 10.1007/s00280-010-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JY, Hong SM, Klimstra DS, et al. Pdx1 Expression in Pancreatic Precursor Lesions and Neoplasms. Appl Immunohistochem Mol Morphol. 2011 doi: 10.1097/PAI.0b013e318206d958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parwani AV, Geradts J, Caspers E, et al. Immunohistochemical and genetic analysis of non-small cell and small cell gallbladder carcinoma and their precursor lesions. Mod Pathol. 2003;16:299–308. doi: 10.1097/01.MP.0000062656.60581.AA. [DOI] [PubMed] [Google Scholar]
- 47.Reyes CV, Wang T. Undifferentiated small cell carcinoma of the pancreas: a report of five cases. Cancer. 1981;47:2500–2502. doi: 10.1002/1097-0142(19810515)47:10<2500::aid-cncr2820471032>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 48.Sangoi AR, Ohgami RS, Pai RK, et al. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol. 2011;24:412–424. doi: 10.1038/modpathol.2010.176. [DOI] [PubMed] [Google Scholar]
- 49.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 50.Sjogren S, Inganas M, Norberg T, et al. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J Natl Cancer Inst. 1996;88:173–182. doi: 10.1093/jnci/88.3-4.173. [DOI] [PubMed] [Google Scholar]
- 51.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC press; 2004. [Google Scholar]
- 52.Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development. 2004;131:4299–4310. doi: 10.1242/dev.01232. [DOI] [PubMed] [Google Scholar]
- 53.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yachida S, Zhong Y, Patrascu R, et al. Establishment and characterization of a new cell Line, A99, from a primary small cell carcinoma of the pancreas. Pancreas. 2011 doi: 10.1097/MPA.0b013e3182207a58. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 56.Zhou XP, Loukola A, Salovaara R, et al. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439–447. doi: 10.1016/S0002-9440(10)64200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


