Abstract
High over-expression of somatostatin receptors in neuroendocrine tumors allows imaging and radiotherapy with radiolabelled somatostatin analogues. To know if a tumor is suitable for in vivo somatostatin receptor targeting, its somatostatin receptor expression has to be determined. There are specific indications to use immunohistochemistry for the somatostatin receptor subtype 2A (sst2A), but this has up to now been limited by the lack of an adequate reliable antibody. The aim of the present study was to correlate immunohistochemistry using the new monoclonal anti-sst2A antibody UMB-1 with the gold standard in vitro method quantifying somatostatin receptor levels in tumor tissues. A UMB-1 immunohistochemistry protocol was developed, and tumoral UMB-1 staining levels were compared with somatostatin receptor binding site levels quantified with in vitro 125I-[Tyr3]-octreotide autoradiography in 89 tumors. This allowed defining an immunohistochemical staining threshold permitting to distinguish tumors with somatostatin receptor levels high enough for clinical applications from those with low receptor expression. The presence of more than 10% positive tumor cells correctly predicted high receptor levels in 95% of cases. Conversely, no UMB-1 staining at all truly reflected low or no somatostatin receptor expression in 96% of tumors. If 1–10% of tumor cells were stained, a weak staining intensity was suggestive of low somatostatin receptor levels. This study allows for the first time a reliable recommendation concerning eligibility of an individual patient for in vivo somatostatin receptor targeting based on somatostatin receptor immunohistochemistry. Under optimal methodological conditions, UMB-1 immunohistochemistry may be equivalent to in vitro receptor autoradiography.
Keywords: Somatostatin receptors, monoclonal anti-sst2A antibody, neuroendocrine tumors, immunohistochemistry, somatostatin receptor autoradiography
Introduction
Somatostatin receptors represent molecular tumor targets of increasing clinical importance (17). They are highly expressed particularly in neuroendocrine tumors of the gastroenteropancreatic tract. This allows radiologic visualization of these tumors with high specificity and sensitivity with somatostatin analogues labelled with 111In, such as OctreoScan®, or 68Ga used for 68Ga-DOTATOC PET/CT (5, 10, 12). This represents an important tool for patient staging and follow-up. Moreover, the same tumors can be subjected to peptide receptor radionuclide therapy (PRRT) with somatostatin analogues coupled with toxic nuclides like 90Y or 177Lu in specialized centers (11). Although often several of the five somatostatin receptor subtypes sst1, sst2A, sst3, sst4 and sst5 are concomitantly present in neuroendocrine tumors, sst2A is most important as it shows the highest expression (26). Correspondingly, the somatostatin analogues applied in clinical practice display highest affinity for this subtype (22).
An important prerequisite for a successful in vivo somatostatin receptor targeting for imaging or therapeutic purposes is a high tumoral somatostatin receptor expression. Therefore, the somatostatin receptor levels in an individual patient’s tumor have to be determined in order to decide if he or she is eligible for these applications. This can be achieved with either in vivo or in vitro methods. Tumoral somatostatin receptors can be measured directly in vivo by performing a preoperative OctreoScan® or 68GA-DOTATOC PET/CT. The advantage of this approach is that the entire tumor mass is evaluated and that the necessary radiotherapeutic tracer dose can be calculated. A preoperative scan is, however, not performed when it is not easily available or the diagnosis of a neuroendocrine tumor is not suspected. Then the somatostatin receptor expression has to be measured in vitro in the resected tumor tissue. The gold standard method to do this is in vitro somatostatin receptor autoradiography. It represents the in vitro correlate of an in vivo scan, as receptor binding sites are assessed with the same somatostatin analogues used in vivo (10). Moreover, it is highly sensitive and specific, and receptor levels can be quantified. Limitations of this method include the restricted availability in highly specialized laboratories and the dependence on frozen tissue which is often not collected. An alternative is immunohistochemistry which is widely available, fast and cheap, and can be performed on formalin-fixed, paraffin-embedded (FFPE) tissue, even retrospectively on archival material.
Presently, only the somatostatin receptor subtype sst2A is assessed with immunohistochemistry as it shows by far the highest expression in tumors. However, the value of sst2A immunohistochemistry has been limited. Until recently, two acceptable anti-sst2A antibodies existed, namely the excellent and well characterized, but non-commercial R2-88 antibody (A Schonbrunn, Houston, Tx) (18), and the commercially available SS-800 antibody (27) (Gramsch Laboratories, Schwabhausen, Germany). Although these antibodies yield satisfactory sst2A staining (9), they are afflicted with several disadvantages. First, they are polyclonal antibodies. They therefore display heterogeneity from batch to batch the excellent and extensively used R2-88 originates from an animal that died almost two decades ago; while it has been possible during more than ten years to successfully detect sst2A receptors with R2-88 in a variety of normal and tumor tissues (8, 9, 18, 19, 24, 25), the quality of R2-88 immunohistochemistry for human tissue was found to strongly decline in recent time (Waser, Körner, Schonbrunn and Reubi, unpublished data). Second, both antibodies show cross-reactivity with other antigens (21). In summary, it has been difficult to establish a useful quantification scheme for staining results for these two antibodies that would allow reliable prediction of tumoral somatostatin receptor levels.
Recently, a monoclonal anti-sst2A antibody, UMB-1, became commercially available (Biotrend Chemikalien GmbH, Köln, Germany) which exhibits a considerably more effective and cleaner staining than polyclonal antisera (4). The aim of the present study was to test if immunohistochemistry with this antibody may allow quantification of somatostatin receptor levels in tumor tissues and therefore be used as reliable tool in routine diagnostics to evaluate tumoral sst2A expression. In particular, it was sought to develop a sensitive immunohistochemistry protocol and to define a simple and easily applicable evaluation scheme for UMB-1 staining results that permits the selection of tumors with somatostatin receptor levels high enough for in vivo somatostatin receptor targeting. For this purpose, UMB-1 staining was compared with somatostatin receptor levels quantified with in vitro receptor autoradiography as reference method in 89 tumors. A retrospective study design was chosen due to the infrequency of the tumors of interest.
Materials and Methods
Tumors
Eighty-nine tumors from 84 patients were studied. They are listed in table 1. They comprise tumor types with generally high somatostatin receptor levels which are subjected to in vivo somatostatin receptor targeting in routine clinical practice or in clinical trials. Informed consent was available for all patients. The study conformed to the ethical guidelines of the Medical Faculty of the University of Berne and was reviewed by the Review Board of the Institute of Pathology.
Table 1.
Tumors tested with sst2A immunohistochemistry and in vitro 125I-[Tyr3]-octreotide autoradiography
| Gastrointestinal neuroendocrine tumors* | |
| Pancreas | 2 non-functioning neuroendocrine tumors |
| 17 non-functioning well differentiated neuroendocrine carcinomas | |
| 11 insulin-producing neuroendocrine tumors | |
| 1 insulin-producing well differentiated neuroendocrine carcinoma | |
| 1 glucagon-producing well differentiated neuroendocrine carcinoma | |
| 1 serotonin-producing neuroendocrine tumor | |
| 1 somatostatin-producing well differentiated neuroendocrine carcinoma | |
| Extrahepatic bile duct | 1 well differentiated neuroendocrine carcinoma |
| Gall bladder | 1 well differentiated neuroendocrine carcinoma |
| Liver | 1 well differentiated neuroendocrine carcinoma |
| Stomach | 1 well differentiated neuroendocrine carcinoma |
| Ileum | 10 well differentiated neuroendocrine carcinomas |
| Vermiform appendix | 1 well differentiated neuroendocrine carcinoma |
| Sigmoid colon | 1 well differentiated neuroendocrine carcinoma |
| Extra-gastrointestinal neuroendocrine tumors | |
| Adrenal gland | 5 pheochromocytomas |
| 1 ganglioneuroblastoma | |
| Extraadrenal paraganglia | 2 retroperitoneal paragangliomas |
| 1 mediastinal ganglioneuroblastoma | |
| Mesentery | 1 well differentiated neuroendocrine carcinoma |
| Lung | 10 typical carcinoid tumors |
| Thymus | 1 carcinoid tumor |
| Non-neuroendocrine tumors | 4 meningiomas |
| 14 breast carcinomas | |
Classification of retrospectively analyzed neuroendocrine tumors according to WHO 2004
All tumor samples were obtained from surgical resection specimens. In each case, an FFPE tissue block was available for sst2A immunohistochemistry and a frozen sample stored at −80°C for in vitro somatostatin receptor autoradiography. From the FFPE material, a tissue micro-array (TMA) was additionally constructed which was used to establish the UMB-1 immunohistochemistry protocol.
Sst2A immunohistochemistry
Sst2A immunohistochemistry was performed on 4 µm thick sections of FFPE tissue blocks with two different primary antibodies, namely the new monoclonal antibody UMB-1 (Biotrend) and the original non-commercial polyclonal antibody R2-88 (Schonbrunn, Houston, TX) (3, 7). For UMB-1 immunohistochemistry, different conditions were tested on the TMA, including a range of antibody concentrations (1:500 - 1:20) and various antigen retrieval methods (boiling in the pressure cooker in 10 mM citrate buffer, pH 6.0, for 5 or 10 minutes, cooking in the microwave in 5% urea buffer, pH 9.0, or 10 mM citrate buffer, pH 6.0, for 18 minutes and digestion with 0.1% trypsin or 0.1% pronase). R2-88 immunohistochemistry was carried out as described before with pre-treatment in the microwave in 5% urea buffer, pH 9.0, and a concentration of the primary antibody of 1:1000 (9). The tissue sections were incubated with the primary antibodies over-night at room temperature. As secondary antibody, a biotinylated goat anti-rabbit immunoglobulin was used. Antibody binding was visualized with the ABComplex/HRP (DAKO, Zug, Switzerland). Staining was performed with DAB and counterstaining with hemalum. In each experiment, a well-characterized gastroenteropancreatic neuroendocrine tumor was included as positive control. In every case, antibody preabsorption with 100 nM of the immunogen peptide was performed as negative control for both R2-88 and UMB-1 immunohistochemistry.
Analysis of immunohistochemical staining
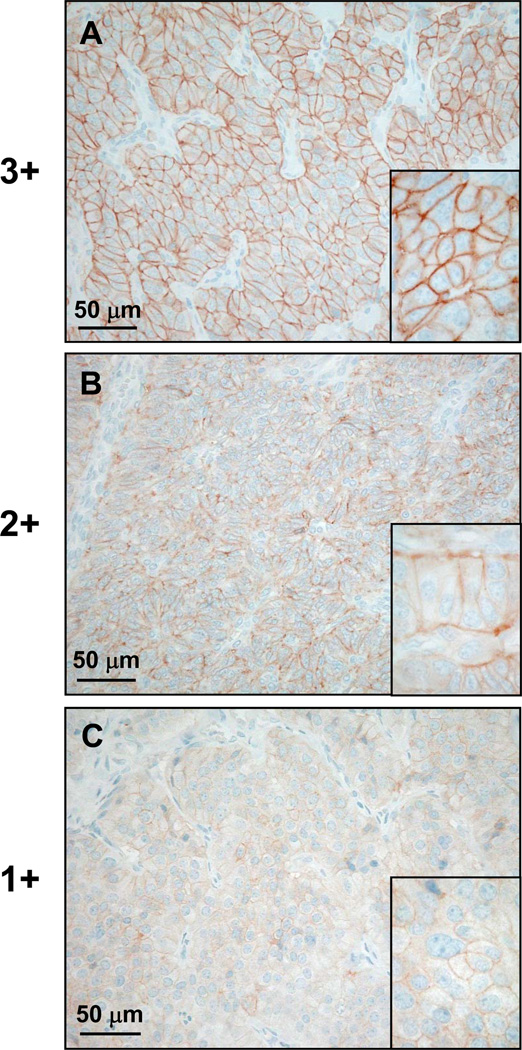
The immunohistochemical staining results were semi-quantitatively assessed. Only membranous staining was considered. The proportion of stained tumor cells was expressed in percentages with increments of 10 (i.e. 0%, 1–10%, 11–20%, … of tumor cells stained). The staining intensity was analysed using the following scoring system and as depicted in figure 1: 1+ = faint staining at 100× magnification; 2+ = strong staining at 100× magnification, not entire circumference of tumor cell membranes stained at 400× magnification; 3+ = strong staining at 100× magnification, entire circumference of tumor cell membranes stained at 400× magnification (adapted from (13, 28)).
Figure 1.
Scoring of immunohistochemical staining intensity in typical examples (A: ileal neuroendocrine carcinoma, B: neuroendocrine carcinoma of the gall bladder, C: pulmonary carcinoid tumor). A: 3+ staining intensity represents strong staining at low magnification and fully circumferential staining of tumor cells membranes at high magnification (inset). B: 2+ staining intensity equals strong staining at low magnification, but no staining of the entire tumor cell circumference at high magnification (inset). C: 1+ staining intensity is defined by weak staining of the tumor cell membranes at low and high (inset) magnification.
Western blot analysis
Western blot analysis was performed on five tumors, four with and one without somatostatin receptor expression based on in vitro somatostatin receptor autoradiography. The procedure was carried out as published previously (9), using a UMB-1 antibody concentration of 1:200.
In vitro somatostatin receptor autoradiography
In vitro somatostatin receptor autoradiography was carried out as described before (20). Briefly, 20 µm thick cryostat sections were incubated with the sst2A-preferring radioligand 125I-[Tyr3]-octreotide for 2 hours at room temperature. Non-specific binding was assessed by incubating serial tissue sections with the radioligand in presence of 10−6 M of unlabeled octreotide. The slides were then exposed to Kodak films Biomax MR® for 7 days at 4°C. Radioligand binding to the tumors was analyzed in correlation with morphology using a corresponding hematoxylin and eosin-stained tissue section. The somatostatin receptor binding site density was measured using tissue standards containing known amounts of isotope and cross-calibrated to tissue-equivalent ligand concentrations (2, 14) as well as a computer-assisted imaging system (Interfocus, Mering, Germany).
Statistical evaluation
Linear regression analysis and the Spearman’s rank correlation coefficient r2 were used to correlate immunohistochemistry and autoradiography results. P < 0.05 was considered to be statistically significant.
Results
Immunohistochemical sst2A staining with the UMB-1 antibody was tested on a TMA under different experimental conditions in order to identify the optimal antigen retrieval method and antibody concentration. Heat pre-treatment either in the pressure cooker in citrate buffer for 10 minutes (PC-C) or in the microwave in urea buffer (MW-U) yielded the best results: a large fraction of autoradiographically positive tumors was stained, and there was a distinct membranous staining of tumor cells and negligible cytoplasmatic background staining. PC-C appeared to be slightly superior to MW-U. Of the 95 evaluable spots in the TMA expected to be positive based on somatostatin receptor autoradiography, a maximum of 78 (82%) could be stained with PC-C as compared to 76 (80%) with MW-U. Moreover, pre-treatment with PC-C often resulted in staining of a larger fraction of tumor cells than pre-treatment with MW-U, while the staining intensity was comparable. The other tested antigen retrieval methods were unsatisfactory, yielding a staining in only a small fraction of tumors expected to be positive. Relatively high UMB-1 concentrations had to be applied for optimal staining results. A concentration of 1:20 was found to be most appropriate: It stained a maximum of 78 of the 95 evaluable positive spots (82%) compared to a 1:100 concentration which stained only 65 spots (68%) using PC-C pre-treatment.
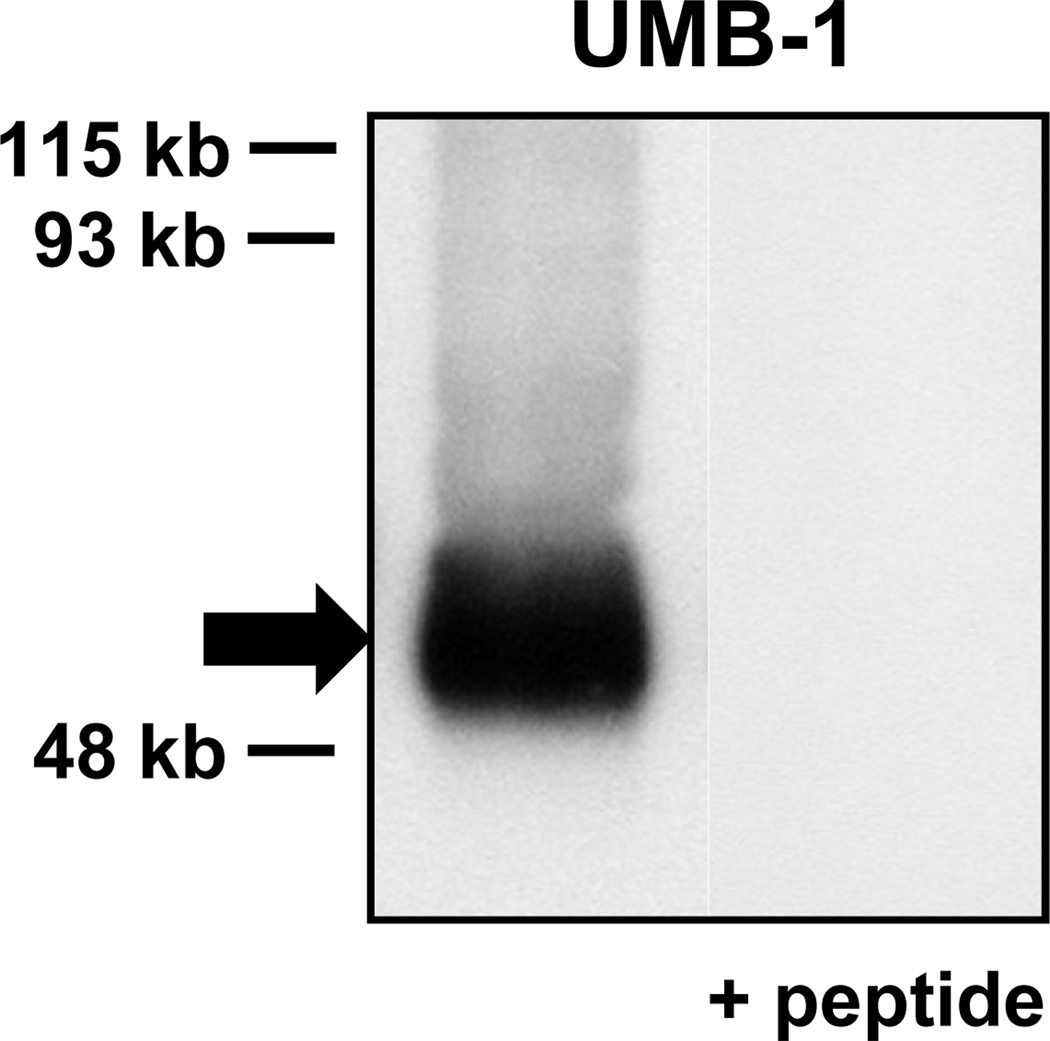
Several lines of evidence were provided for the specificity of UMB-1 staining for sst2A. First, antibody binding to the tumor cells was completely abolished by pre-absorption with the immunogen peptide. Second, all but one of the 26 evaluable spots in the TMA expected to be negative based on receptor autoradiography were not stained. Lastly, UMB-1 Western blot analysis of somatostatin receptor expressing tumors yielded a single specific band which migrated at the expected molecular weight and was completely pre-absorbed with the corresponding immunogen peptide (figure 2). UMB-1 Western blots gave congruent results with immunohistochemistry and receptor autoradiography in all studied tumors.
Figure 2.
UMB-1 Western blot analysis providing evidence of the specificity of UMB-1 staining for sst2A in an insulinoma. There is a single broad band (arrow) migrating at approximately 53 kb, the expected size of sst2A. This band is completely abolished when adding the immunogen peptide.
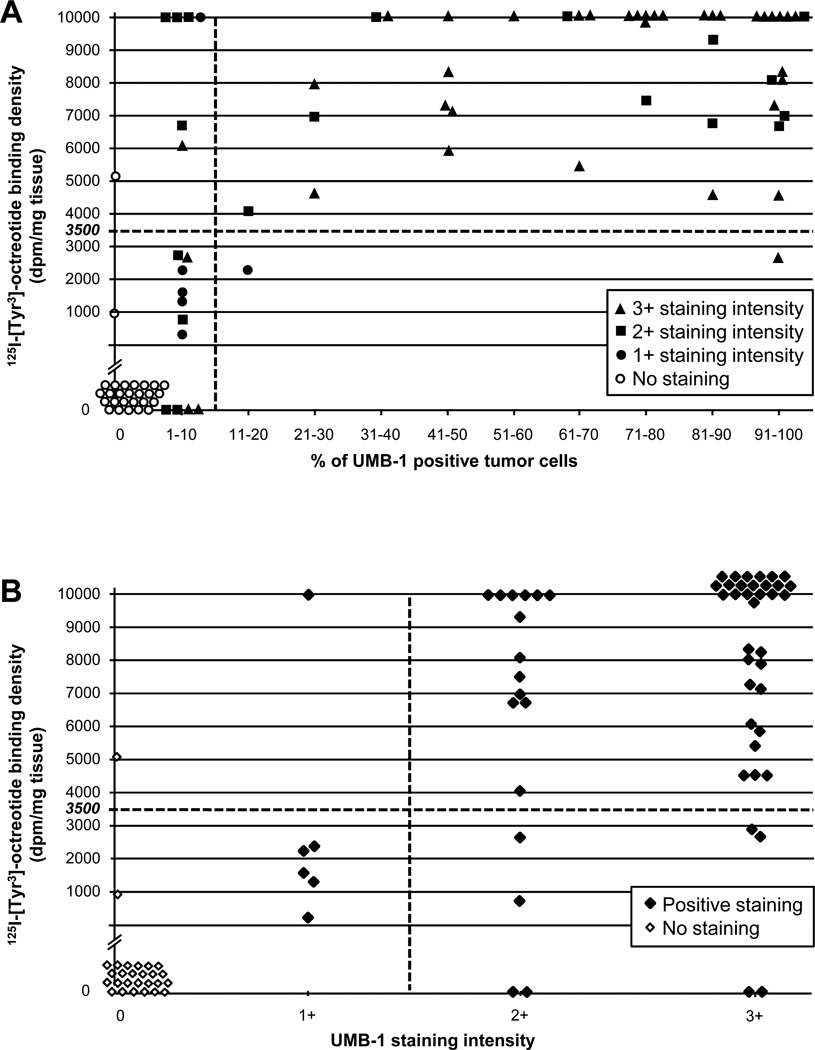
Immunohistochemistry for sst2A using PC-C pre-treatment and a UMB-1 concentration of 1:20 was performed on large tissue sections of the 89 tumors. Results were semi-quantitatively evaluated and compared with those of in vitro receptor autoradiography as reference method. There was a good correlation of the results obtained with these two methods, as shown in figure 3 and illustrated with typical examples in figure 4. In all but two of the 60 tumors expressing somatostatin receptors by autoradiography (97%), sst2A immunohistochemistry was positive. Likewise, 25 of the 29 autoradiographically negative tumors (86%) were also negative by immunohistochemistry. Moreover, the immunohistochemical staining levels correlated well with the 125I-[Tyr3]-octreotide binding density in the same tumor. Tumors with strong radioligand binding usually showed also a wide-spread and strong immunohistochemical staining (cases 1 and 2 of figure 4), while tumors with low autoradiographic receptor expression often displayed only weak UMB-1 staining in few tumor cells (case 3 of figure 4). Statistically, the r2 values for the associations between autoradiographic 125I-[Tyr3]-octreotide binding density on the one hand and the proportions of immunohistochemically positive tumor cells and staining intensities on the other hand were 0.464 (p<0.0001) and 0.428 (p<0.0001), respectively.
Figure 3.
Relation between immunohistochemical UMB-1 staining (concentration 1:20, pre-treatment PC-C) and 125I-[Tyr3]-octreotide binding levels in 89 tumors. A cut-off value of 3’500 dpm/mg is applied to distinguish between no or low levels and high levels of 125I-[Tyr3]-octreotide binding. A: If a tumor exhibits UMB-1 staining in more than 10% of tumor cells, the likelihood that it expresses 125I-[Tyr3]-octreotide binding levels above 3’500 dpm/mg and, therefore, is eligible for somatostatin receptor targeting is very high (high positive predictive value). B: Almost all tumors with 125I-[Tyr3]-octreotide binding above 3’500 dpm/mg exhibit strong (>1+) staining of tumor cells (high sensitivity).
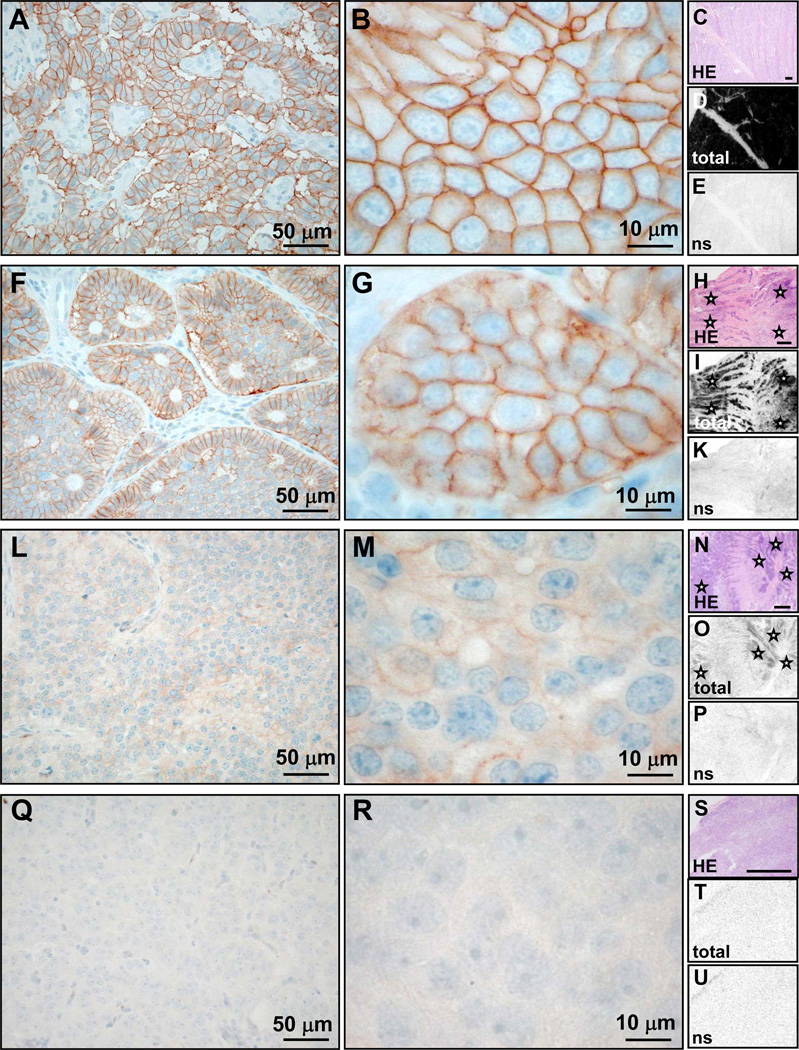
Figure 4.
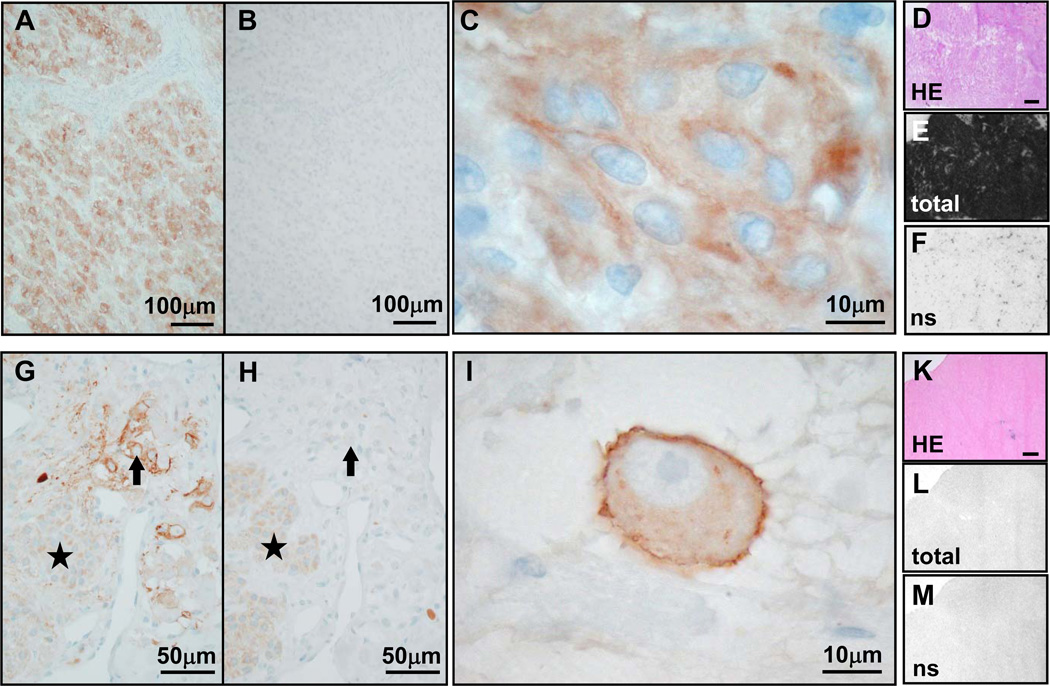
Comparison of UMB-1 immunohistochemistry (concentration 1:20, pre-treatment PC-C; first two columns) with 125I-[Tyr3]-octreotide receptor autoradiography (last column; bars indicate 1mm) in typical examples of tumors with high somatostatin receptor levels (rows 1 and 2), low somatostatin receptor levels (row 3) and no somatostatin receptor expression (row 4). A–E, malignant insulinoma: Strong membranous UMB-1 staining of most tumor cells (A) and often of entire tumor cell circumference (3+; B). Receptor autoradiography on serial tissue sections of the same case showing the tumor tissue stained with HE in C, very strong total 125I-[Tyr3]-octreotide binding to the entire tumor sample in D and complete displacement of 125I-[Tyr3]-octreotide by cold octreotide, providing evidence of specificity of somatostatin receptor binding in E (ns = non-specific 125I-[Tyr3]-octreotide binding in presence of excess cold octreotide). There is an excellent correlation between strong UMB-1 staining and high 125I-[Tyr3]-octreotide binding levels. F–K, ileal neuroendocrine carcinoma: Strong membranous UMB-1 staining of a large proportion of tumor cells (F), in some tumors cells affecting the entire tumor cell circumference (3+; G). This corresponds well to the strong specific 125I-[Tyr3]-octreotide binding to the entire tumor (asterisks) in the autoradiography experiment (H–K). L–P, ileal neuroendocrine carcinoma: Weak membranous UMB-1 staining at low (L) and high (M) magnification, correlating well with weak 125I-[Tyr3]-octreotide binding to the tumor tissue (asterisks; N–P). Q–U, benign insulinoma: No UMB-1 staining (Q, R), which matches well with negative receptor autoradiography (S–U).
We then analyzed how far UMB-1 immunohistochemistry was able to select tumors expressing enough somatostatin receptors for in vivo somatostatin receptor targeting. For this purpose, the tumors were divided into those with high autoradiographic receptor binding site levels anticipated to yield a positive in vivo scan and those with no or low levels of receptor binding sites expected to result in a negative scan. A 125I-[Tyr3]-octreotide binding density of at least 3’500 dpm/mg was considered to be necessary for an unambiguously positive scan, based on previous comparative data (10, 23). As apparent in figures 3A and B and table 2A, most tumors with high autoradiographic receptor levels showed a specific membranous UMB-1 staining in more than 10% of tumor cells and with an intensity of 2+ or 3+, while the majority of tumors with no or low autoradiographic receptor expression exhibited less than 10% stained tumor cells and a weak staining intensity of 1+. The statistical performances of the cut-off levels of >10% stained tumor cells and >1+ staining intensity to identify tumors with a high somatostatin receptor expression are summarized in table 2B. A cut-off level of 10% UMB-1 positive tumor cells was excellent in predicting 125I-[Tyr3]-octreotide binding density above 3’500 dpm/mg, as only two of the 45 cases with >10% stained tumor cells exhibited a low autoradiographic receptor expression (positive predictive value 95.4%). These two tumors showed still relatively high 125I-[Tyr3]-octreotide binding levels between 2’000 and 3’000 dpm/mg. Conversely, only two of the 39 cases with low or no 125I-[Tyr3]-octreotide binding displayed >10% stained tumor cells (specificity 95%). Forty-three of 50 cases with high autoradiographic receptor levels were correctly identified with this cut-off level, resulting in a sensitivity of 86%. The negative predictive value amounted to 96%: seven of the 44 cases with 0–10% stained tumor cells showed in fact high somatostatin receptor levels by autoradiography. In comparison, the cut-off level of >1+ staining intensity exhibited a higher sensitivity (96%) and a higher negative predictive value (94%). The best negative predictive value was obtained when looking for the absence of any immunohistochemical staining: if there was no staining at all, the likelihood of low autoradiographic receptor levels was 96%. Only one single case with negative immunohistochemistry, a serotonin-producing tumor, showed a discrepant high 125I-[Tyr3]-octreotide binding.
Table 2.
| A) Immunohistochemical sst2A staining levels in relation with undetectable or low levels (< 3’500 dpm/mg) and high levels (≥ 3’500 dpm/mg) of 125I-[Tyr3]-octreotide binding in tumor tissues under different experimental conditions and for different antibodies | |||
|---|---|---|---|
| Antibody | Immunoreactivity | 125I-[Tyr3]-octreotide autoradiography | |
| No or low receptor expression |
High receptor expression |
||
| UMB-1 (PC-C)1) | 0–10% of tumor cells stained | 37 | 7 |
| >10% of tumor cells stained | 2 | 43 | |
| Staining intensity 0, 1+ | 31 | 2 | |
| Staining intensity 2+, 3+ | 8 | 48 | |
| No tumor cell staining | 26 | 1 | |
| Any tumor cell staining | 13 | 49 | |
| UMB-1 (MW-U)2) | 0–10% of tumor cells stained | 38 | 9 |
| >10% of tumor cells stained | 1 | 41 | |
| Staining intensity 0, 1+ | 33 | 4 | |
| Staining intensity 2+, 3+ | 3 | 49 | |
| No tumor cell staining | 16 | 2 | |
| Any tumor cell staining | 13 | 48 | |
| R2–88 | No tumor cell staining | 36 | 10 |
| Any tumor cell staining | 3 | 40 | |
| B) Sensitivity, specificity, and positive and negative predictive values of semi-quantitatively evaluated UMB-1 immunohistochemistry to detect 125I-[Tyr3]-octreotide binding levels ≥ 3’500 dpm/mg in tumor tissues | ||||
|---|---|---|---|---|
| Immunoreactivity | Sensitivity1) | Specificity2) | Positive predictive value3) |
Negative predictive value4) |
| >10% tumor cells stained | 86.0% | 94.9% | 95.4% | 84.1% |
| Staining intensity 2+ or 3+ | 96.0% | 79.5% | 85.7% | 93.9% |
| Any tumor cell staining | 98.0% | 66.7% | 79.0% | 96.3% |
Pre-treatment in pressure cooker in 10 mM citrate buffer (pH 6.0)
Pre-treatment in microwave in 5% urea buffer (pH 9.0)
Sensitivity: true positive rate, fraction of correctly as positive diagnosed cases of all truly positive cases
Specificity: true negative rate, fraction of correctly as negative diagnosed cases of all truly negative cases
Positive predictive value: fraction of all truly positive cases of all positively tested cases
Negative predictive value: fraction of all truly negative cases of all negatively tested cases
Only a small fraction of cases was misdiagnosed with the cut-off levels of >10% tumor cells with UMB-1 staining and of >1+ UMB-1 staining intensity. This included in particular seven tumors with <10% UMB-1 positive tumor cells, but in fact very high autoradiographic somatostatin receptor levels (figure 3A). Of note, in two of these cases, namely meningiomas, there was indeed a strong UMB-1 staining present which was, however, not clearly membranous but rather indistinct. An example is depicted in figure 5 in the top row. This tumor displays strong immunohistochemical UMB-1 staining in the vast majority of tumor cells, however in less than 10% of tumor cells in a clearly membranous distribution. Autoradiographically, the tumor exhibits very high somatostatin receptors levels which appear to originate from a larger fraction of tumor cells than only those with membranous staining. Could in tumors with <10% stained cells assessment of UMB-1 staining intensity be helpful in the differentiation between low and high tumoral somatostatin receptor levels? Indeed, a 1+ staining intensity was preferably present in the cases with low 125I-[Tyr3]-octreotide binding site levels, while 2+ and 3+ staining intensities equally occurred in tumors with low and high autoradiographic receptor amounts (figure 3A).
Figure 5.
Cases requiring cautious interpretation of immunohistochemical UMB-1 staining (concentration 1:20; pre-treatment PC-C; columns 1–3) in view of 125I-[Tyr3]-octreotide autoradiography results (last column; bars indicate 1mm). A–F, meningioma: Wide-spread and strong UMB-1 positivity at low magnification (A) that is completely abolished by the immunogen peptide (B). At high magnification (C), there is often a blurred staining in the area of the cell membrane, but only rarely a clear membranous staining. This staining pattern is probably due to prominent intercellular interdigitations. Autoradiographically, there is strong specific 125I-[Tyr3]-octreotide binding to the entire tumor tissue (D–F). Although <10% of tumor cells show membranous staining, this tumor is an excellent candidate for in vivo somatostatin receptor targeting. G–M, ganglioneuroblastoma: UMB-1 staining is present in single ganglionic tumor cells (arrow; G) in a membranous distribution (I, high magnification), but not in larger areas of neuroblastic tumor cells (asterisk; G). UMB-1 staining of ganglionic tumor cells is completely abolished by the immunogen peptide (H), providing proof of specific UMB-1 staining. No specific 125I-[Tyr3]-octreotide binding with receptor autoradiography (K–M). Despite strong tumor cell staining, this tumor is apparently not suitable for in vivo somatostatin receptor targeting, as the total receptor number per tumor mass is too low.
Furthermore, a small proportion (14%) of tumors with 2+ or 3+ staining intensity exhibited only low 125I-[Tyr3]-octreotide binding site levels under 3’500 dpm/mg or even completely negative autoradiography (figure 3B). Of interest, in all but one of these tumors less than 10% of tumor cells were stained. A typical example is shown in figure 5 in the bottom row. In this ganglioneuroblastoma, well-differentiated ganglionic tumor cells are strongly stained with UMB-1, comprise, however, only a small part of the tumor, while the poorly differentiated neuroblastic tumor cells are negative for UMB-1. Receptor autoradiography is completely negative.
Finally, it was assessed how variations of the experimental conditions and the use of different antibodies could affect the ability of immunohistochemistry to select tumors with a high autoradiographic somatostatin receptor expression. This is shown in table 2A. For UMB-1 immunohistochemistry, the two best antigen retrieval methods, PC-C and MW-U, were compared. It was confirmed that with pre-treatment with PC-C slightly more tumoral staining was obtained, with more tumors exhibiting >10% cells stained or a staining intensity of >1+. In contrast, when comparing UMB-1 with the original anti-sst2A antibody R2-88, the latter failed to stain a considerably larger fraction of tumors with high autoradiographic somatostatin receptor levels, namely 20% as opposed to 4%.
Discussion
The study shows that immunohistochemistry using the newly available monoclonal antibody UMB-1 is excellent and at present the best available tool for the semi-quantitative characterization of tumoral sst2A expression in formalin-fixed, paraffin-embedded tissues. It is highly specific, and a very clean and easily identifiable membranous staining pattern can be obtained. Moreover, UMB-1 staining levels correlate well with tumoral somatostatin receptor binding site amounts as measured with in vitro receptor autoradiography, the in vitro correlate of in vivo receptor imaging. In fact, the first evaluation scheme of sst2A immunohistochemistry is proposed that reliably selects tumors with high somatostatin receptor expression suitable for in vivo somatostatin receptor targeting and that, moreover, is simple and easy to apply. The presence of more than 10% tumor cells stained with UMB-1 correctly predicted high somatostatin receptor levels in over 95% of cases. Conversely, no staining at all truly reflected low or no somatostatin receptor expression in 96% of tumors. UMB-1 immunohistochemistry is thus equivalent to analogous tests for molecular targets in paraffin-embedded tissues such as the HercepTest in breast cancer (15). Only few limitations of this semi-quantitative evaluation of UMB-1 immunohistochemistry exist that have to be recognized. These include the dependence on a good immunohistochemical procedure, interpretational pitfalls, and the possibility of selecting false negative or false positive cases.
The usefulness of sst2A immunohistochemistry for tumor diagnostics strongly depends on a good antibody and an optimal technical procedure. UMB-1, at present the only commercially available monoclonal antibody against sst2A, was found to show the best staining characteristics in comparison with other reference antibodies. It produced the clearest membranous staining and virtually no cytoplasmatic background staining in a large proportion of somatostatin receptor positive cases. In accordance with our observations, UMB-1 had initially been demonstrated to yield a significantly more effective and cleaner staining than polyclonal antisera (4). Moreover, UMB-1 shows all advantages of a monoclonal antibody, such as no variation from batch to batch and unlimited supply. In contrast, SS-800, the only other appropriate commercial anti-sst2A antibody, and the non-commercial R2-88 antibody, up to now the gold standard antibody for sst2A immunohistochemistry, exhibit less distinct membranous and more diffuse cytoplasmatic background staining, as reported previously (9). Moreover, R2-88 showed in the present series significantly weaker tumor staining than in previous studies (9, 18, 19) and compared poorly with UMB-1. R2-88, generated in the early nineteen nineties, permitted for many years the successful characterization of sst2A receptors in a variety of tissues (8, 9, 18, 19, 24, 25), but is likely to have lost its good performance for immunohistochemistry in recent time.
Also the choice of the antigen retrieval method has an important effect on immunohistochemical sst2A detection. In our hands, best staining results were obtained with selected heat pre-treatments. While only negligible differences in the all-over excellent staining were observed between cooking in the pressure cooker in citrate buffer for 10 minutes and boiling in the microwave in urea buffer, a significantly poorer result was obtained using the citrate buffer for microwave boiling or protease digestion. Importantly, all pre-treatment methods yielded positive results in some samples, but the rate of false negative stainings dramatically increased under suboptimal pre-treatments.
In order to determine how immunohistochemistry can identify tumors with high somatostatin receptor expression, the prerequisite for successful in vivo somatostatin receptor targeting, UMB-1 staining results needed to be correlated with levels of the actual clinical target, i.e. the receptor binding sites. The latter can be measured with two established methods, either in vivo with a somatostatin receptor scan or in vitro with receptor autoradiography. There is a good correlation of results obtained by in vivo somatostatin receptor scan and in vitro somatostatin receptor autoradiography, based on previous investigations (6, 10) and own experience of more than two decades. For the present study, in vitro receptor autoradiography was chosen as reference method. It allowed determining with high specificity and selectivity the somatostatin receptor expression in tumor cells, as it can be correlated with morphology and controlled for non-specific radioligand binding by displacement experiments with cold somatostatin analogue. In contrast, a positive in vivo scan may not always be tumor-specific. Possible tracer up-take in tissues other than tumor cells, either specifically in somatostatin receptor expressing physiologic targets like ectopic spleen or tumor infiltrating leukocytes or non-specifically in tumor necrosis or scars, cannot be distinguished. Moreover, not all tumors of diagnostic interest are well visible on an in vivo scan, depending on their location and size. A last important advantage of chosing receptor autoradiography was the possibility to include also tumors with low somatostatin receptor expression below the detection threshold of an in vivo scan.
Only few previous studies compared results of sst2A immunohistochemistry with tumoral somatostatin receptor binding site levels. The latter were always defined by in vivo scan. In most of these series, the correlation was considerably worse than in the present one (1, 13, 28). This is probably largely explained by the use of the polyclonal anti-sst2A antibody SS-800 which shows poorer staining characteristics than UMB-1. The results of the single published investigation using UMB-1 are in agreement with the present findings (16): Müssig et al. obtained a similar correlation between sst2A immunohistochemistry and somatostatin receptor binding site densities, with only little lower r2 values. However, the sensitivity of immunohistochemistry for a positive scan was with 64% significantly lower. This may be explained by different immunohistochemical procedures (Müssig et al. used a lower antibody concentration and another antigen retrieval method), a different study population or, theoretically, a lower sensitivity of in vitro receptor autoradiography compared with in vivo scan.
Based on the comparison with in vitro somatostatin receptor autoradiography, UMB-1 immunohistochemistry with an optimized technical procedure represents the most reliable in vitro method for FFPE material to identify tumors with high somatostatin receptor levels suitable for in vivo somatostatin receptor targeting. Only a small fraction of cases remains where itUMB-1 immunohistochemistry cannot correctly predict somatostatin receptor levels. In those few inconclusive cases, in vivo imaging or in vitro receptor autoradiography would then be the solution of choice. Otherwise, underestimation of tumoral somatostatin receptors would exclude patients who would benefit from in vivo somatostatin receptor targeting. In the present series, 14% of tumors with in fact high somatostatin receptor levels had only a small fraction of less than 10% of tumors cells stained with UMB-1. Various explanations for this false negativity can be discussed. Theoretically, some tumors may predominantly express large amounts of somatostatin receptor subtypes 3 or 5 that weakly bind octreotide, but are not detected by sst2A immunohistochemistry. However, it was previously shown that expression of only sst2A, but not other somatostatin receptor subtypes correlated with octreotide binding (1, 16). Alternatively, tumors may display a focal somatostatin receptor expression in only one of the different samples subjected to immunohistochemistry and receptor autoradiography, respectively. Furthermore, poor fixation of the FFPE material may significantly impair immunohistochemical sst2A staining. In fact, we have repeatedly observed UMB-1 staining only in peripheral but not in central zones especially of large tumors. Another pitfall in the evaluation of tumoral UMB-1 staining is the poor membranous but rather blurred staining of meningiomas which actually express high sst2A levels (26). This particular staining pattern of meningiomas is likely explained by the prominent interdigitation of intercellular membranes typical of meningioma tumor cells which yields an indistinct cell membrane staining at the light microscopic level. In these few instances of minor diagnostic importance, the cytoplasmatic staining better reflects the actual somatostatin receptor levels and may well correspond to receptors located in the strongly interdigitated cell membrane.
Conversely, overestimation of tumoral somatostatin receptor levels by immunohistochemistry may also represent a diagnostic problem. In the present series, this occurred mainly when UMB-1 staining intensity alone was considered. In particular, in four of the 29 tumors (5%) that were completely negative by receptor autoradiography, strong (2+, 3+) tumor cell staining was present. Of importance, in all these cases, <10% of tumor cells were stained. It appears logic that high tumoral receptor levels allowing a positive in vitro or in vivo scan are the result not of only few tumor cells which individually express a large number of receptors, but of a receptor expression in a substantial number of tumor cells in a larger area. This is illustrated with the example of the ganglioneuroblastoma. In this case, it is not surprising that the few sst2A expressing ganglionic tumor cells are below the detection level of autoradiography which is completely negative. It thus emerges that in the interpretation of immunohistochemical sst2A staining, priority should be given to the fraction of stained tumor cells over the staining intensity of individual cells. In order to obtain representative specificity values, more than 40% of the cases included in the study were tumors with autoradiographically no or low somatostatin receptor expression. In only 5% of these cases, UMB-1 staining of more than 10% of tumor cells was present.
The experience collected by comparing the excellent UMB-1 immunohistochemistry with tumoral in vitro 125I-[Tyr3]-octreotide binding site levels allows new recommendations for the use of sst2A immunohistochemistry in daily diagnostics for an optimally tailored patient management. These recommendations are provided in table 3. Table 3 also proposes an interpretation of the staining results regarding the suitability of individual tumors for in vivo somatostatin receptor targeting. Of note, the cut-off level of 10% stained tumor cells is based on the experimental conditions in our laboratory. Cut-off staining levels may vary from laboratory to laboratory, depending on the sensitivity of the immunohistochemical procedure applied. In order to know the performance of an individual immunohistochemical test, an interlaboratory comparison on a reference set of cases with known somatostatin receptor binding site levels defined by in vitro or in vivo binding methods could be helpful. The proposals in table 3 may represent the currently best option for a recommendation if an individual patient is eligible for in vivo somatostatin receptor targeting based on somatostatin receptor immunohistochemistry.
Table 3.
Updated recommendations for sst2A immunohistochemistry on formalin-fixed, paraffin-embedded tumor tissues
| Method | Use UMB-1 antibody |
| Define the best experimental conditions (antibody concentration, antigen retrieval method) in individual laboratories | |
| Use representative large tissue sections of well-fixed tumors | |
| Analysis of tumor cell staining | Assess only membranous staining |
| Assess percentage of stained tumor cells | |
| Assess staining intensity (according to figure 1) | |
| Interpretation of results |
|
In conclusion, UMB-1 immunohistochemistry may reach a sensitivity virtually equivalent to that of in vitro receptor autoradiography and may even replace autoradiography if the optimal methodological conditions are identified and an external quality control is performed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: A.S. is currently receiving a grant from NIH. The other authors have no conflicts of interest or funding to disclose.
References
- 1.Asnacios A, Courbon F, Rochaix P, et al. Indium-11-pentreotide scintigraphy and somatostatin receptor subtype 2 expression: new prognostic factors for malignant well-differentiated endocrine tumors. J Clin Oncol. 2008;26:963–970. doi: 10.1200/JCO.2007.12.7431. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Wimpy TH. Calibration of [14C]plastic standards for quantitative autoradiography of [125I]labeled ligands with Amersham Hyperfilm beta-max. Neurosci Lett. 1989;104:171–177. doi: 10.1016/0304-3940(89)90350-9. [DOI] [PubMed] [Google Scholar]
- 3.Dournaud P, Gu YZ, Schonbrunn A, et al. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer T, Doll C, Jacobs S, et al. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab. 2009;93:4519–4524. doi: 10.1210/jc.2008-1063. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 6.Garden OA, Reubi JC, Dykes NL, et al. Somatostatin receptor imaging in vivo by planar scintigraphy facilitates the diagnosis of canine insulinomas. J Vet Intern Med. 2005;19:168–176. doi: 10.1892/0891-6640(2005)19<168:sriivb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Gu YZ, Schonbrunn A. Coupling specificity between somatostatin receptor sst2A and G proteins: isolation of the receptor-G protein complex with a receptor antibody. Mol Endocrinol. 1997;11:527–537. doi: 10.1210/mend.11.5.9926. [DOI] [PubMed] [Google Scholar]
- 8.Gugger M, Waser B, Kappeler A, et al. Cellular detection of sst2A receptors in human gastrointestinal tissue. Gut. 2004;53:1431–1436. doi: 10.1136/gut.2004.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Körner M, Eltschinger V, Waser B, et al. Value of immunohistochemistry for somatostatin receptor subtype sst2A in cancer tissues: lessons from the comparison of anti-sst2A antibodies with somatostatin receptor autoradiography. Am J Surg Pathol. 2005;29:1642–1651. doi: 10.1097/01.pas.0000174013.14569.90. [DOI] [PubMed] [Google Scholar]
- 10.Krenning EP, Kwekkeboom DJ, Pauwels S, et al. Somatostatin receptor scintigraphy. In: Freeman LM, editor. Nuclear Medicine Annual 1995. New York: Raven Press; 1995. pp. 1–50. [Google Scholar]
- 11.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 12.Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J Nucl Med. 2011;52:841–844. doi: 10.2967/jnumed.110.084236. [DOI] [PubMed] [Google Scholar]
- 13.Miederer M, Seidl S, Buck A, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 14.Miller JA, Zahniser NR. The use of 14C-labeled tissue paste standards for the calibration of 125I-labeled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- 15.Moelans CB, Kibbelaar RE, van den Heuvel MC, et al. Validation of a fully automated HER2 staining kit in breast cancer. Cell Oncol. 2010;32:149–155. doi: 10.3233/CLO-2010-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müssig K, Öksüz MÖ, Dudziak K, et al. Association of somatostatin receptor 2 immunohistochemical expression with [111In]-DTPA octreotide scintigraphy and [68Ga]-DOTATOC PET/CT in neuroendocrine tumors. Horm Metab Res. 2010;42:599–606. doi: 10.1055/s-0030-1253354. [DOI] [PubMed] [Google Scholar]
- 17.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 18.Reubi JC, Kappeler A, Waser B, et al. Immunohistochemical localization of somatostatin receptors sst2A in human tumors. Am J Pathol. 1998;153:233–245. doi: 10.1016/S0002-9440(10)65564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reubi JC, Kappeler A, Waser B, et al. Immunohistochemical localization of somatostatin receptor sst2A in human pancreatic islets. J Clin Endocrinol Metab. 1998;83:3746–3749. doi: 10.1210/jcem.83.10.5314. [DOI] [PubMed] [Google Scholar]
- 20.Reubi JC, Kvols LK, Waser B, et al. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990;50:5969–5977. [PubMed] [Google Scholar]
- 21.Reubi JC, Laissue JA, Waser B, et al. Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: facts and artifacts. J Clin Endocrinol Metab. 1999;84:2942–2950. doi: 10.1210/jcem.84.8.5878. [DOI] [PubMed] [Google Scholar]
- 22.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 23.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC, Waser B, Lamberts SWJ, et al. Somatostatin (SRIH) messenger ribonucleic acid expression in human neuroendocrine and brain tumors using in situ hybridization histochemistry: Comparison with SRIH receptor content. J Clin Endocrinol Metab. 1993;76:642–647. doi: 10.1210/jcem.76.3.8095268. [DOI] [PubMed] [Google Scholar]
- 25.Reubi JC, Waser B, Liu Q, et al. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab. 2000;85:3882–3891. doi: 10.1210/jcem.85.10.6864. [DOI] [PubMed] [Google Scholar]
- 26.Reubi JC, Waser B, Schaer JC, et al. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 27.Schulz S, Schulz S, Schmitt J, et al. Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res. 1998;4:2047–2052. [PubMed] [Google Scholar]
- 28.Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20:1172–1182. doi: 10.1038/modpathol.3800954. [DOI] [PubMed] [Google Scholar]