Abstract
Basic science literature abounds with molecules that promise to ameliorate almost any disease, from curing cancer to slowing the aging process itself. However, most of these compounds will never even be evaluated in humans, let alone proven effective. Here, we use resveratrol as an example to highlight the enormous difficulties in understanding pharmacokinetics, determining side effects, and, ultimately, establishing mechanisms of action for a natural compound. Despite extensive interest and effort, and continuing promising results from basic science groups, very little is known even today about the effects of resveratrol in humans. Part of the problem is the unattractiveness of natural compounds to large, well-funded companies that could run clinical trials because developing their own molecules affords much greater protection for their intellectual property. In fact, selling unpatentable material motivates smaller nutraceutical companies to complicate the scientific problem even more—each creates its own proprietary blend, making it extremely difficult to compare their data with those of other companies, or of academic labs using pure compounds. But even beyond these problems lies a deeper one; resveratrol, and almost every natural compound, is likely to have many clinically relevant targets with different dose–response profiles, tissue distributions, and modifiers. Tackling this type of problem efficiently, and even beginning to address the spectrum of other molecules with claimed benefits, is likely to require the development of new paradigms and approaches. Examples include better molecular modeling to predict interactions, large-scale screens for toxic or other common effects, affinity-based methods to identify drug-interacting proteins, and better synthesis of existing data, including legislation to promote the release of trial results, and tracking of voluntary supplement usage. The evidence for benefits of resveratrol in humans remains too sparse to be conclusive; yet, the limited data that are available, combined with a growing list of animal studies, provide a strong justification for further study.
Keywords: Clinical trials, Translational, Nutraceutical, Metabolism, Polyphenol
Solving problems in rodents is deceptively easy. Diabetes has been cured numerous times (1–3), exercise can be delivered in pill form (4), and just a few months ago, scientists were able to reverse age-related degeneration (5). Yet, few of these effects ever seem to lead directly to human therapies. In some cases, the reason is readily apparent—for example, the study that reversed age-related degeneration in mice did so by reactivating telomerase, an intervention that is widely believed to concomitantly increase the risk of developing cancer (6). In other cases, animal models may fail to accurately reflect the corresponding human disease (7,8). But in many cases, the interventions simply have not been tested thoroughly enough, getting bogged down by confusing or conflicting results, or abandoned in favor of a new approach. This review will focus on resveratrol as an example of the challenges involved in moving a nutraceutical (naturally occurring molecule found in foods that has health benefits [9]) that has shown promise in animals into the realm of human therapeutics.
Resveratrol (Figure 1) is a small polyphenol found in various berries, nuts, and other plants sources, including traditional Asian medicines (10). It has received attention in recent years for a number of reasons: First, the most important dietary source for most individuals is red wine, leading to speculation that resveratrol might be responsible for some of wine’s health benefits (11). Second, it was found to inhibit cyclooxygenases, which can play a role in cancer progression, and was subsequently established to prevent carcinogenesis at multiple stages in mice (12). Third, it was found in an in vitro screen for activators of sirtuin enzymes, which regulate longevity in lower organisms, and was subsequently shown to extend life in yeast (13), worms (14), flies (although some controversy remains for this organism [15,16]), and a short-lived species of fish (17). The resulting interest in resveratrol has inspired an enormous number of studies, leading to the identification of many additional target enzymes and cell culture effects that are beyond the scope of this review, having been discussed at length elsewhere (18,19).
Figure 1.
Structure of resveratrol. Resveratrol is a naturally occurring polyphenol with the chemical name trans-3,5,4’-trihydroxystilbene.
In mice, resveratrol has produced a number of effects that would be very desirable for humans. In obese animals, resveratrol has been shown to restore normal insulin sensitivity, tissue function, gene expression profiles, and life span, even at doses that do not have a major effect on body weight (20). These changes appear to be mediated at least in part by improvements in mitochondrial number and function, which also result in a near doubling of endurance in obese or lean mice (21). The higher doses used in the endurance studies are sufficient to cause weight loss, and other studies provide evidence that resveratrol can protect against ischemic injuries and promote cardiovascular health (22). Combined with the previous observation that resveratrol can prevent cancers, especially skin and colon cancer, in rodent models (23,24) and its anti-inflammatory effects (25,26), these studies have provided powerful fodder for nutraceutical companies selling various formulations that contain the molecule. They have also spurred the announcements of numerous clinical trials; yet, questions about dosing and mechanism have hampered progress, and very little human data, other than short-term pharmacokinetic and safety studies, have been published.
The first international conference on resveratrol and health, Resveratrol2010, was held in September of 2010 in Helsingor, Denmark, with the purpose of assessing the current state of knowledge in the field, and making recommendations for human use and future study of resveratrol. A clear theme of the conference, which was attended by all of the authors, was that multiple mechanisms are likely to contribute to the beneficial effects of resveratrol, making it difficult to agree on a specific dose, biomarker, or outcome that can define the molecule (27, 28). In addition, studies in the clinical area highlighted the difficulty in comparing proprietary formulations and delivery routes, and in establishing consistent guidelines or obtaining funding for large-scale studies of natural compounds. This review will highlight some of the issues raised at the conference and elsewhere, in order to illustrate the challenges facing researchers focused on resveratrol or other molecules being developed outside of large pharmaceutical companies.
Bioavailability and Metabolism of Resveratrol
One of the most important questions to ask, and one of the most difficult to answer, is, “what dose of resveratrol should be used?” This is true from in vitro studies, to animal models, to clinical trials, and certainly true for the average consumer facing a bewildering array of nutraceutical offerings. A panel of experts, including two of the authors (O.V. and J.A.B.), convened to address this and other questions at the Resveratrol2010 conference. Some of the ideas in the following paragraphs are elaborations on these discussions, which have been summarized more completely in a dedicated review (Vang O, Ahmad N, Baile CA, et al., unpublished data, 2011).
The bioavailability and the pharmacokinetics of resveratrol have been studied in humans and experimental animals. In humans, a rapid uptake of resveratrol is observed, as the plasma concentration of resveratrol peaks about 30 minutes after low dose intake. Administration of higher doses of resveratrol, as piceid (a glucose conjugate of resveratrol) or under fasting conditions delayed the peak to 1.5 or 2 hours. Up to 50% of the ingested resveratrol has been found to be bioavailable in rats based on the level in the plasma (29) and a correspondingly high level is found in humans (∼70%) (30). An enterohepatic cycle of the metabolism of resveratrol has been proposed in both rats and humans. Resveratrol is quickly taken up by enterocytes and metabolized to glucuronide and sulfate conjugates, which are secreted back to the intestine where they may be deconjugated and reabsorbed or excreted in the feces (29,30). The enterohepatic cycle reduces the concentration of the free compound reaching the body lumen. Thus, the enterohepatic cycle, along with a rapid metabolism in the liver, likely explains the low concentration of resveratrol in the blood stream. The glucuronide and sulfate conjugates, including disulfates and mixed sulfate-glucuronides, are the major metabolites of resveratrol, but other metabolites are formed, including dihydroresveratrol (31) and metabolites that still need to be identified. It is clear that only a small fraction of the ingested resveratrol reaches the body as the parent compound. Furthermore, the amount of resveratrol ingested from dietary sources, such as red wine, juices, and so on, would very rarely exceed 5 mg, resulting in plasma levels that are either not detectable or orders of magnitude below the micromolar concentrations employed in vitro. Administration of about 25 mg resveratrol results in plasma concentrations of the free form that range from 1 to 5 ng/mL (32); administration of higher doses (up to 5 g) produced concentrations of free resveratrol of up to about 500 ng/mL, or just over 2 μM (33).
The low concentrations of resveratrol observed in plasma after ingestion are a puzzle, as the target concentrations implied by most in vitro experiments are not reached. Resveratrol is lipophilic, meaning that it mixes very well with lipids, including membranes and lipoproteins. Therefore, the tissue or subcellular concentration of resveratrol may be higher than suggested based on the concentrations measured in the plasma. This is biochemically very relevant because several known targets of this compound are membrane components. Furthermore, it has been shown in vitro that cellular effects of resveratrol are observed at time points where resveratrol is not measurable in the growth media, indicating that resveratrol might be tightly associated with various macromolecules and cellular structures (O.V., unpublished results). In addition, an increased uptake of resveratrol was observed in PTEN-knockout mice when resveratrol was delivered as a liposome solution (34) and the liposome–resveratrol reduced the incidence of adenomas in these mice. This suggests that liposome-mediated delivery of resveratrol could enhance its activity in humans, but one should bear in mind that the safety and biologic responses of such formulations would need to be tested carefully. The influence of dietary lipids on the delivery of natural resveratrol in foods is poorly understood but may be quite important to its effects (35). Finally, some of the biologic effects of resveratrol are observed even at low concentrations (36,37), and resveratrol could also act directly on the intestinal tissue, affecting the rest of the body through secondary effects that are not dependent on plasma levels of the compound (10).
The low bioavailability and extensive metabolism of resveratrol are a constant source of contention about whether the concentrations used for most in vitro studies are even relevant to the in vivo situation (30). In rodent models, the doses employed routinely range over four orders of magnitude, from about 0.1 to 1,000 mg/kg, with even higher or lower doses occasionally being used (10). It is clear that this is more than a matter of simply dialing up the dose to reach an effective level; resveratrol frequently exhibits biphasic effects (38). For example, resveratrol at low doses (∼5 mg/kg/d) has been shown to cause weight gain in mice fed a high-fat diet (39), whereas at high doses (∼400 mg/kg/d), there is marked weight loss (21). Moreover, cardioprotective effects of resveratrol that are observed at 2.5 or 5 mg/kg/d are reversed when the dose is increased to 25 or 50 mg/kg/d (40). Having chosen a rodent dose, one is still faced with the question of how to extrapolate to a human equivalent. Direct extrapolation by body weight is often used, but as others have pointed out, using body surface area has performed better in the past (41), and there is simply no way to be confident without performing the human studies.
Dosing, Interactions, and Side Effects of Resveratrol
It was generally agreed by the expert panel of Resveratrol2010 that at least some portion of the population likely obtains 1–2 mg/day of resveratrol from dietary sources and that this level is almost certain to be safe for chronic consumption. At the other extreme, at least one study reported gastrointestinal side effects in a high proportion of participants taking 2.5 g/day or more (42), suggesting that doses at or above this level are unlikely to be tolerated chronically. Two different commercial suppliers of resveratrol, DSM Nutritional Products and Fluxome, have each reached the conclusion that 450 mg/day can be considered safe for a 60-kg individual, based on their own studies in rats and literature review ([43] and www.fluxome.dk, respectively). Although the panel as whole was not prepared to adopt this position without further human data, it is certainly consistent with the small number of clinical trials that have been published, several of which have included doses at or above 1 g/day without serious side effects (although the studies were short term, so long-term side effects are not known); (42,44). The panel concluded that the optimal dose of resveratrol remains unclear and is likely different depending on which of the reported benefits one chooses as an end point.
The potential for interactions with other polyphenols, dietary components, or prescription drugs makes it even harder to confidently recommend a dosage for nutraceutical formulations. Resveratrol may act in an additive or synergistic manner with other polyphenols and may influence the metabolism or activity of other drugs. Synergism of various polyphenols with resveratrol has been observed experimentally (45–49) and is the specific intention of many nutraceutical formulations. In addition, resveratrol can directly interact with many drug-metabolizing enzymes and induces expression of others transcriptionally (10). Both of these effects appear to be beneficial under some circumstances; inhibition of CYP1A1 by resveratrol suppresses benzo[a]pyrene-induced tumorigenesis (50), and many of the transcripts upregulated by resveratrol are directly involved in oxidative stress resistance (51). However, CYPs and other drug-metabolizing enzymes determine the pharmacokinetics of many drugs, which could change dramatically in the presence of resveratrol. There is also the potential to antagonize disease states with what would otherwise be relatively mild side effects. A clinical trial in which multiple myeloma patients were given a proprietary formulation of resveratrol at 5 g/day was recently terminated after 5 of 24 participants developed cast nephropathy. This is a normal complication of multiple myeloma (52), and it was reported in the Wall Street Journal (December 1, 2010) that dehydration due to vomiting and diarrhea in the resveratrol-treated patients may have exacerbated the underlying disease (52). Unfortunately, primary data from the trial are not available and are not likely to be, but this example should serve as a stark reminder to carefully consider the context in which resveratrol, or any nutraceutical, is being delivered.
Commercial Products and Regulatory Issues Complicate Scientific Research
A given nutraceutical may be found in various forms: as pure compound, partially purified, or as one component of a crude extract or mix of defined compounds. Resveratrol found in supplements generally comes from grapes (Vitisvinifera), Polygonum cuspidatum, synthetic sources, or a combination of these (54). Some of these supplements are pure trans-resveratrol, whereas others include a combination of compounds that demonstrate synergism with resveratrol (45–49). Synergism-based products may be labeled to include very specific molecules (eg, quercetin) or a vague blend of compounds (eg, proprietary blend of red wine extract) (54). The vast array of formulations combined with lack of scientific testing makes it difficult for experts to apply general research findings to recommend a given product for general health, let alone treatment for a specific condition. This emphasizes the need for extensive human clinical trials evaluating the optimal approach for resveratrol administration, but there are many barriers to overcome in this realm.
Despite the numerous proprietary formulations available, most supplement companies have not performed well-controlled clinical trials on their product but rather make claims using research not specific to their product, or even humans. A number of companies boldly proclaim their product is effective in fighting aging and preventing disease through extrapolating findings from publications demonstrating increased health and survival in obese mice (20), despite the lack of human trials, and the fact that life-span extension was not observed even in mice, when lean, healthy animals were used (39,55). The focus of the media has largely been on such breakthrough findings, as well as business-related issues, such as the 2008 acquisition of the formerly resveratrol-centered company Sirtris by GlaxoSmithKline for $720 million. Such publicity may provide validation in the minds of consumers and reduce the motivation for companies to provide compelling but expensive clinical data. Small companies may not have budgetary resources for research, and large companies may intentionally abstain from performing research or publishing results because easily generalized findings may also be used by their competitors. This further encourages companies to develop proprietary formulations, widely varying in resveratrol and synergist content, to separate their product from the competition. The situation makes it extremely perplexing to compare human supplementation data to laboratory findings utilizing pure compounds.
Registration of clinical trials and public disclosure of complete study results is voluntary (56) (although registration is required for publication in most medical journals), which creates a number of ethical issues. Failure to release full results, including possible adverse effects of the compound or blend, can place the participants of future studies at unnecessary risk and inhibits the ability of researchers to design subsequent trials effectively. The value of timely and detailed reporting to the medical community is clear, but any potential detriment to the reputation or financial interests of a company may prevent such open disclosure, leading to reporting bias (57). This problem is magnified with nutraceutical supplements, where clinical trials are not even required. Without the strict regulation, supplement companies have the liberty of treating clinical trials as an optional component of development and marketing. Negative or neutral clinical trials of nutraceuticals could easily be hidden, allowing further product development or sales of an unsafe or ineffective supplement to continue. As such, extensive safety information, including other supplement and drug interactions and disease contraindications, is largely unknown at the time of nutraceutical product release.
A thorough evaluation of nutraceutical supplements is essential, given widespread supplement usage combined with a relative lack of knowledge on the mechanisms of many supplements. Estimates suggest that 10%–52% of the U.S. population uses dietary supplements (58), with some research suggesting more than half of supplement users do so to treat a specific condition (59). This is especially alarming, considering most individuals taking dietary supplements do not report their use to medical professionals and also use pharmaceutical products (60). Although Food and Drug Administration (FDA) regulations on pharmaceutical products have increased the public’s awareness of the negative consequences of drugs, consumers equate “natural” with “safe,” which increases the risk of supplement misuse and can provide consumers a false sense of security (61). The increasing sale of nutraceutical products in pharmacies further adds to their image of safety and efficacy, despite the fact that pharmacists themselves generally do not have an adequate understanding of these products (62). Because nutraceuticals are not subject to the FDA’s rigorous standards, their safety is not as widely established and adverse events may go unreported, which consequentially may provide the public with an inflated sense of confidence in their usage.
The Dietary Supplement Health and Education Act of 1994 ended the treatment of nutraceuticals as drugs and gave supplement manufacturers the responsibility of evaluating safety and efficacy data. Under these less stringent regulations, dietary supplement labels cannot legally make any claims regarding preventing, treating, mitigating, or curing a disease (63) but may provide basic information about the mechanism by which the supplement is intended to “affect the structure or function of humans,” provided that the FDA disclaimer, “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.” is included (64). The boundaries of such regulation are frequently tested, particularly when non-human data are available. For instance, resveratrol is frequently advertised as a weight loss product, a claim that is likely inspired by a study that employed very high doses in mice (21). Notably, none of the available studies have reported weight loss in humans, and a lower dose, more comparable with those obtained using supplements, actually caused weight gain in mice (39). Nutraceuticals may go through the process of becoming a “botanical drug product” and upon approval, be labeled for treating a disease and sold as an over-the-counter drug (63). Botanical drugs have stricter regulations than dietary supplements but still do not face the scrutiny of prescription drugs (63). On the bottom line, both government and consumers need to strongly encourage better clinical research on nutraceuticals to identify safe and effective compounds or blends.
Understanding Mechanisms of Action: How Much Knowledge Is Enough?
One common concern with many drugs and supplements is that the exact mechanism of action is often unknown. From a clinical standpoint, a thorough understanding of mechanism of action is not necessarily required for widespread use. This is best exemplified in the use of general anesthetics, which are not fully understood (65–68) yet are an essential component of modern medicine. Nonetheless, the lack of mechanistic knowledge in such cases increases the risk of unexpected adverse events. Resveratrol is a prime example of a widely used nutraceutical with a complexity of mechanisms responsible for its benefits and potential risks. Although much is known about mechanisms that contribute to resveratrol’s effects, our understanding of its actions continues to rapidly evolve (18,19). The many benefits of resveratrol may be attributable to general effects as antioxidant activity (69), anti-inflammatory activity (70), modulation of drug-metabolizing enzymes (71), and more specific effects on proteins or signaling cascades, such as activation of SIRT1 (72) and AMPK (73), inhibition of cyclooxygenases (74), and a host of other direct effects (eg, Table 1). Many of these enzymes themselves play complex roles in regulating multiple aspects of physiology, which in part may explain the multiple effects observed for resveratrol. For example, SIRT1 controls a number of other key regulatory factors associated with metabolism and inflammation, including p53, FoxO1, NF-κB, and PGC-1α (75). This complexity makes it difficult to pinpoint the events mediating beneficial effects of resveratrol or to prospectively identify potential adverse effects. Nutraceutical research can benefit from certain aspects of pharmaceutical research to better understand mechanisms of action. One of the most effective tools to consider in advancing nutraceutical research is high-throughput in vitro bioassays (76), which have regularly been successfully employed in identifying natural compounds with promise for cancer chemoprevention while also providing safety information (54). Such screening methods were also employed to identify a series of molecules, including resveratrol, as SIRT1 activators (13). However, the ultimate proof for both safety and efficacy must lie in well-controlled clinical studies.
Table 1.
Some Activities of Resveratrol
| General Activities | Specific Proteins/Pathways |
| Suppresses inflammation | Upregulates Fas pathway |
| Has antioxidant effects | Inhibits Rb/E2FDP pathway |
| Acutely inhibits mitochondria | Activates p53 pathway |
| Induces mitochondrial biogenesis | Activates adenylyl-cyclase pathway |
| Modulates xenobiotic metabolism | Inhibits NF-κB pathway |
| Suppresses angiogenesis | Inhibits AP-1 pathway |
| Induces apoptosis | Regulates Egr-1 pathway |
| Inhibits cell proliferation | Inhibits MAPK pathway |
| Suppresses mutagenesis | Inhibits protein kinase C |
| Inhibits COX-2 | |
| Inhibits lipooxygenases | |
| Activates Sirt1 | |
| Activates AMPK | |
| Modulates estrogen receptors |
Are Data From Experimental Animals Enough When Human Data Are Not Available?
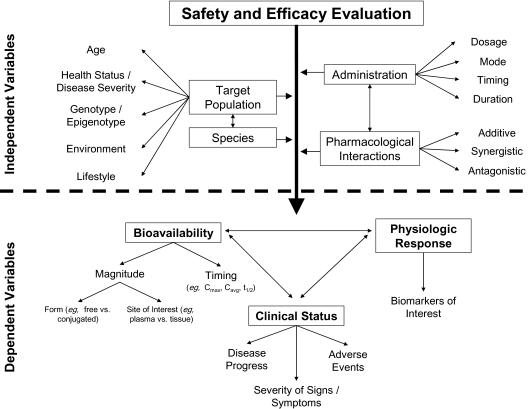
While in vitro and laboratory animal studies are critical phases for testing safety and efficacy during preclinical drug development, these models do not perfectly foretell their ultimate success in human medicine. Rodents and other laboratory animals approximate some aspects of human physiology and can serve as models for invasive and highly controlled research, which could not be reasonably be performed using human volunteers, but the results obtained from such experiments should not be equated with human clinical data. Outcomes and interpretation vary considerably with the disease of interest and laboratory model used (77,78), as well as many other factors that ultimately influence dependent variables (Figure 2). Though interspecies differences in physiology are unavoidable, experimental design may be improved to maximize the validity of the animal studies (78,79). In the past 5 years, resveratrol supplementation has provided some impressive results in rodent models, whereas the human research in this realm is in its infancy.
Figure 2.
Factors influencing safety and efficacy. A difference in any of the independent variables (above the dotted line) can potentially influence any of the dependent variables.
The complex issues involved in extrapolating from rodents to humans may be exemplified through study of resveratrol’s effects on mitochondrial biogenesis. Because exercise increases mitochondrial biogenesis through a SIRT1-dependent mechanism (21,80), one might predict that resveratrol supplementation would promote mitochondrial biogenesis, and this has indeed been observed in mice (21). Although these data have prompted the use of resveratrol among human athletes, there is currently insufficient evidence to support or refute an effect in humans due to lack of research in this area. Likewise, quercetin has been demonstrated to increase markers of muscle and brain mitochondrial biogenesis, as well as endurance performance, in mice (81) but such has not been observed in humans (82,83). Interestingly, despite the lack of effect on mitochondrial biogenesis, increased exercise performance has been reported in some (83,84), but not all (85) studies of humans taking quercetin. When discrepancy exists between animal and human studies, it may be due to true species differences in physiological responses or it may simply reflect dissimilar experimental designs. The underwhelming evidence for grapeskin polyphenols to promote mitochondrial biogenesis in humans, based on quercetin research (82,83), may reflect opposing mechanisms of action. Resveratrol is thought to increase mitochondrial biogenesis through increasing SIRT1 activity, which ultimately increases PGC-1α activity (86). However, resveratrol’s inherent antioxidant property should attenuate oxidative stress, which itself is a major component of the hormetic response driving mitochondrial biogenesis (87,88). There is direct evidence that supplementation with other antioxidants impairs the hormetic response, thus impairing mitochondrial biogenesis (89,90), though this issue remains controversial. If resveratrol does in fact induce opposing effects on mitochondrial biogenesis pathway, the end response may be dependent upon multiple factors that cannot be accurately modeled in rodents, such as supplement timing, genetic variation, overall oxidative stress, and the subjects’ physical conditioning.
Clearly, animal data are not sufficient to determine the safety and efficacy of a drug in humans, and we would argue that short-term safety trials in humans are not enough either. Recalls of major pharmaceutical products illustrate the need for ongoing testing and evaluation of any molecule that is administered to humans. For example, Avandia (rosiglitazone) promotes insulin sensitivity in diabetic patients (91) but may also increase the risk for cardiovascular disease (92,93). Rare or mild adverse effects are often impossible to predict or detect based on animal studies, and require analysis of thousands of clinical cases to prove (94). Unfortunately, safety data for dietary supplements are often collected during efficacy studies (95), or aggregated from poison control centers following adverse events (58), with little effort to follow-up in larger populations. For example, it took decades to link Ephedra to cardiovascular-related deaths, which ultimately resulted in its removal from the market in 2004 (96). Moreover, supplements can have potentially dangerous drug interactions (97,98), which may not be adequately tested during formal clinical trials. Although there have been very few adverse events reported following resveratrol supplementation in healthy humans (42,99), there is a clear need to remain vigilant as larger populations become exposed to this, or any other nutraceutical.
Non-human studies should continue to examine the physiological mechanisms that may ultimately lead to increased risks in humans, especially in cases where there is existing evidence of such risk. In the case of resveratrol, it would be prudent to further explore the potential for renal toxicity in animal models, given the recent incidents with SRT501 in multiple myeloma patients, and to investigate the molecule’s effects on the gastrointestinal system in greater detail, given reports of gastrointestinal side effects (42). Such studies may not fully prevent adverse events but can potentially allow for more focused research in human subjects and more vigilant monitoring for potential danger, or even lead to new uses. As a hypothetical example, perhaps gastrointestinal symptoms caused by resveratrol are rooted in a heretofore-unknown mechanism of increasing gastric motility, which could be useful in the treatment of gastrointestinal disorders. However, these examples also illustrate the importance of controlled and cautious testing in humans, which cannot be replaced by any amount of animal data. We further suggest that more extensive use of species with a physiology closer to that of humans, such as pigs, would provide valuable insight prior to the completion of full clinical testing.
Improving Research Design to Optimize Clinical Relevance
The emerging data from human clinical trials suggest that resveratrol may have benefits on human health. However, even clinical trials that confirm effectiveness relative to placebo are not sufficient to warrant the use of resveratrol in humans because the real question is one of comparative efficacy to other available treatments (100). This is especially important from a clinical standpoint, given that cost-effectiveness is a key component of effective health care but is not often considered in clinical trials (101). As addressed earlier, this issue is complicated by the many formulations available, as well as unresolved questions about optimal dosage. Furthermore, nutraceuticals (including resveratrol) are often suggested to prevent the development of a disease, rather then serve as a primary treatment, and therefore require long-term intervention studies to prove efficacy. Other important issues for researchers include selecting appropriate end points when testing efficacy and considering variation between subjects. This is exemplified by the case of thiazolidinedione diabetic drugs, which show single-nucleotide polymorphism-dependent changes in insulin sensitivity but not fasting glucose (102). Additionally, laboratory animal researchers should ensure their experimental design mimics the real-life conditions of human patients as closely as possible to maximize translational carryover. For instance, resveratrol has been demonstrated to improve glucose tolerance in mice, but human diabetics often have a number of comorbidities, such as obesity, cardiovascular disease, and neuropathy that should be considered when evaluating laboratory models. If a nutraceutical or drug is efficacious for treating one aspect of a disease, but exacerbates a comorbidity, this should be taken into consideration. SRT501’s issues may serve as a cautionary tale in this regard. This does not necessarily preclude the use of such nutraceuticals or drugs but emphasizes the need to preemptively address potential risks.
Starting Points to Address These Problems
Evidence continues to emerge in support of the benefits of resveratrol and other natural compounds; however, there is a major void in research directly comparing nutraceuticals to prescription drugs. Given the skepticism toward industry-funded research, there is a need for independent unbiased research in this realm; however, there are many hurdles to overcome. The issues we have been discussing are not easily resolved and may ultimately require innovations that we have not foreseen. However, we would like to offer the following suggestions as starting points to address the problem:
High-throughput molecular modeling—Although accurately fitting a small molecule to the entire proteome is well beyond our current knowledge or computational power, continuing to push technology toward this ultimate goal and exploiting software that is currently available to compliment in vivo studies should be a high priority.
Standardized screens for common toxic or biologically relevant effects—Compounds submitted to the FDA are routinely put through a standard series of tests for toxicity before any further action is taken; yet, no such standards exist for molecules that fall outside of FDA oversight (ie, nutraceuticals). The funding of grants to develop high-throughput screens could lead to the creation of online databases of receptor-binding activities, cell killing, and key transcriptional effects for hundreds of thousands of molecules.
Purification and identification of drug-interacting proteins—This method has been applied to resveratrol to identify quinone reductase 2 and many other targets that are still being characterized (103). In principle, it should be possible to attach any molecule of interest to a column and elute interacting proteins under various conditions. Further investment in this area should bring costs down and reliability up, making it suitable for more routine use.
Better tracking of supplement usage—Clinical trials for other purposes rarely record detailed information on supplement usage (dosage, brand, frequency of use). Larger cohort studies may record supplement usage, but to our knowledge, there has been no systematic attempt to extract information on resveratrol or most other molecules beyond vitamins, fish oil, and antacids.
Regulation to promote or require the release of clinical trial data—We believe that the public has a right to the most up-to-date information that is available to assist them in making decisions about whether or not to take any pharmaceutical or nutraceutical. Unfortunately, data from clinical trials are treated as proprietary information, preventing the free flow of potentially crucial information. We believe that it should be the position of the FDA and similar organizations in other countries that enrolling patients from the general population requires full disclosure of results to the general population.
Improved communication between laboratory animal researchers, human researchers, and clinicians—We believe that translational research is a valuable component of nutraceutical development, despite its potential limitations, but that it must be carefully planned to optimize its ultimate impact on human medicine. A coordinated effort with a logical sequence of valid research studies must be developed to ensure that laboratory animal research can effectively guide the progress of human medical research.
Greater studies focusing on comparative efficacy—Future research should not simply examine whether or not a given nutraceutical product is efficacious but rather how it compares to alternative treatments. Claims of synergism or improved formulations should require comparison with a standardized extract or pure compound, which would also facilitate comparisons between studies. These changes would greatly assist the scientific and medical communities in establishing the clinical relevance of nutraceuticals.
Conclusions
Establishing the chronic effects of any molecule in humans is difficult, time consuming, and expensive. There is very little precedent for successfully creating an evidence-based therapeutic intervention outside of large pharmaceutical companies, and even molecules produced by such companies frequently perform more poorly than expected or have side effects. Despite the large effort that has been put forth by the international research community to understand the mechanisms of resveratrol’s beneficial effects, the precise dosing, risks, and outcomes in humans remain uncertain. Resveratrol itself may have enough momentum to carry it through additional human trials that will provide more satisfying data on safety and efficacy, but it is only one of approximately 8,000 polyphenols found in our food and of perhaps hundreds of thousands of potentially beneficial molecules. Effectively evaluating large numbers of these molecules, or even defining the precise in vivo mechanism of resveratrol’s effects, is likely to require the development of new paradigms and approaches that can deal with multiple targets and multiple outcomes. There remain many challenges that need to be overcome to ensure that potentially beneficial medicines are made available efficiently and safely.
FUNDING
Joseph Baur is partially supported by the National Institute on Aging, National Institutes of Health (R00 AG031182).
References
- 1.Feral CC, Neels JG, Kummer C, Slepak M, Olefsky JM, Ginsberg MH. Blockade of alpha4 integrin signaling ameliorates the metabolic consequences of high-fat diet-induced obesity. Diabetes. 2008;57:1842–1851. doi: 10.2337/db07-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu M, Patsouris D, Li P, et al. A new antidiabetic compound attenuates inflammation and insulin resistance in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2010;298:E1036–E1048. doi: 10.1152/ajpendo.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Suarez E, Geserick C, Flores JM, Blasco MA. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24:2256–2270. doi: 10.1038/sj.onc.1208413. [DOI] [PubMed] [Google Scholar]
- 7.Elsea SH, Lucas RE. The mousetrap: what we can learn when the mouse model does not mimic the human disease. ILAR J. 2002;43:66–79. doi: 10.1093/ilar.43.2.66. [DOI] [PubMed] [Google Scholar]
- 8.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalra EK. Nutraceutical—definition and introduction. AAPS PharmSci. 2003;5:E25. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 11.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Eno Vitic. 1992;43:49–52. [Google Scholar]
- 12.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 13.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 14.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 16.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 19.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Das M, Das DK. Resveratrol and cardiovascular health. Mol Aspects Med. 2010;31:503–512. doi: 10.1016/j.mam.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila). 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm Res. 2009;26:211–217. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 25.Csiszar A, Labinskyy N, Olson S, et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Fidalgo S, Cardeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Vang O, Das DK. Resveratrol and health. Ann N Y Acad Sci. 2011;1215 [Google Scholar]
- 28.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE. doi: 10.1371/journal.pone.0019881. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 30.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Hang T, Wu C, Liu W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:97–106. doi: 10.1016/j.jchromb.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Almeida L, Vaz-da-Silva M, Falcao A, et al. Pharmacokinetic and safety profile of transresveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 33.Boocock DJ, Patel KR, Faust GE, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 35.Vitaglione P, Sforza S, Galaverna G, et al. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 36.Waite KA, Sinden MR, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum Mol Genet. 2005;14:1457–1463. doi: 10.1093/hmg/ddi155. [DOI] [PubMed] [Google Scholar]
- 37.Pearce VP, Sherrell J, Lou Z, Kopelovich L, Wright WE, Shay JW. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene. 2008;27:2365–2374. doi: 10.1038/sj.onc.1210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabrese EJ, Mattson MP, Calabrese V. Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol. 2010;29:980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- 39.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley J, Das S, Mukherjee S, Das DK. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–452. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 42.Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majumdar AP, Banerjee S, Nautiyal J, et al. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer. 2009;61:544–553. doi: 10.1080/01635580902752262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikstacka R, Rimando AM, Ignatowicz E. Antioxidant effect of trans-resveratrol, pterostilbene, quercetin and their combinations in human erythrocytes in vitro. Plant Foods Hum Nutr. 2010;65:57–63. doi: 10.1007/s11130-010-0154-8. [DOI] [PubMed] [Google Scholar]
- 47.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila). 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 48.Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, Salbego C, Lenz G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009;100:1655–1662. doi: 10.1111/j.1349-7006.2009.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park HJ, Yang JY, Ambati S, et al. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008;11:773–783. doi: 10.1089/jmf.2008.0077. [DOI] [PubMed] [Google Scholar]
- 50.Revel A, Raanani H, Younglai E, et al. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 51.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iggo N, Winearls CG, Davies DR. The development of cast nephropathy in multiple myeloma. QJM. 1997;90:653–656. doi: 10.1093/qjmed/90.11.653. [DOI] [PubMed] [Google Scholar]
- 53.Loftus P. Glaxo Ends Study of ‘Red-Wine’ Drug, No More Studies Planned The Wall Street Journal. New York: Dow Jones Newswires; 2010. [Google Scholar]
- 54.Pezzuto JM. Resveratrol as an inhibitor of carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 55.Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2010;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 57.McGauran N, Wieseler B, Kreis J, Schuler YB, Kolsch H, Kaiser T. Reporting bias in medical research—a narrative review. Trials. 2010;11:37. doi: 10.1186/1745-6215-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardiner P, Sarma DN, Low Dog T, et al. The state of dietary supplement adverse event reporting in the United States. Pharmacoepidemiol Drug Saf. 2008;17:962–970. doi: 10.1002/pds.1627. [DOI] [PubMed] [Google Scholar]
- 59.Kennedy J. Herb and supplement use in the US adult population. Clin Ther. 2005;27:1847–1858. doi: 10.1016/j.clinthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Gardiner P, Graham R, Legedza AT, Ahn AC, Eisenberg DM, Phillips RS. Factors associated with herbal therapy use by adults in the United States. Altern Ther Health Med. 2007;13:22–29. [PubMed] [Google Scholar]
- 61.Rietjens IM, Slob W, Galli C, Silano V. Risk assessment of botanicals and botanical preparations intended for use in food and food supplements: emerging issues. Toxicol Lett. 2008;180:131–136. doi: 10.1016/j.toxlet.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 62.Boon H, Hirschkorn K, Griener G, Cali M. The ethics of dietary supplements and natural health products in pharmacy practice: a systematic documentary analysis. Int J Pharm Pract. 2009;17:31–38. doi: 10.1211/ijpp.17.1.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rockville MD FDA. Guidance for Industry: Botanical Drug Products. U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 64.U.S. Department of Health and Human Services. Dietary Supplement Health and Education Act of 1994. http://www.health.gov/dietsupp/ch1.htm. Accessed March 2, 2011. [Google Scholar]
- 65.Mashour GA, Forman SA, Campagna JA. Mechanisms of general anesthesia: from molecules to mind. Best Pract Res Clin Anaesthesiol. 2005;19:349–364. doi: 10.1016/j.bpa.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Saniova B, Drobny M. Consciousness and general anaesthesia: a recent view. Neuro Endocrinol Lett. 2008;29:822–830. [PubMed] [Google Scholar]
- 68.Ishizawa Y. Mechanisms of anesthetic actions and the brain. J Anesth. 2007;21:187–199. doi: 10.1007/s00540-006-0482-x. [DOI] [PubMed] [Google Scholar]
- 69.Aftab N, Likhitwitayawuid K, Vieira A. Comparative antioxidant activities and synergism of resveratrol and oxyresveratrol. Nat Prod Res. 2010;24:1726–1733. doi: 10.1080/14786410902990797. [DOI] [PubMed] [Google Scholar]
- 70.Bereswill S, Munoz M, Fischer A, et al. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5:e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an auto-feedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010;59:551–553. doi: 10.2337/db09-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wendeburg L, de Oliveira AC, Bhatia HS, Candelario-Jalil E, Fiebich BL. Resveratrol inhibits prostaglandin formation in IL-1beta-stimulated SK-N-SH neuronal cells. J Neuroinflammation. 2009;6:26. doi: 10.1186/1742-2094-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uttamchandani M, Wang J, Yao SQ. Protein and small molecule microarrays: powerful tools for high-throughput proteomics. Mol Biosyst. 2006;2:58–68. doi: 10.1039/b513935j. [DOI] [PubMed] [Google Scholar]
- 77.Perel P, Roberts I, Sena E, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334:197. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I. Where is the evidence that animal research benefits humans? BMJ. 2004;328:514–517. doi: 10.1136/bmj.328.7438.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Jong M, Maina T. Of mice and humans: are they the same? Implications in cancer translational research. J Nucl Med. 2010;51:501–504. doi: 10.2967/jnumed.109.065706. [DOI] [PubMed] [Google Scholar]
- 80.Dumke CL, Mark Davis J, Angela Murphy E, et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol. 2009;107:419–427. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 81.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 82.Nieman DC, Henson DA, Maxwell KR, et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc. 2009;41:1467–1475. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 83.Nieman DC, Williams AS, Shanley RA, et al. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2010;42:338–345. doi: 10.1249/MSS.0b013e3181b18fa3. [DOI] [PubMed] [Google Scholar]
- 84.Davis JM, Carlstedt CJ, Chen S, Carmichael MD, Murphy EA. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int J Sport Nutr Exerc Metab. 2010;20:56–62. doi: 10.1123/ijsnem.20.1.56. [DOI] [PubMed] [Google Scholar]
- 85.Bigelman KA, Fan EH, Chapman DP, Freese EC, Trilk JL, Cureton KJ. Effects of six weeks of quercetin supplementation on physical performance in ROTC cadets. Mil Med. 2010;175:791–798. doi: 10.7205/milmed-d-09-00088. [DOI] [PubMed] [Google Scholar]
- 86.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji L, Dickman JR, Kang C, Koening R. Exercise-induced hormesis may help healthy aging. Dose Response. 2010;8:73–79. doi: 10.2203/dose-response.09-048.Ji. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji L, Gomez-Cabrera M-C, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 89.Gomez-Cabrera M-C, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 90.Teixeira VH, Valente HF, Casal SI, Marques AF, Moreira PA. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med Sci Sports Exerc. 2009;41:1752–1760. doi: 10.1249/MSS.0b013e31819fe8e3. [DOI] [PubMed] [Google Scholar]
- 91.Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabet Med. 1999;16:179–192. doi: 10.1046/j.1464-5491.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 92.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 93.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 94.Cohen D. Rosiglitazone: what went wrong? BMJ. 2010;341:c4848. doi: 10.1136/bmj.c4848. [DOI] [PubMed] [Google Scholar]
- 95.Berman J. Clinical development of dietary supplements: the perils of starting at phase III. Fitoterapia. 2010:80–84. doi: 10.1016/j.fitote.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Woolf AD, Watson WA, Smolinske S, Litovitz T. The severity of toxic reactions to ephedra: comparisons to other botanical products and national trends from 1993-2002. Clin Toxicol (Phila). 2005;43:347–355. doi: 10.1081/clt-200066075. [DOI] [PubMed] [Google Scholar]
- 97.Cohen PA, Ernst E. Safety of herbal supplements: a guide for cardiologists. Cardiovasc Ther. 2010;28:246–253. doi: 10.1111/j.1755-5922.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald L, Foster BC, Akhtar H. Food and therapeutic product interactions—a therapeutic perspective. J Pharm Pharm Sci. 2009;12:367–377. doi: 10.18433/j30p4c. [DOI] [PubMed] [Google Scholar]
- 99.Boocock DJ, Faust GES, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemoprotective agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 100.O’Connor AB. Building comparative efficacy and tolerability into the FDA approval process. JAMA. 2010;303:979–980. doi: 10.1001/jama.2010.257. [DOI] [PubMed] [Google Scholar]
- 101.Allan GM, Korownyk C, LaSalle K, et al. Do randomized controlled trials discuss healthcare costs? PLoS One. 2010;5:e12318. doi: 10.1371/journal.pone.0012318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Distefano JK, Watanabe RM. Pharmacogenetics of anti-diabetes drugs. Pharmaceuticals (Basel). 2010;3:2610–2646. doi: 10.3390/ph3082610. [DOI] [PMC free article] [PubMed] [Google Scholar]