Abstract
Carotid angioplasty is associated with adverse events in elderly patients; it is unclear whether this is related to an altered inflammatory axis. The carotid arteries of young (6 months) or aged (22–24 months) Fischer 344 rats were balloon injured. Aged rats had reduced lumen area (0.18 ± 0.03 vs 0.24 ± 0.01 mm2, p = .02) and increased neointimal thickening (0.15 ± 0.04 vs 0.08 ± 0.03 mm2, p = .006). Aged rats had increased circulating monocytes (96 ± 21 vs. 54 ± 7; p = .002) as well as increased numbers of monocytes at the post-angioplasty site. Aged rats had sustained monocyte chemotactic protein-1 expression after angioplasty but young rats did not. Aged arteries also exhibited defective vasorelaxation and abnormal eNOS localization. Aged (≥80 years) human patients with high-grade carotid stenosis had increased number of monocytes (9.1% ± 0.4%) compared with younger (65–80 years) patients (8.1% ± 0.3%, p = .013). Aged rats develop neointimal hyperplasia after carotid angioplasty with increased numbers of monocytes, and elderly humans with carotid stenosis have increased numbers of circulating monocytes. These preliminary results may suggest a role for monocytes in the response to carotid angioplasty.
Keywords: Carotid angioplasty, Balloon angioplasty, Aging, Monocyte, MCP-1
Despite technological advances in carotid artery angioplasty and stenting (CAS), elderly patients continue to have inferior outcomes after CAS in comparison to younger patients, with higher rates of stroke (1–6). For example, the Carotid Revascularization Endarterectomy versus Stenting Trial showed that elderly patients had an approximately eightfold increased risk of complications compared with younger patients (1,2). Because increasing age is a major risk factor for the development of vascular disease and the numbers of elderly patients continue to increase, the question as to how to best treat carotid disease in elderly patients remains unanswered and controversial (7–10).
Lumen reduction and restenosis following arterial injury are mediated by both neointimal hyperplasia as well as inward remodeling (11,12). Neointimal hyperplasia reduces lumen area by increasing the area of the intima, delimited by the internal elastic lamina, via a space-occupying lesion; the immediate response to injury induces a cascade of cytokine and inflammatory mediators from platelets, endothelial, and smooth muscle cells (SMC), stimulating SMC proliferation and migration into the intima, synthesizing, and secreting extracellular matrix to increase the intimal area with both cells and matrix (13–17). However, lumen reduction may also be mediated by “inward” or “negative” remodeling, the reduction in vessel size as measured by smaller area delimited by the external elastic lamina. Inward vessel remodeling is an endothelial-dependent compensatory response to chronic low blood flow and shear stress (18–21); multiple studies suggest that inward remodeling is a significant factor and must be treated successfully to address clinically significant restenosis (22–28).
Few studies address the effects of aging on neointimal hyperplasia and inward remodeling. Torella and colleagues (29) reported the results of balloon angioplasty performed in young and aged Wistar rats. This study showed that aged Wistar rats have reduced neointima but significantly increased inward remodeling compared with young rats; in addition, aged rats have reduced rates of endothelialization, reduced Akt and eNOS activation and expression, and increased apoptosis, suggesting that aging decreases the capacity of vascular arterial cells to survive and proliferate after injury (29). However, this study does not suggest a mechanism by which elderly patients have increased adverse events after CAS. In addition, it is unclear whether results in Wistar rats are applicable to humans; studies using the Fischer 344 rat, a commonly used and validated animal model of aging (30), have shown increased neointimal hyperplasia after endothelial injury in aged compared with young rats (31).
We have previously shown that elderly patients have increased arch calcification, a potential long-term result of inflammation within the vascular wall (32). In addition, we have previously shown that symptomatic carotid plaques are inflammatory (33). Therefore, we hypothesized that the increased number of adverse events after CAS in elderly patients may be related to increased inflammatory cells at the site, leading to a more friable post-angioplasty site. It has been previously reported that aged arteries and SMC contain more monocyte chemotactic protein (MCP)-1 and CC chemokine receptor-2 (CCR-2) mRNA and protein compared with younger arteries (34). MCP-1 is a CC chemokine expressed by macrophages, endothelial cells, and SMC that binds to CCR-2 (35,36). The role of MCP-1 and its receptor CCR-2 in neointimal formation have been extensively studied in experimental models of mechanical vascular injury (37). Therefore, we examined whether the transcription of MCP-1 and CCR-2 is differentially altered in young and aged animals after balloon angioplasty and whether these alterations are associated with differential deposition of monocytes at the angioplasty site.
MATERIALS AND METHODS
Animals
All experiments were approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine. Young (6-months) and aged (22-24-months) Fischer 344 rats were obtained from the National Institute of Aging Rodent Colony (Bethesda, MD). Rats were raised in pathogen-free conditions at the National Institute of Aging and housed locally at least 1 week prior to surgery. Rat balloon angioplasty was performed, as previously described (38). Briefly, under general anesthesia, the right internal and external carotid arteries were exposed and a 2F Fogarty balloon catheter was inserted in retrograde fashion via an arteriotomy made in the right external carotid artery. The balloon was inserted into the appropriate position of the common carotid artery, and the balloon was inflated to 2 atmospheres for 1 minute and then deflated for 1 minute and then repeated for a total of three times. The balloon was removed and the external carotid artery ligated. The injured carotid artery was harvested and examined 3 days or 2 weeks postoperatively. In some animals, samples of whole blood were collected from the heart immediately prior to specimen harvesting, as previously described (39). Contralateral common carotid arteries were used for control.
Histological Analysis
For histological analysis, animals were exsanguinated under anesthesia and specimens were collected using pressure perfusion fixation, as previously described (39). The harvested carotid artery was fixed in 10% formalin and embedded in paraffin. All sections were stained with hematoxylin and eosin as well as Masson’s trichrome stain. For immunohistochemical staining, all sections were treated with Proteinase K solution for antigen retrieval, subsequently permeabilized with 0.3% Triton X-100 phosphate-buffered saline, and blocked with 3% normal goat serum containing phosphate-buffered saline. Sections were then incubated with the primary antibody: Monoclonal Anti Alpha Smooth Muscle Actin (Sigma–Aldrich), non-muscle Myosin Heavy Chain (NM-MHC; Abcam), or F4/80 (AbD Serotec). Immunohistochemical detection was performed using DAB as well as NovaRED substrate (Vector). Sections were counterstained with Mayer’s hematoxylin. Morphological analysis was measured by computer morphometry using Image J software (NIH), and signal density of immunohistochemical stains was measured in blinded fashion using Metamorph software (Molecular Devices, Sunnyvale, CA).
In Situ Whole Mount Staining
Whole mount staining for en face observation was performed, as previously described (40). The common carotid arteries were harvested and fixed with cooled 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 phosphate-buffered saline, and blocked with 3% normal goat serum containing phosphate-buffered saline. The arteries were probed with rabbit anti-eNOS antibody and mouse anti-GM130 antibody (BD Transduction Laboratories). Alexa Fluor 488 anti-rabbit IgG and 568 anti-mouse IgG (Invitrogen) were used as secondary antibodies. Stained carotid arteries were opened under a dissection microscope and immediately captured images with an Axioimager A1 (Carl Zeiss) under identical conditions.
Real-Time Reverse Transcription–Polymerase Chain Reaction
The harvested carotid artery was immediately processed by snap freezing with liquid nitrogen and transferred to −80°C storage. Total RNA was extracted by TRIzol Reagent (Invitrogen), and RNA was cleaned using the RNeasy Mini Kit with DNase-I (Qiagen) following the manufacturer’s instructions. Total RNA concentration and purity were measured with a spectrophotometer with absorbance at 260 nm. RNA quality was confirmed by the 260/280 nm absorbance ratio.
First strand cDNA was synthesized by Superscript III Reverse Transcriptase (Invitrogen). Quantitative polymerase chain reaction was performed with SYBR Green Master Mix (Bio-Rad). Primers for genes of interest were designed by Primer-BLAST at the National Center for Biotechnology Information Web site (Table 1). Amplification specificity was confirmed by 2% agarose gel electrophoresis, and primer efficiencies were determined by melting curve analysis. All data were normalized by GAPDH as internal standard.
Table 1.
Primer Sequences for Real-Time Polymerase Chain Reaction
| Forward Primer | Reverse Primer | |
| MCP-1 | 5′-TGTCTCAGCCAGATGCAGTT-3′ | 5′-TTCCTTATTGGGGTCAGCAC-3′ |
| CCR-2 | 5′-CTGCCCCTACTTGTCATGGT-3′ | 5′-GGCCTGGTCTAAGTGCATGT-3′ |
| Ephrin-B2 | 5′-CTGTGCCAGACCAGACCA AGA-3′ | 5′-CAGCAGAACTTGCATCTTGTC-3′ |
| GAPDH | 5′-AGACAGCCGCATCTTCTTGT-3′ | 5′-CCACAGTCTTCTGAGTGGCA-3′ |
Measurement of Isometric Vessel Tension
The isometric tension was measured in uninjured young and aged common carotid arteries, as previously described (40). Dissected common carotid arteries were cut into 3 mm–long segments. The rings are suspended by two tungsten wires mounted in a vessel myograph system (Danish Myotechnologies). The arteries were bathed in oxygenated Krebs buffer and submitted to a resting tension of 3 mN. After equilibration, concentration–response curves for high potassium (KCl) and phenylephrine (PE; 10−9 to 10−4 mol/L) were generated. To study vasodilator responses, the rings were preconstricted with submaximal concentrations of KCl (35 mM) and PE (1 μM), and acetylcholine (Ach; 10−9 to 10−4 mol/L) was added at the plateau of the PE-induced contraction.
Human Monocyte Counts
After informed consent was obtained, serum was drawn from patients prior to undergoing carotid endarterectomy at St. Mary’s Hospital (Waterbury, CT). Patients were considered to be younger if they were less than 65 years old, older if they were 65–80 years old, and elderly if they were more than 80 years old. Monocyte counts were determined as part of the differential count using a Beckman Coulter LH780 Analyzer.
Statistical Analysis
Statistical analysis was carried out with StatView version 5.0 (SAS Institute, Cary, NC). Results are presented as mean value ± SEM, and statistical significance was determined using analysis of variance with post hoc tests analyzed by Fisher’s Protected Least Significant Difference (PLSD) test with the value of p ≤ .05 considered to be statistically significant.
RESULTS
Increased Neointimal Thickness After Angioplasty in Aged Rats
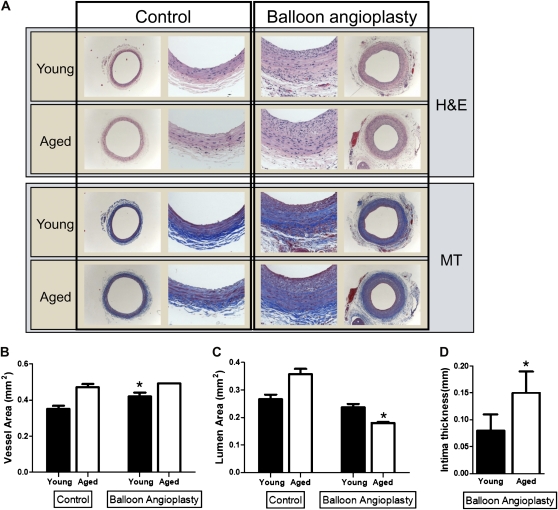
Beccause elderly human patients, that is, greater than 80 years old, have worse clinical outcome after balloon angioplasty of the carotid arteries compared with younger patients, the morphology of carotid arteries derived from young and aged Fischer 344 rats, before and after balloon angioplasty, was examined (Figure 1). At baseline, carotid arteries in aged rats were slightly larger than carotid arteries in young rats (Figure 1A and B). Two weeks after balloon angioplasty, both young and aged carotid arteries had similar vessel areas (aged: 0.49 ± 0.02 mm2; young: 0.4 ± 0.02 mm2; p = .2; n = 5–6; Figure 1A and B). However, after angioplasty, aged carotid arteries had significantly reduced lumen area (aged: 0.18 ± 0.03 mm2; young: 0.24 ± 0.01 mm2; p = .02; Figure 1A and C), corresponding to increased neointimal thickening compared with young carotid arteries (aged: 0.15 ± 0.04 mm2; young: 0.08 ± 0.03 mm2; p = .006; Figure 1A and D). The intima:media ratio was also increased in aged arteries compared with young arteries (0.94 vs 0.58). These results are consistent with preserved Glagov’s phenomenon in young but not aged carotid arteries, that is, aged arteries failed to remodel outwardly to preserve lumen blood flow (41).
Figure 1.
Effect of balloon angioplasty in young and aged Fischer 344 rats. (A) Representative photomicrographs, low, and high magnification of young and aged carotid arteries, both control arteries and arteries 2 weeks after balloon angioplasty. H&E, hematoxylin and eosin; MT, Masson’s trichrome. (B) Bar graph of vessel area in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 5–6; *p < .05. (C) Bar graph of lumen area in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 5–6; *p = .02. (D) Bar graph of intimal thickness in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 5–6; *p = .006.
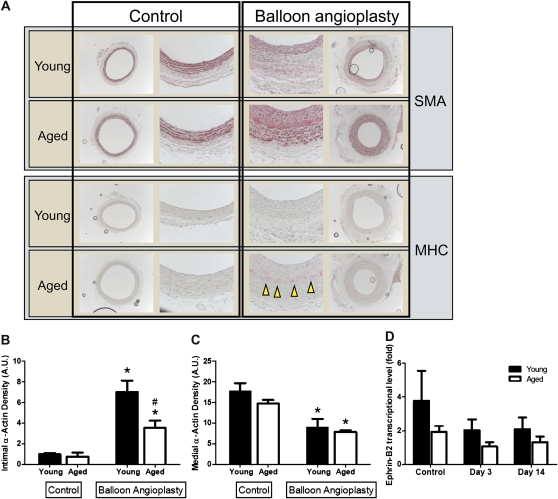
To determine the effect of balloon angioplasty on SMC in young and aged carotid arteries, the carotid arteries were examined with immunohistochemistry (Figure 2). There were no differences between young and aged SMC in control arteries examined by histology (Figure 2A–C). Following balloon angioplasty, there were increased numbers of intimal SMC in both young and aged arteries, although the density of alpha-actin staining was not as intense in the intima of aged arteries compared with young arteries (Figure 2B). This reduced intimal alpha-actin density in aged arteries may be due to the presence of immature NM-MHC–positive SMC in aged intima, whereas these immature SMC are not present in young intima [Figure 2A (42)]. The number of SMC was reduced in the media of both young and aged arteries, although there was no difference in the reduction between young and aged arteries (p = .07; Figure 2C). To confirm the reduction in SMC after angioplasty, we examined the expression of the arterial marker Ephrin-B2 before and after angioplasty (39,43,44). There was a trend toward reduced expression of Ephrin-B2 in both young and aged arteries after angioplasty (Figure 2D), which may reflect the loss of SMC after angioplasty observed with immunohistochemistry (Figure 2A–C); however, lack of statistical significance of reduced Ephrin-B2 expression may also reflect expression of Ephrin-B2 in endothelial cells, and we did not measure potential differences in the rates of post-angioplasty reendothelialization between young and aged rats. Taken together, these data show that the response to balloon angioplasty in aged Fischer 344 rats differs from that in younger rats, and these differences in aged rats, that is, increased neointima composed of immature SMC with concomitant failure to remodel outwardly, suggest structural changes in aged arteries that could be associated with adverse events after angioplasty.
Figure 2.
Effect of balloon angioplasty on SMC in young and aged Fischer 344 rats. (A) Representative photomicrographs, low, and high magnification of young and aged carotid arteries, both control arteries and arteries 2 weeks after balloon angioplasty. SMA, smooth muscle actin; MHC, myosin heavy chain. Arrowheads show presence of MHC reactivity. (B) Bar graph of intimal alpha-actin density in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 5–6; *p < .0001, #p = .002. (C) Bar graph of medial alpha-actin density in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 5–6; *p = .03. (D) Bar graph of Ephrin-B2 relative transcription level in young and aged carotid arteries in response to balloon angioplasty (2 weeks). Error bars denote SEM; n = 8; p = .08, p = .07 for reduction in post-angioplasty groups versus control in aged arteries. A.U., arbitrary units.
Increased Monocyte Infiltration in Aged Rats After Angioplasty
Since it has been previously reported that aged arteries and SMC contain more MCP-1 and CCR-2 mRNA and protein compared with younger arteries (34), we examined whether there were increased numbers of circulating monocytes in aged animals compared with younger animals. We have previously shown that young and aged Fischer 344 rats have similar heart rates, blood pressure, and cardiac output, as well as similar carotid artery diameter, flow, shear stress, and compliance at baseline, that is, prior to intervention (39). Examination of the blood count taken from young and aged rats before angioplasty is shown in Table 2. There was no difference in mean hemoglobin (14.7 ± 0.1 vs. 13.5 ± 0.7, p = .11) or platelet count (811 ± 30 vs. 887 ± 54, p = .26) between young and aged rats. The only significant difference was the elevated number of circulating monocytes present in aged rats (Table 2). Three days after angioplasty, there was a trend toward increased numbers of circulating monocytes in aged rats (5% ± 1.7%) compared with young rats (2% ± 0.5%), but this increase was not significant (p = .104). The increased number of circulating monocytes present in aged rats suggests that monocytes may play a role in different response to angioplasty in young and aged rats.
Table 2.
Values of Fischer 344 Rat Blood Testing (n = 6–7)
| Young | Aged | p Value | |
| WBC | 2,700 ± 327 | 2,543 ± 343 | .75 |
| Neutrophils (%) | 837 ± 116 | 901 ± 123 | .31 |
| 31 ± 3 | 36 ± 3 | ||
| Lymphocytes (%) | 1,763 ± 230 | 1,514 ± 241 | .24 |
| 65 ± 3 | 59 ± 3 | ||
| Monocytes (%) | 54 ± 7 | 96 ± 21 | .002 |
| 2 ± 0 | 3.6 ± 0.4 | ||
| Eosinophils (%) | 23 ± 5 | 19 ± 6 | .38 |
| 1 ± 0.3 | 0.7 ± 0.2 | ||
| Basophils (%) | 22 ± 5.6 | 12 ± 6 | .16 |
| 0.8 ± 0.2 | 0.4 ± 0.2 |
Note: WBC = white blood cell.
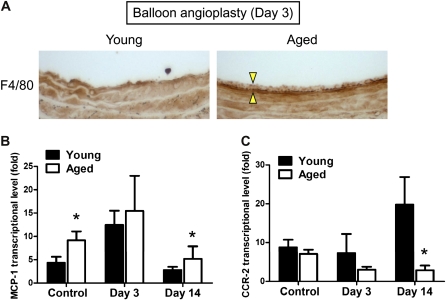
To determine whether there is a difference in infiltrating monocytes at the site of angioplasty, young and aged rat carotid arteries were examined 3 days after angioplasty. Immunohistochemistry showed increased numbers of monocytes at the site of angioplasty in aged rats (Figure 3A). Because aged rats have increased numbers of monocytes after angioplasty compared with young rats, we examined whether MCP-1, the monocyte protein that attracts monocytes into arteries, or its endothelial receptor CCR-2 were differentially expressed. There was increased basal expression of MCP-1 in aged rats compared with young rats (Figure 3B), consistent with the increased numbers of circulating monocytes in aged rats (Table 2). MCP-1 was increased in both young and aged rats early (Day 3) after balloon angioplasty; MCP-1 levels returned to baseline in young rats but remained elevated in aged rats (Figure 3B), consistent with the finding of increased monocytes at the site of angioplasty in aged rats (Figure 3A). On the other hand, basal expression of CCR-2, the receptor for MCP-1, was similar between young and aged rats at baseline (Figure 3C). After balloon injury, young arteries have increased expression of CCR-2 by Day 14, whereas aged arteries have diminished expression of CCR-2 (Figure 3C).
Figure 3.
Effect of balloon angioplasty on monocytes in young and aged Fischer 344 rats. (A) Representative photomicrographs of young and aged carotid arteries, 3 days after balloon angioplasty. Arrowheads show presence of F4/80 reactivity. (B) Bar graph of MCP-1 expression in young and aged carotid arteries in response to balloon angioplasty. Error bars denote SEM; n = 8. The difference between young and aged arteries is significant (p = .05, analysis of variance), both at control and Day 14 (p = .01, post hoc test). (C) Bar graph of CCR-2 expression in young and aged carotid arteries in response to balloon angioplasty. Error bars denote SEM; n = 8. The difference between young and aged arteries is significant (p = .01, analysis of variance), at Day 14 (p = 0.03, post hoc test) but not Day 3 (p = .41).
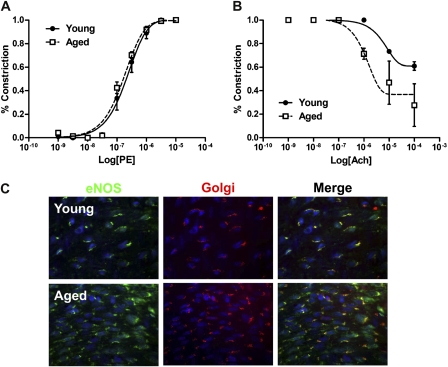
Because CCR-2 receptors are present on the arterial endothelium, diminished expression of CCR-2 after angioplasty in aged rats but not young rats suggests either significant endothelial dysfunction in aged endothelium or significant residual endothelial denudation after angioplasty in the aged arteries. To determine whether there was any basal endothelial dysfunction in aged arteries compared with young arteries, uninjured young and aged arteries were examined with a myograph. Both young and aged arteries showed endothelial-dependent constriction in response to phenylephrine (Figure 4A), but aged arteries showed significant impairment of vasorelaxation in response to acetylcholine (Figure 4B). These results suggest that endothelium is present at 28 days after balloon injury but that aged rats have basal endothelial dysfunction. To confirm endothelial dysfunction in aged arteries, we examined the localization of eNOS in young and aged arteries. Immunofluorescence showed that aged arteries had impaired localization of eNOS in aged arteries compared with young arteries, with reduced colocalization to the Golgi apparatus (Figure 4C) and caveolae (data not shown) in aged arteries. Abnormal localization of eNOS in aged endothelium is consistent with endothelial dysfunction in aged arteries.
Figure 4.
Abnormal endothelial function in aged arteries. (A) Vasoconstriction of uninjured young and aged arteries in response to phenylephrine (PE). Error bars denote SEM; n = 3. The difference between young and aged arteries is not significant (p = .47, paired t test). (B) Vasorelaxation of uninjured young and aged arteries in response to acetylcholine (Ach). Error bars denote SEM; n = 3. The difference between young and aged arteries is significant (p = .01, paired t test). (C) Representative photomicrographs of uninjured young and aged carotid arteries examined en face with immunofluorescence; n = 3.
Increased Circulating Monocytes in Elderly Humans With Carotid Stenosis
These results suggest that monocytes may play a role in the differential response of young and aged animals to balloon angioplasty. To determine whether this response may play a role in the human response to angioplasty, serum was evaluated from human patients with significant carotid stenosis prior to surgical intervention. Because clinical reports of inferior outcome of carotid angioplasty in elderly patients are generally cited for patients over 80 years old, patients were grouped into three groups: younger patients (<65 years old), older patients (65–80 years old), and elderly patients (>80 years old). The mean age of younger patients (n = 18) was 60.3 ± 1.3 years, older patients (n = 95) was 73.1 ± 0.5 years, and the mean age of elderly patients (n = 52) was 85.8 ± 0.5 years (p < .0001). Elderly human patients (>80) with carotid stenosis had increased number of circulating monocytes compared with older (65–80 years) patients (p = .025, post hoc test; Table 3). These results suggest that monocytes may also play a role in the differential response of younger and elderly humans following balloon angioplasty.
Table 3.
White Blood Cell Counts in Patients With Carotid Stenosis Prior to Undergoing Carotid Endarterectomy
| Younger Group (<65 y) | Older Group (65–80 y) | Elderly Group (>80 y) | p Value | |
| n | 18 | 95 | 52 | |
| WBC | 8.1 ± 0.5 | 7.4 ± 0.2 | 7.3 ± 0.3 | .38 |
| Neutrophils (%) | 64 ± 2.3 | 62 ± 1.2 | 63 ± 1.2 | .88 |
| Lymphocytes (%) | 23.0 ± 2.0 | 25.6 ± 1.0 | 23.5 ± 1.1 | .33 |
| Monocytes (%) | 9.7 ± 0.6 | 8.1 ± 0.3 | 9.1 ± 0.4 | .013 |
| Eosinophils (%) | 2.8 ± 0.4 | 3.1 ± 0.2 | 3.5 ± 0.3 | .41 |
| Basophils (%) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | .67 |
Note: p Value is the value of the analysis of variance test. WBC = white blood cell.
DISCUSSION
We show that aged Fischer 344 rats have increased neointimal hyperplasia after balloon injury compared with the neointima that forms in younger rats, with a neointima composed, at least in part, of immature SMC. In addition, aged rats exhibit increased circulating monocytes compared with young rats, and after angioplasty, there are increased numbers of monocytes found at the injured site, colocalizing with increased MCP-1 expression. Diminished CCR-2 expression in aged rats, abnormal vasorelaxation, and abnormal endothelial eNOS localization suggests basal endothelial dysfunction in aged arteries. Finally, aged human patients with carotid stenosis have increased numbers of circulating monocytes compared with younger patients.
Balloon injury induces SMC and adventitial fibroblasts to migrate into the intima, forming the neointimal lesion (45). A previous report examining the response to balloon injury in aged Wistar rats demonstrated negative remodeling with minimal neointimal hyperplasia (29). As this study was performed in Wistar rats, it is not clear whether this represents the typical response associated with aging. Studies of wire injury in the carotid artery of aged mice show increased neointimal hyperplasia, increased platelet-derived growth factor receptor-α expression, increased SMC proliferation in response to platelet-derived growth factor-BB, and diminished apoptosis compared with young mice (46). As such, the effects of aging on the response to arterial injury are not clearly established. We use the Fischer 344 rat, a validated animal model of aging that reflects some of the changes present during human aging (30). We show increased neointimal hyperplasia without negative remodeling in response to arterial injury (Figures 1 and 2), which agrees with the findings in mice (46). Of significance, all of these studies including this one show that aging is accompanied by a pathological remodeling response compared with that present in young adult arteries. As such, it is possible that the arterial environment may remodel abnormally after CAS when performed in elderly patients and may be a source of adverse events in elderly patients with other predisposing factors.
We believe that the host inflammatory axis is a critical aspect of the remodeling response. Regulation of inflammatory genes after balloon injury has been reported (47). Inflammation after balloon injury is likely to induce production of reactive oxygen species and endothelial dysfunction. As such, it is not surprising that adenovirus delivery of superoxide dismutase and catalase decreases restenosis after balloon angioplasty (48). Furthermore, our group has shown that flow-mediated arterial remodeling depends on superoxide-initiated MyD88-dependent inflammation (49). We have also recently shown that arterial remodeling in mice to hemodynamic stresses critically depends on IP-10– and CXCR3-dependent accumulation of macrophages (50). As such, the link between arterial remodeling and inflammation is becoming established. These studies support such a concept, in that aging is associated with abnormal vascular responses (Figure 4) as well as abnormal accumulation of monocytes at the site of injury (Figure 3). A critical role for monocytes in the development of restenosis after angioplasty has been shown (51).
In addition, our finding that aged rats have increased number of circulating monocytes prior to injury (Table 2) as well as basal arterial endothelial dysfunction (Figure 4) is also in agreement with this hypothesis; additional cell types, such as T cells or dendritic cells, should also be analyzed in future studies. Taken together, all these data suggest that strategies to control inflammation may promote more physiological remodeling after angioplasty in elderly patients and possibly diminish post-CAS events. It is tempting to speculate that such anti-inflammatory agents may improve the results of post-CAS remodeling just as statins have reduced post-CEA events (52).
We also demonstrate that elderly human patients (≥80 years old) with significant carotid stenosis have higher monocyte counts compared with patients 65–80 years old (Table 3). These data suggest that elderly human patients may have a different potential to remodel carotid plaques after angioplasty compared with patients <80 years old. Although we were not able to assess the monocyte counts of patients with carotid stenosis undergoing carotid angioplasty and stenting due to the expected extremely few numbers of elderly patients currently undergoing this procedure, we were able to assess a comparable group of patients, that is, those with carotid stenosis undergoing carotid endarterectomy. Therefore, we believe that these data are relevant to this study. Interestingly, younger patients, that is, patients with aggressive atherosclerotic disease, also had elevated circulating monocytes (Table 3); younger patients have also been noted to have increased restenosis after CEA (53,54). As such, both very young and elderly patients have inferior outcomes compared with the majority of middle-aged patients undergoing CEA, and both of these groups have elevated monocytes compared with the majority of patients. Future studies will need to prospectively assess the correlation of age and monocyte count with outcomes following the procedure.
We show that aged rats have increased numbers of monocytes incorporated into the vessel wall following balloon angioplasty and that elderly humans with carotid stenosis have increased numbers of circulating monocytes compared with younger patients. Correlation of animal data with human data should be made with caution; if these results can be repeated, then they suggest that monocytes may play a role in remodeling after carotid angioplasty and that monocytes may be relevant to the clinically worse results of elderly patients after this procedure.
FUNDING
This work was supported by the National Institute of Health (R01-HL095498-01 to A.D.); the American Vascular Association William J. von Liebig Award; as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
References
- 1.Hobson RB, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106–1111. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher ES, Malenka DJ, Solomon NA, Bubolz TA, Whaley FS, Wennberg JE. Risk of carotid endarterectomy in the elderly. Am J Public Health. 1989;79:1617–1620. doi: 10.2105/ajph.79.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winslow CM, Solomon DH, Chassin MR, Kosecoff J, Merrick NJ, Brook RH. The appropriateness of carotid endarterectomy. N Engl J Med. 1988;318:721–727. doi: 10.1056/NEJM198803243181201. [DOI] [PubMed] [Google Scholar]
- 5.Roubin GS, New G, Iyer SS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;103:532–537. doi: 10.1161/01.cir.103.4.532. [DOI] [PubMed] [Google Scholar]
- 6.Chastain HD, II, Gomez CR, Iyer S, et al. Influence of age upon complications of carotid artery stenting. UAB Neurovascular Angioplasty Team. J Endovasc Surg. 1999;6:217–222. doi: 10.1583/1074-6218(1999)006<0217:IOAUCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III. Cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 8.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. I. Aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LT, Cooke JP, Dzau VJ. The vasculopathy of aging. J Gerontol. 1994;49:B191–B196. doi: 10.1093/geronj/49.5.b191. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson TB, Adams MR, Weingand KW, Miller LC, Heydrick S. Effect of age on atherosclerosis progression in nonhuman primates. In: Bates SR, Ganghoff EC, editors. Atherogenesis and Aging. New York, NY: Spring-er-Verlag; 1987. pp. 57–71. [Google Scholar]
- 11.Kuntz RE, Gibson CM, Nobuyoshi M, Baim DS. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol. 1993;21(1):15–25. doi: 10.1016/0735-1097(93)90712-a. [DOI] [PubMed] [Google Scholar]
- 12.Paszkowiak JJ, Maloney SP, Kudo FA, et al. Evidence supporting changes in Nogo-B levels as a marker of neointimal expansion but not adaptive arterial remodeling. Vascul Pharmacol. 2007;46:293–301. doi: 10.1016/j.vph.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 14.Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997;40(2):107–116. doi: 10.1016/s0033-0620(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi N, Matsumori A, Furukawa Y, et al. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2000;20(12):2521–2526. doi: 10.1161/01.atv.20.12.2521. [DOI] [PubMed] [Google Scholar]
- 16.Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000;102(14):1697–1702. doi: 10.1161/01.cir.102.14.1697. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RS. Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am J Cardiol. 1998;81(7A):14E–17E. doi: 10.1016/s0002-9149(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 18.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 19.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5(3):413–420. [PubMed] [Google Scholar]
- 20.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16(10):1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 21.Tuttle JL, Nachreiner RD, Bhuller AS, et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281(3):H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 22.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239(1):H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- 23.Kohler TR, Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb. 1992;12(8):963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- 24.Kakuta T, Currier JW, Haudenschild CC, Ryan TJ, Faxon DP. Differences in compensatory vessel enlargement, not intimal formation, account for restenosis after angioplasty in the hypercholesterolemic rabbit model. Circulation. 1994;89(6):2809–2815. doi: 10.1161/01.cir.89.6.2809. [DOI] [PubMed] [Google Scholar]
- 25.Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89(6):2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- 26.Post MJ, de Smet BJ, van der Helm Y, Borst C, Kuntz RE. Arterial remodeling after balloon angioplasty or stenting in an atherosclerotic experimental model. Circulation. 1997;96(3):996–1003. doi: 10.1161/01.cir.96.3.996. [DOI] [PubMed] [Google Scholar]
- 27.Lafont A, Guzman LA, Whitlow PL, Goormastic M, Cornhill JF, Chisolm GM. Restenosis after experimental angioplasty. Intimal, medial, and adventitial changes associated with constrictive remodeling. Circ Res. 1995;76(6):996–1002. doi: 10.1161/01.res.76.6.996. [DOI] [PubMed] [Google Scholar]
- 28.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156(5):1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torella D, Leosco D, Indolfi C, et al. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am J Physiol Heart Circ Physiol. 2004;287:2850–2860. doi: 10.1152/ajpheart.01119.2003. [DOI] [PubMed] [Google Scholar]
- 30.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol. 2000;55A:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariri RJ, Alonso DR, Hajjar DP, Coletti D, Weksler ME. Aging and arteriosclerosis. I. Development of myointimal hyperplasia after endothelial injury. J Exp Med. 1986;164:1171–1178. doi: 10.1084/jem.164.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased aortic arch calcification in patients older than 75 years: implications for carotid artery stenting in elderly patients. J Vasc Surg. 2007;46(5):841–845. doi: 10.1016/j.jvs.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Bazan HA, Lu Y, Thoppil D, Fitzgerald TN, Hong S, Dardik A. Diminished omega-3 fatty acids are associated with carotid plaques from neurologically symptomatic patients: Implications for carotid interventions. Vascul Pharmacol. 2009;51(5-6):331–336. doi: 10.1016/j.vph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 35.Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- 36.Charo IF. CCR2: from cloning to the creation of knockout mice. Chem Immunol. 1999;72:30–41. doi: 10.1159/000058724. [DOI] [PubMed] [Google Scholar]
- 37.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 38.Indolfi C, Esposito G, Di Lorenzo E, et al. Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation. 1995;92(5):1230–1235. doi: 10.1161/01.cir.92.5.1230. [DOI] [PubMed] [Google Scholar]
- 39.Kudo FA, Muto A, Maloney SP, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 40.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204(10):2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 42.Aikawa M, Sakomura Y, Ueda M, et al. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96(1):82–90. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 43.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 44.Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 46.Vazquez-Padron RI, Lasko D, Li S, et al. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg. 2004;40(6):1199–1207. doi: 10.1016/j.jvs.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Li JM, Zhang X, Nelson PR, et al. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J Cell Biochem. 2007;101(2):399–410. doi: 10.1002/jcb.21190. [DOI] [PubMed] [Google Scholar]
- 48.Durand E, Al Haj Zen A, Addad F, et al. Adenovirus-mediated gene transfer of superoxide dismutase and catalase decreases restenosis after balloon angioplasty. J Vasc Res. 2005;42(3):255–265. doi: 10.1159/000085658. [DOI] [PubMed] [Google Scholar]
- 49.Tang PC, Qin L, Zielonka J, et al. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205(13):3159–3171. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, Tang PC, Qin L, et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207(9):1951–1966. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori E, Komori K, Yamaoka T, et al. Essential role of monocyte chemoattractant protein-1 in development of restenotic changes (neointimal hyperplasia and constrictive remodeling) after balloon angioplasty in hypercholesterolemic rabbits. Circulation. 2002;105(24):2905–2910. doi: 10.1161/01.cir.0000018603.67989.71. [DOI] [PubMed] [Google Scholar]
- 52.McGirt MJ, Perler BA, Brooke BS, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg. 2005;42(5):829–836. doi: 10.1016/j.jvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 53.Rockman CB, Svahn JK, Willis DJ, et al. Carotid endarterectomy in patients 55 years of age and younger. Ann Vasc Surg. 2001;15(5):557–562. doi: 10.1007/s10016-001-0029-4. [DOI] [PubMed] [Google Scholar]
- 54.Levy PJ, Olin JW, Piedmonte MR, Young JR, Hertzer NR. Carotid endarterectomy in adults 50 years of age and younger: a retrospective comparative study. J Vasc Surg. 1997;25(2):326–331. doi: 10.1016/s0741-5214(97)70354-9. [DOI] [PubMed] [Google Scholar]