Abstract
The genotoxic and anti-genotoxic effects of Stachys petrokosmos leaf extracts (Sp) were investigated in human lymphocytes. The cells were treated with 1.5, 3.0 and 6.0 μL/mL concentrations of Sp leaf extracts for 24 and 48 h treatment periods in the absence and presence of metabolic activator (S9mix). In the absence of S9mix, Sp alone did not induce chromosome aberrations and formation of micronucleus while inducing the mean sister chromatid exchange at the highest concentration. In addition, Sp decreased the mutagenic effect of mitomycin-c. Sp alone showed a cytotoxic effect determined by a decrease in the proliferation index, mitotic index and nuclear division index. On the other hand a mixture of Sp and mitomycin-c resulted in a higher cytotoxic effect especially for 48 h treatment period. In the presence of S9mix, Sp was not genotoxic and cytotoxic however, it showed an anti-genotoxic effect by decreasing the effects of cyclophosphamide.
Keywords: Stachys petrokosmos, Sister chromatid exchange, Chromosome aberration, Micronucleus, Genotoxicity, Anti-genotoxicity
Introduction
Stachys petrokosmos (Sp) is one of the 450 species of the Stachys genus that is the largest genus in the flowering plants belonging to the Lamiaceae family. Sp is endemic in Turkey and it is a 5–10 cm long plant with bushes, flowering at spring and lives at 900–1,100 m, in rocky places of Turkey. The members of the Stachys genus have been used as a medicine for centuries because it contains useful substances such as alpha- and beta-phellandrene, elemol, betulinic acid, D-camphor, delphinidin, hyperoside, saponins, tannins, manganese, urcolic acid, apigenin, rosmarinic acid, oleanolic acid, chlorogenic acid, phenidon, naphthalene and Lavandulifolioside B (Delazar et al. 2005; Ivanova et al. 2009; Savic et al. 2010; Ali et al. 2010; Delazar et al. 2011). In addition, there are a lot of reports on substances characterized by GC–MS such as phenol, flavonoids, germacrene, stachysosides E–H, alpha and beta-pinene, iridoid glycoside and stachyssaponin which are specific to the Stachy genus (Lenherr et al. 1984; Sajjadi and Amiri 2007; Ahmad et al. 2008; Murata et al. 2008; Giuliani et al. 2009; Ozturk et al. 2009). Serbetci et al. (2010) reported that 63 compounds were identified in Stachys cretica subspecies and Cavar et al. (2010) also reported that more than 100 compounds were identified in three different samples of Stachys menthifolia Vis. collected from various natural habitats. These substances have been used in tea prepared from Stachys species for treatment of diarrhea, fevers, sore mouth and throat, internal bleeding, weaknesses of the liver and heart. In addition Hollman et al. (1996) reported that the herbal flavonoids contain anti-inflammatory, anti-mutagenic and anti-allergic activities. Couladis et al. (2003) reported that Stachys spruneri has an anti-oxidant activity against alpha-tocoferol. Stachys annula tea has been used for centuries against cancer (Aksoy et al. 1988). Also, extract of this plant showed an anti-mutagenic effect against sodium azide in Salmonella typhimurium TA 100 strain (Karakaya and Kavas 1999).
In contrast, the unconscious use of the plants in medicine could cause toxic (Qu et al. 1992; Chiang et al. 1997), mutagenic or cancerogenic effects (Gurley et al. 2010; Háznagy-Radnai et al. 2008; Rencuzogullari et al. 2009; Buyukleyla and Rencuzogullari 2009; Kayraldiz et al. 2010; Kocaman et al. 2011). Azirak and Rencuzogullari (2008) also reported that thymol and carvacrol induced chromosomal abnormalities in bone marrow cells of rats.
To date these studies in the literature did not include the genotoxic and antigenotoxic effects of Stachys petrokosmos extract. Madle et al. (1993) reported that using human lymphocytes for the mutagenicity studies could explain the best results for humans. To consider the genotoxicity of substances, genotoxicity of them should be investigated in human lymphocytes (Madle et al. 1993). Thus, the aim of the present study was to evaluate the genotoxic and anti-genotoxic effects of Sp extract by the in vitro test systems using human peripheral lymphocytes in the absence and presence of a metabolic activator.
Materials and methods
In the present study, Sp leaf extract was used as the test substance. The leaves of Sp were collected from Karaisali province of Adana, Turkey. The leaves were cut out from the base of the plant, cleaned and dried at room temperature. Five gr of plant leaves were extracted with 100 mL methanol kept in the ultrasonic water bath for 15 min. Then 100 mL methanol was added and the extract was kept in ultrasonic water bath for 15 min more. After this the extract was filtered and the methanol was evaporated. Ten mg extract was dissolved in 10 mL methanol to obtain the concentrations that were used in the present study.
The test without metabolic activator (-S9mix)
The methods of Evans (1984) and Perry and Thompson (1984) were followed in the preparation of CA (chromosomal aberration) and SCE (sister chromatid exchanges) tests with minor modifications. This study was conducted according to guidelines of the International Programme on Chemical Safety (IPCS) (Albertini et al. 2000).
Whole blood (0.2 mL) from two healthy donors (one male and one female, non-smokers, age: 20), was added to 2.5 mL chromosome medium B (Biochrom, F5023) supplemented with 10 μg/mL bromodeoxyuridine (Sigma, B5002). The cultures were incubated at 37 °C for 72 h. The cells were treated with 1.5, 3.0 and 6.0 μL/mL concentrations of methanol extract of Sp for 24 h (Sp was added 48 h after initiating the culture) and 48 h (Sp was added 24 h after initiating the culture). A negative control (untreated cultures) and a positive control (0.2 μg/mL mitomycin-C (MMC, Kyowa, Hakko, Japan)) were also used. The cells were exposed to colchicine (0.06 μg/mL, Sigma C9754) for 2 h before harvesting. The suspension was centrifuged for 10 min at 1200 rpm, and cells were resuspended in 0.4% KCl at 37 °C for 5 min, and then fixed in cold methanol: glacial acetic acid (3:1) for 20 min at room temperature. The treatment with fixative was repeated three times. Then the cells were spread on glass slides and air-dried. The staining of air dried slides was performed following the standard method using 5% Giemsa stain for CA and modified fluorescence plus Giemsa method for SCE (Speit and Haupter, 1985). The slides was irradiated with 30 W, 254 nm UV lamp at 15 cm distance in Sorensen buffer, then incubated with 1× SSC (standard saline citrate) at 60 °C for 45–60 min and stained with 5% Giemsa prepared with Sorensen buffer.
The number of CA was obtained by calculating the percentage of metaphases from each concentration and treatment period that showed structural and/or numerical alterations. The CA was classified according to the ISCN (International System for Human Cytogenetic Nomenclature) (Paz-y-Miño et al. 2002). Chromosome aberrations were evaluated in 100 well spread metaphases per donor (totally 200 metaphases per concentration). Gaps were not evaluated as CA according to Mace et al. (1978). The scoring of SCE was carried out according to the IPCS guidelines (Albertini et al. 2000). In order to score SCE, totally 50 s-division metaphases (25 cells per sample) were analyzed. The results were used to determine the mean number of SCE (SCE/cell). In addition, a total of 200 cells (100 cells from each donor) were scored for the proliferation index (PI). The mitotic index (MI) was also determined by scoring 3,000 cells from each donor. The MI explained the effects of the chemicals on G2 stage of the cell cycle, and the PI reflects the effects of chemicals on S and G2 stages of the cell cycles (Arslan et al. 2008). The PI was calculated according to the formula as follows: PI = (M1 × 1) + (M2 × 2) + (M3 × 3)/total scored cells. M1, M2 and M3 are the fraction of cells undergoing the first, second and third mitosis during 72 h cell culture period.
For the analysis of micronucleus in binucleated lymphocytes, 0.2 mL of fresh blood was used to establish cultures. The cells were treated with 1. 5, 3.0 and 6.0 μL/mL concentrations of Sp alone and with MMC (0.2 μg/mL) for 24 and 48 h treatment periods.
Cytochalasin B (Sigma, C6762) was added at 44 h of incubation to a final concentration of 6 μg/mL to block cytokinesis. After the additional 24 h incubation at 37 °C, cells were harvested by centrifugation and processed for micronucleus (MN) test in peripheral lymphocytes (Fenech 2000; Kirsch-Volders et al. 2003). In all subjects, 2,000 binucleated lymphocytes were scored from each donor (4,000 binucleated cells were scored per concentration). For each donor, in total 1,000 viable cells were scored to determine the frequency of cells with 1, 2, 3 or 4 nuclei and calculate the NDI (nuclear division index) for cytotoxicity of Sp using the formula: NDI = (1 × M1) + (2 × M2) + (3 × M3) + (4 × M4)/N; where M1–M4 represent the number of cells with one to four nuclei and N is the total number of viable cells scored (Fenech 2000).
The modified methods of Roncada et al. (2004) and Mendelsohn (1992) were used for evaluating anti-genotoxicity of the Sp. In the present study, mitomycin C was used as a mutagenic agent. To investigate the anti-genotoxic effect of Sp against the mutagenicity induced by MMC, the cultures were co-treated with 0.2 μg/mL of MMC and with different concentrations of the Sp for 24 and 48 h treatment times.
The test with metabolic activator (+S9mix)
The 3-methylcolanthrene induced rat liver microsomal fraction was used as the metabolic activator (S9mix) (Maron and Ames 1983).
Albino male rats (Rattus norvegicus var. albinos) weighing 200 gr were pre-treated with 80 mg/kg concentration of 3-methylcholanthrene (dissolved in sunflower oil) for 5 days and the S9 fraction and S9mix were prepared following the procedure of Maron and Ames (1983). The freshly prepared S9 fraction is distributed in 1 mL portions into small plastic tubes frozen immediately and stored at −80 °C. The S9mix was prepared fresh for each mutagenicity assay. For preparation of S9mix, NADP (4 mM), glucose-6-phosphate (5 mM), MgCl2 (8 mM), KCl (33 mM) and 6.2 mL phosphate buffer (0.2 mM) were completed to 18 mL with sterile bidistilled water supplemented with 2 mL of microsome fraction (S9). 0.5 mL of S9mix was used for each culture tube (0.5 mL S9mix/tube).
48 h after initiating the culture, the cells were treated with Sp and S9mix or with Sp+ cyclophosphamide (Cyp) and S9mix for the 3 h treatment period. Then, the cells were centrifuged at 2,000 rpm for 5 min washed with RPMI 1640 medium (Sigma) two times and resuspended with Chromosome Medium B supplemented with 10 μg/mL bromodeoxyuridine and incubated for 21 h for CA, SCE and MN assays. The harvesting of the cells was carried out according to methods described above.
Statistical significance
The t-test was used for the statistical significance of all the parameters after Anova one-way analysis of variance test using Minitab 14 statistical software. Dose response relationships were determined from the correlation (r) and regression coefficients for the percentage of structural CA, percentage of abnormal cells, mean SCE, MN, PI, MI and NDI.
Results
According to Anova one-way analysis of variance the CA, the percentage of abnormal cell, the mean of SCE and the frequency of MN showed significance. However, the RI and the NDI did not significantly decreased following the Anova one-way analysis of variance for the 24 h treatment periods. For the 24 h treatment periods, only the MI significantly decreased except for the 48 h treatment periods all parameters decreased using one-way analysis of variance.
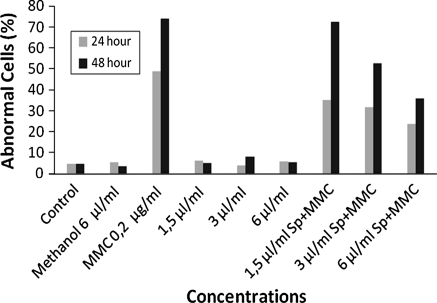
In the absence of S9mix, Sp did not induce structural CAs and an increase of the percentage of abnormal cells in all concentrations for the 24 and 48 h treatment periods except for the 3 μL/mL concentrations for the 48 h treatment (Table 1, Figs. 1, 2). On the other hand Sp decreased the genotoxic effect of MMC by decreasing the CA and the percentage of abnormal cell for both treatment periods of 24 and 48 h. The percentage of abnormal celsl was decreased in a dose dependent manner in the cultures treated with Sp plus MMC for 24 h (r = 0.999, P < 0.02).
Table 1.
The structural CAs and percentage of abnormal cells in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
| Test substance | Treatment time (h) | Concentrations (μL/mL) | Abnormalities++ | Structural CA/cell ± SE+ | Abnormal cells ± SE (%)+ | |
|---|---|---|---|---|---|---|
| B’ type | B’’ type | |||||
| Control | – | – | 4 | 7 | 0.05 ± 0.01 | 4.50 ± 1.50 |
| Methanol | 24 | 6.0 | 7 | 5 | 0.06 ± 0.01 | 5.50 ± 0.50 |
| MMC | 24 | 0.2 μg/mL | 87 | 43 | 0.65 ± 0.02 | 49.0 ± 3.00 |
| Sp | 24 | 1.5 | 8 | 5 | 0.06 ± 0.02 | 6.00 ± 1.00 |
| 24 | 3.0 | 4 | 5 | 0.04 ± 0.00 | 4.00 ± 1.00 | |
| 24 | 6.0 | 10 | 2 | 0.06 ± 0.00 | 5.95 ± 0.05 a1 | |
| Sp+MMC | 24 | 1.5 Sp+MMC* | 50 | 43 | 0.46 ± 0.03 a1b1 | 35.0 ± 1.00 a1b1c1 |
| 24 | 3.0 Sp+MMC* | 77 | 41 | 0.59 ± 0.01 a1b1 | 31.5 ± 0.50 a1b1c1 | |
| 24 | 6.0 Sp+MMC* | 52 | 24 | 0.38 ± 0.02 a1b1c1d | 23.5 ± 1.50 a1b1c1d | |
| Methanol | 48 | 6.0 | 6 | 2 | 0.04 ± 0.01 | 3.50 ± 0.50 |
| MMC | 48 | 0.2 μg/mL | 127 | 149 | 1.38 ± 0.02 | 74.0 ± 7.00 |
| Sp | 48 | 1.5 | 7 | 4 | 0.06 ± 0.01 | 5.00 ± 1.00 |
| 48 | 3.0 | 13 | 4 | 0.08 ± 0.00 a2 | 7.95 ± 0.05 a2b2 | |
| 48 | 6.0 | 9 | 4 | 0.06 ± 0.00e | 5.50 ± 1.50e | |
| Sp+MMC | 48 | 1.5 Sp+MMC* | 219 | 181 | 2.00 ± 0.12 a1b1 | 72.5 ± 0.58 a2b2 |
| 48 | 3.0 Sp+MMC* | 116 | 60 | 0.88 ± 0.01 a2b2c1 | 52.5 ± 1.50 a1b1c1 | |
| 48 | 6.0 Sp+MMC* | 51 | 49 | 0.50 ± 0.04 a1b1c1f | 36.0 ± 1.00 a1b1c1f | |
a: significant from control; b: significant from methanol control; c: significant from positive control, MMC; a1b1: P < 0.05; a2b2: P < 0.01
* MMC (Mitomycin C): 0.2 μg/mL
+d:164, e: 184 and f: 135 cells were scored for excessive toxicity
++B’ type: chromatid breakage; B’’ type: chromosome breakage
Fig. 1.
The structural CAs in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
Fig. 2.
The percentage of abnormal cells in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
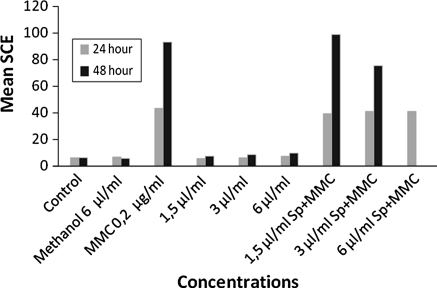
Sp alone increased the mean SCE at the highest concentration (6.0 μL/mL) for the 24 h treatment, also at the two highest concentrations (3.0 and 6.0 μL/mL) for the 48 h treatment periods in the absence of metabolic activator (S9mix) (Table 2, Fig. 3).
Table 2.
The mean SCE in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
| Test substance | Treatment time (h) | Concentrations (μL/mL) | Min–max SCE | SCE/cell ± SE |
|---|---|---|---|---|
| Control | – | – | 1–16 | 5.84 ± 0.72 |
| Methanol | 24 | 6.0 | 2–16 | 6.46 ± 0.70 |
| MMC | 24 | 0.2 μg/mL | 13–64 | 43.29 ± 1.79 |
| Sp | 24 | 1.5 | 1–12 | 5.48 ± 0.48 |
| 24 | 3.0 | 1–13 | 5.96 ± 0.12 | |
| 24 | 6.0 | 2–14 | 7.06 ± 0.02 a2b1 | |
| Sp+MMC | 24 | 1.5 Sp+MMC* | 21–65 | 39.29 ± 1.66 a1b1 |
| 24 | 3.0 Sp+MMC* | 16–64 | 41.09 ± 1.69 a1b1 | |
| 24 | 6.0 Sp+MMC* | 1–63 | 41.00 ± 2.52 a1b1 | |
| Methanol | 48 | 6.0 | 1–15 | 5.62 ± 0.14 |
| MMC | 48 | 0.2 μg/mL | 53–121 | 92.88 ± 2.32 |
| Sp | 48 | 1.5 | 2–17 | 7.24 ± 0.24 |
| 48 | 3.0 | 1–20 | 8.46 ± 0.22 a1b1 | |
| 48 | 6.0 | 2–22 | 9.68 ± 0.32 a1b1 | |
| Sp+MMC | 48 | 1.5 Sp+MMC* | 60–127 | 98.80 ± 2.36 a1b1 |
| 48 | 3.0 Sp+MMC* | 52–133 | 75.42 ± 3.34 a1b1 | |
| 48 | 6.0 Sp+MMC* | – | 0.0 ± 0.0+ |
a: significant from control; b: significant from methanol control; c: significant from positive control, MMC; a1b1: P < 0.05; a2b2: P < 0.01
* MMC (Mitomycin C): 0.2 μg/mL
+Not scorable for excessive toxicity
Fig. 3.
The mean SCE in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
In the absence of metabolic activator, Sp did not induce the formation of micronucleus for the 24 and 48 h treatment periods (Table 3, Fig. 4). There were no scorable cells at the highest concentration of Sp alone and Sp+MMC at the 48 h treatment period for excessive toxicity.
Table 3.
The frequency of MN in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
| Test substance | Treatment time (h) | Concentration (μL/mL) | Micronucleated binuclear cells (%) ± SE |
|---|---|---|---|
| Control | – | – | 0.150 ± 0.030 |
| Methanol | 24 | 6.0 | 0.225 ± 0.025 |
| MMC | 24 | 0.2 μg/mL | 2.150 ± 0.250 |
| Sp | 24 | 1.5 | 0.400 ± 0.100 |
| 24 | 3.0 | 0.275 ± 0.025 | |
| 24 | 6.0 | 0.625 ± 0.125 | |
| Sp+MMC | 24 | 1.5 Sp+MMC* | 1.625 ± 0.075 a1b1 |
| 24 | 3.0 Sp+MMC* | 1.535 ± 0.015 a2b2c1d | |
| 24 | 6.0 Sp+MMC* | 1.800 ± 0.070 a1b1e | |
| Methanol | 48 | 6.0 | 0.375 ± 0.225 |
| MMC | 48 | 0.2 μg/mL | 1.785 ± 0.065 |
| Sp | 48 | 1.5 | 0.410 ± 0.050 |
| 48 | 3.0 | 0.277 ± 0.026 | |
| 48 | 6.0 | 0.0 ± 0.0f | |
| Sp+MMC | 48 | 1.5 Sp+MMC* | 1.892 ± 0.117 a1b1 |
| 48 | 3.0 Sp+MMC* | 1.500 ± 0.120 a1b1 | |
| 48 | 6.0 Sp+MMC* | 0.0 ± 0.0f |
a: significant from control; b: significant from methanol control; c: significant from positive control, MMC; a1b1: P < 0.05; a2b2: P < 0.01
* MMC (Mitomycin C): 0.2 μg/mL
+d: 3,592 and e: 2,978 cells were scored for excessive toxicity, f: not scorable
Fig. 4.
The frequency of MN in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
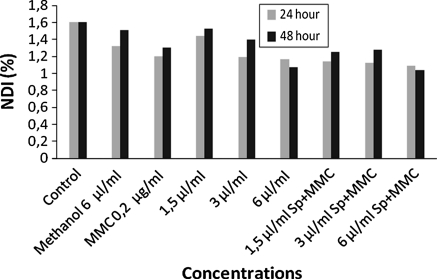
In absence of S9mix, Sp alone decreased the PI at the highest concentration for the 24 and 48 h treatment periods (Table 4, Fig. 5). In addition MI was decreased at the highest concentration for the 24 h and at all concentrations for the 48 h treatment periods. NDI was also decreased for both treatment periods (Table 4, Figs. 6, 7). On the other hand Sp and MMC as a mixture showed a higher cytotoxic effect by decreasing the PI, MI and NDI especially for the 48 h treatment period. There was a dose-dependent effect of Sp alone on decreasing the NDI for the 24 and 48 h treatment periods (r = 0.994, P < 0.04 and r = 0.997, P < 0.03, respectively).
Table 4.
The PI, MI and NDI in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
| Test substance | Treatment time (h) | Concentration (μL/mL) | PI ± SE | MI ± SE | NDI ± SE |
|---|---|---|---|---|---|
| Control | – | – | 2.17 ± 0.41 | 3.38 ± 0.55 | 1.60 ± 0.04 |
| Methanol | 24 | 6.0 | 2.12 ± 0.31 | 2.57 ± 0.14 | 1.32 ± 0.04 |
| MMC | 24 | 0.2 μg/mL | 1.53 ± 0.03 | 0.79 ± 0.13 | 1.20 ± 0.01 |
| Sp | 24 | 1.5 | 1.79 ± 0.50 | 2.01 ± 0.11 | 1.44 ± 0.16 |
| 24 | 3.0 | 1.79 ± 0.18 | 2.24 ± 0.31 | 1.19 ± 0.00 a1b1 | |
| 24 | 6.0 | 1.60 ± 0.00 a2b2 | 0.81 ± 0.08 a1b1 | 1.17 ± 0.01 a1b1 | |
| Sp+MMC | 24 | 1.5 Sp+MMC* | 1.50 ± 0.14 | 0.60 ± 0.10 a1b1 | 1.14 ± 0.00 a2b1 |
| 24 | 3.0 Sp+MMC* | 1.37 ± 0.01 a2b2c1 | 0.78 ± 0.05 a1b1 | 1.12 ± 0.01 a1b1 | |
| 24 | 6.0 Sp+MMC* | 1.49 ± 0.01 a1b1 | 0.66 ± 0.03 a2b1 | 1.09 ± 0.01 a1b1 | |
| Methanol | 48 | 6.0 | 2.32 ± 0.01 | 3.33 ± 0.27 | 1.51 ± 0.00 |
| MMC | 48 | 0.2 μg/mL | 1.49 ± 0.16 | 1.08 ± 0.15 | 1.30 ± 0.05 |
| Sp | 48 | 1.5 | 2.35 ± 0.12 | 2.53 ± 0.03 a1b1 | 1.53 ± 0.01 |
| 48 | 3.0 | 2.21 ± 0.09 | 2.71 ± 0.01 a1b1 | 1.40 ± 0.01 a1 | |
| 48 | 6.0 | 1.34 ± 0.01 a2b2 | 1.01 ± 0.01 a2b2 | 1.07 ± 0.03 a1b1 | |
| Sp+MMC | 48 | 1.5 Sp+MMC* | 1.35 ± 0.07 a1b1 | 0.86 ± 0.06 a1b1 | 1.25 ± 0.00 a2b1c1 |
| 48 | 3.0 Sp+MMC* | 1.46 ± 0.06 a1b1 | 1.11 ± 0.05 a1b1 | 1.28 ± 0.01 a1b1 | |
| 48 | 6.0 Sp+MMC* | 0.0 ± 0.0+ | 0.68 ± 0.02 a2b2c1 | 1.04 ± 0.02 a1b1c1 |
a: significant from control; b: significant from methanol control; c: significant from positive control, MMC; a1b1: P < 0.05; a2b2: P < 0.01
* MMC (Mitomycin C): 0.2 μg/mL
+Not scorable for excessive toxicity
Fig. 5.
The PI in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
Fig. 6.
The MI in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
Fig. 7.
The NDI in human lymphocytes treated with Sp alone or with Sp+MMC in the absence of S9mix
In the presence of S9mix, Sp alone or Sp+Cyp did not induce structural CA and did not increase the percentage of abnormal cell formation (Table 5, Figs. 8, 9). Sp decreased the genotoxic effect of Cyp on CA without a statistical significance.
Table 5.
The structural CA and percentage of abnormal cells in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
| Test substance | Concentration (μL/mL) | Abnormalities | Structural CA/cell ± SE | Abnormal cells ± SE (%)+ | |
|---|---|---|---|---|---|
| B’ type | B’’ type | ||||
| Control | – | 8 | 1 | 0.04 ± 0.00 | 4.50 ± 0.50 |
| Methanol | 6.0 | 6 | 3 | 0.05 ± 0.00 | 5.50 ± 0.05 |
| Cyp | 28 μg/mL | 28 | 14 | 0.21 ± 0.04 | 18.50 ± 1.50 |
| Sp | 1.5 | 11 | 3 | 0.07 ± 0.01 | 6.50 ± 1.50 |
| 3.0 | 17 | 10 | 0.13 ± 0.01 | 12.50 ± 0.50 a1 | |
| 6.0 | 12 | 10 | 0.11 ± 0.01 | 10.00 ± 1.00 | |
| Sp+Cyp | 1.5 Sp+Cyp* | 28 | 14 | 0.21 ± 0.01 a1b1 | 19.50 ± 1.50 a1 |
| 3.0 Sp+Cyp* | 18 | 8 | 0.13 ± 0.01 a1 | 11.00 ± 1.00 a1 | |
| 6.0 Sp+Cyp* | 24 | 9 | 0.16 ± 0.01 a1 | 15.00 ± 1.50 a1 | |
a: significant from control; b: significant from methanol control; c: significant from positive control, Cyp; a1b1: P < 0.05; a2b2: P < 0.01
* Cyp (Cyclophosphamide): 28 μg/mL
Fig. 8.
The structural CA and percentage of MN in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
Fig. 9.
The percentage abnormal cell and mean SCE in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
Sp alone did not increase the mean SCE, however, Sp significantly inhibited the effect of Cyp on mean SCE in the presence of metabolic activator (S9mix) in a dose dependent manner (r = 0.996, P < 0.03) (Table 6, Fig. 9).
Table 6.
The mean SCE in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
| Test substance | Concentration (μL/mL) | Min–max SCE | SCE/Cell ± SE |
|---|---|---|---|
| Control | – | 1–15 | 5.72 ± 0.44 |
| Methanol | 6.0 | 1–25 | 6.26 ± 0.34 |
| Cyp | 28 μg/mL | 7–53 | 24.95 ± 2.83 |
| Sp | 1.5 | 1–12 | 5.28 ± 0.71 |
| 3.0 | 2–12 | 5.56 ± 0.60 | |
| 6.0 | 1–16 | 5.90 ± 0.78 | |
| Sp+Cyp | 1.5 Sp+Cyp* | 2–24 | 10.08 ± 1.00 c1 |
| 3.0 Sp+Cyp* | 3–25 | 9.68 ± 0.80 c1 | |
| 6.0 Sp+Cyp* | 1–19 | 8.64 ± 1.32 c1 |
a: significant from control; b: significant from methanol control; c: significant from positive control, Cyp; a1b1: P < 0.05; a2b2: P < 0.01
* Cyp (Cyclophosphamide): 28 μg/mL
Sp alone induced the formation of MN at the two highest concentrations when compared to the untreated control, but not to the solvent control. However, Sp+Cyp decreased the formation of MN at the highest concentration when compared to Cyp in the presence of S9mix (Table 7, Fig. 8).
Table 7.
The frequency of MN in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
| Test substance | Concentration (μL/mL) | Micronucleated binuclear cell (%) ± SE |
|---|---|---|
| Control | – | 0.250 ± 0.050 |
| Methanol | 6.0 | 0.500 ± 0.100 |
| Cyp | 28 μg/mL | 1.280 ± 0.120d |
| Sp | 1.5 | 0.525 ± 0.075 |
| 3.0 | 1.125 ± 0.075 a1 | |
| 6.0 | 0.800 ± 0.050 a1 | |
| Sp+Cyp | 1.5 Sp+Cyp* | 1.000 ± 0.090 a1 |
| 3.0 Sp+Cyp* | 1.080 ± 0.080 a1e | |
| 6.0 Sp+Cyp* | 0.775 ± 0.045 c1 |
a: significant from control; b: significant from methanol control; c: significant from positive control, Cyp; a1b1: P < 0.05; a2b2: P < 0.01
* Cyp (Cyclophosphamide): 28 μg/mL
d: 3,713 and e: 3,183 cells were scored for excessive toxicity
In the presence of S9mix, Sp alone and Sp+Cyp did not decrease the PI and NDI, however, showed a cytotoxic effect by decreasing the MI (Table 8, Fig. 10). Sp decreased the MI at all concentrations except 3 μL/mL when compared to untreated and solvent controls.
Table 8.
The PI, MI and NDI in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
| Test substance | Concentration (μL/mL) | PI ± SE | MI ± SE | NDI ± SE |
|---|---|---|---|---|
| Control | – | 2.015 ± 0.14 | 2.590 ± 0.17 | 1.381 ± 0,18 |
| Methanol | 6.0 | 1.570 ± 0.04 | 2.320 ± 0.49 | 1.160 ± 0.01 |
| Cyp | 28 μg/mL | 1.440 ± 0.20 | 0.995 ± 0.03 | 1.126 ± 0.01 |
| Sp | 1.5 | 1.500 ± 0.03 a1 | 1.845 ± 0.01 a1b1 | 1.070 ± 0.04 |
| 3.0 | 1.510 ± 0.13 | 2.145 ± 0.21 | 1.195 ± 0.05 | |
| 6.0 | 1.455 ± 0.06 | 1.330 ± 0.10 a1b1 | 1.143 ± 0.01 a1 | |
| Sp+Cyp | 1.5 Sp+Cyp* | 1.470 ± 0.10 | 1.030 ± 0.37 | 1.126 ± 0.03 |
| 3.0 Sp+Cyp* | 1.570 ± 0.21 | 0.985 ± 0.05 a1b1 | 1.135 ± 0.00 a2b1 | |
| 6.0 Sp+Cyp* | 1.650 ± 0.19 | 0.950 ± 0.02 a2b2 | 1.173 ± 0.02 |
a: significant from control; b: significant from methanol control; c: significant from positive control, Cyp; a1b1: P < 0.05; a2b2: P < 0.01
* Cyp (Cyclophosphamide): 28 μg/mL
Fig. 10.
The PI, MI and NDI in human lymphocytes treated with Sp alone or with Sp+Cyp in the presence of S9mix
Discussion
In the absence of S9mix, Sp alone did not induce CA and formation of micronucleus, while it induced the mean SCE at the highest concentration. On the other hand Sp decreased the mutagenic effect of MMC by decreasing the CA, SCE and MN at the two highest concentrations. In absence of S9mix, Sp alone showed a cytotoxic effect by decreasing the PI, MI and NDI at the highest concentration. On the other hand Sp+MMC showed a higher cytotoxic effect especially for the 48 h treatment period. In the presence of S9mix, it was observed that Sp was not genotoxic and cytotoxic however, it had an anti-genotoxic effect by decreasing the effects of cyclophosphamide on SCE and MN frequency.
According to these results it can be concluded that Sp was not genotoxic and cytotoxic. In addition, Sp showed anti-genotoxic effects by decreasing the genotoxic effects of known mutagens (MMC and Cyp) in the absence and presence of S9mix.
Hollman et al. (1996) reported that the plant flavonoids had anti-inflammatory, anti-mutagenic and anti-allergic effects. However, adverse effects such as mutation, cancer, gastric problems and inflammatory disorders for some plant oils were reported (Qu et al. 1992; Chiang et al. 1997; Gurley et al. 2010).
There were a lot of reports for the medicinal use of extracts obtained from various Stachys species. Aksoy et al. (1988) reported that Stachys annula was used as a herbal tea for anti-cancer effects in Anatolia and that this tea contains glycosides, saponins and essential oils. Also, it has been reported that S. annula decreased the mutagenic effects of sodium azide in Salmonella typhimurium TA100 strain (Karakaya and Kavas 1999). Couladis et al. (2003) reported that Stachy spruneri has anti-oxidant effects against α-tocoferol. In addition there were a lot of reports on anti-mutagenic, anti-genotoxic, apoptotic and anti-proliferative effects of Stachy spp (Basaran et al. 1996; Amirghofran et al. 2006; Amirghofran et al. 2007). Kukic et al. (2006) and Tepe et al. (2011) reported that five endemic Stachys species (S. anisochila, S. beckeana, S. plumosa, S. alpina ssp. Dinarica and S. iberica) had strong anti-oxidant effects.
All these reports indicate that a lot of Stachys species have anti-genotoxic, anti-cancerogenic and anti-oxidative effects. The test material of the present study, Sp methanol extract did not only show genotoxic effects but also showed an anti-genotoxic effect in the presence and absence of metabolic activator S9mix. Flavonoids and glycosides with other substances like germacrene, stachysoosides E–H, pinene, iridoid glycoside and stachyssaponin were found in the extracts of Stachys species according to GC–MS analyzing method (Lenherr et al. 1984; Sajjadi and Amiri 2007; Ahmad et al. 2008; Murata et al. 2008; Giuliani et al. 2009; Ozturk et al. 2009). In addition, there are a lot of substances specified to Stachys species such as chlorogenic asit, phenidon, naphthalene, betulinic-acid, D-camphor, delphinidin, hyperoside, manganese, oleanolic acid, rosmarinic acid, urcolic acid, apigenin and tannin (Delazar et al. 2005; Ivanova et al. 2009; Savic et al. 2010). However, the contents of Stachys petrokosmos leaf extract are not reported.
The anti-genotoxic effect of Sp in the presence of S9mix was higher than the effect of Sp in the absence of S9mix. In presence of S9mix Sp decreased the genotoxic effects of Cyp and showed an anti-genotoxic effect. It could be suggested that the high anti-genotoxic effect of Sp in the presence of S9mix was caused by the metabolites of the Stachys extracts. This is because there are a lot of data about the anti-genotoxic, anti- cancerogenic and anti-allergic effects of Stachys species from in vivo and in vitro studies (Basaran et al. 1996; Shin 2004; Delazar et al. 2005; Kukic et al. 2006; Amirghofran et al. 2006; Amirghofran et al. 2007; Haznagy-Radnai et al. 2008). It is reported that the anti-oxidant effects of Stachys species arose from the radical scavenger substances acetyl flavonoids and polyphenols (Hollman et al. 1996; Delazar et al. 2005; Kukic et al. 2006).
Sowjanya et al. (2009) reported that in the liver Cyp was metabolized to active alkylation substances, acrolein and phosphoramid by liver microsome oxydase. At higher doses Cyp formed high mutations by these alkylation substances and caused a cytotoxic effect. Because of this Cyp is used as anti-cancer drug at higher concentrations while Cyp is mutagenic at lower concentrations.
There are a lot of natural compounds preventing toxicity of chemicals and decreasing the cancerogenic effect of them (Milner 2001; Madhavi et al. 2007). In the present study, Sp decreased not only the cytotoxic but also the genotoxic effects of Cyp. According to recent reports, it could be suggested that Sp leaf extracts decreased the effect of Cyp on chromosomal abnormalities because of its free radical scavenger capability. The radical scavenger substances also induced the synthesis of cytochrom P450 and anti-oxidant enzymes, stimulated the DNA repair mechanisms and they prevented mutations by prevention the DNA double-strand crosswise bounding of DNA (Bianchini and Vainio 2001; Khanum et al. 2004; Sowjanya et al. 2009). Amirghofran et al. (2007) reported that Stachys species extracts are capable of inhibiting cancer by inducing apoptosis. These reports explain the anti-genotoxicities of Stachys petrokosmos and the other Stachys species and support the results from this study.
In absence of S9mix, Sp alone showed a cytotoxic effect by decreasing the PI, MI and NDI at the highest concentration. However, in the presence of S9mix, Sp was not cytotoxic. It was reported that Stachys species had an anti-proliferative effect because of their anti-oxidant enzymes (Amirghofran et al. 2007; Haznagy-Radnai et al. 2008). Thus, it can be suggested that Sp protected the cells and especially in the presence of S9mix, decreased the cytotoxicity of Cyp on the cell.
Conclusion
In conclusion, from the previous studies it was clearly understood that the anti-oxidant substances of Stachys species have a medicinal use and protect cells from the action of mutagens. Sp the material of the present study showed an anti-genotoxic effect and decreased the genotoxicity of the known mutagen Cyp.
Acknowledgments
This study was supported by C. U. Research Fund. FEF2009YL1.
References
- Ahmad VU, Arshad S, Bader S, Iqbal S, Khan A, Khan SS, Hussain J, Tareen RB, Ahmed A. New terpenoids from Stachys parviflora Benth. Fitoterapia. 2008;79:595–597. doi: 10.1016/j.fitote.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Aksoy C, Yucecan S, Ciftci N, Tayfur M, Akgun B, Tasci N. Kanser hastalığında tedavi amacıyla kullanılan yöresel bitkiler (The local plants that were used in cancer therapy) Beslenme ve Diyet Dergisi (J Nutr Diet) 1988;17:111–120. [Google Scholar]
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res. 2000;463:111–172. doi: 10.1016/S1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Ali NA, Marongiu B, Piras A, Porcedda S, Falconieri D, Molicotti P, Zanetti S. Essential oil composition of leaves of Stachys yemenensis obtained by supercritical CO2. Nat Prod Res. 2010;24:1823–1829. doi: 10.1080/14786411003754272. [DOI] [PubMed] [Google Scholar]
- Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Anticancer effects of various Iranian native medicinal plants on human tumor cell lines. Neoplasma. 2006;53:428–433. [PubMed] [Google Scholar]
- Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Immunomodulatory and apoptotic effects of Stachys obtusicrena on proliferative lymphocytes. Med Sci Monit. 2007;13:145–150. [PubMed] [Google Scholar]
- Arslan M, Topaktas M, Rencuzogullari E. The effects of boric acid on sister chromatid exchanges and chromosome aberrations in cultured human lymphocytes. Cytotechnology. 2008;56:91–96. doi: 10.1007/s10616-007-9094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azirak S, Rencuzogullari E. The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ Toxicol. 2008;23:728–735. doi: 10.1002/tox.20380. [DOI] [PubMed] [Google Scholar]
- Basaran AA, Yu TW, Plewa MJ, Anderson D. An investigation of some Turkish herbal medicines in Salmonella typhimurium and in the COMET assay in human lymphocytes. Teratog Carcinog Mutagen. 1996;16:125–138. doi: 10.1002/(SICI)1520-6866(1996)16:2<125::AID-TCM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bianchini F, Vainio H. Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ Health Perspect. 2001;109:893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukleyla M, Rencuzogullari E. The effects of thymol on sister chromatid exchange, chromosome aberration and micronucleus in human lymphocytes. Ecotoxicol Environ Safety. 2009;72:943–947. doi: 10.1016/j.ecoenv.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Ćavar S, Maksimović M, Vidic D, Šolić ME. Chemical composition of the essential oil of Stachys menthifolia. Vis Pharm Biol. 2010;48:170–176. doi: 10.3109/13880200903062655. [DOI] [PubMed] [Google Scholar]
- Chiang TA, Wu PF, Wang LF, Lee H, Lee CH, Ko YC. Mutagenicity and polycylic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat Res. 1997;381:157–161. doi: 10.1016/s0027-5107(97)00163-2. [DOI] [PubMed] [Google Scholar]
- Couladis M, Tzakou O, Verykokidou E, Harvala C. Screening of some Greek aromatic plants for antioxidant activity. Phytother Res. 2003;17:194–195. doi: 10.1002/ptr.1261. [DOI] [PubMed] [Google Scholar]
- Delazar A, Celik S, Gokturk RS, Unal O, Nahar L, Sarker SD. Two acylated flavonoid glycosides from Stachys bombycina, and their free radical scavenging activity. Pharmazie. 2005;60:878–880. [PubMed] [Google Scholar]
- Delazar A, Delnavazi MR, Nahar L, Moghadam SB, Mojarab M, Gupta A, Williams AS, Mukhlesur-Rahman M, Sarker SD. Lavandulifolioside B: a new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat Prod Res. 2011;25:8–16. doi: 10.1080/14786411003754330. [DOI] [PubMed] [Google Scholar]
- Evans HJ. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kilbey BJ, Legator M, Nichols W, Ramel C, editors. Handbook of mutagenicity test procedures. 2. Amsterdam: Elsevier Sciences; 1984. pp. 405–427. [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Giuliani C, Pellegrino RM, Tirillini B, Bini LM. Composition of essential oils from leaves and flowers of Stachys germanica subsp salviifolia (Ten.) gams (Labiatae) and related secretory structures. Nat Prod Commun. 2009;4:831–834. [PubMed] [Google Scholar]
- Gurley ES, Rahman M, Hossain MJ, Nahar N, Faiz MA, Islam N, Sultana R, Khatun S, Uddin MZ, Haider MS, Islam MS, Ahmed BN, Rahman MW, Mondal UK, Luby SP. Fatal outbreak from consuming Xanthium strumarium seedlings during time of food scarcity in northeastern Bangladesh. PLoS One. 2010;5:e9756. doi: 10.1371/journal.pone.0009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznagy-Radnai E, Czigle S, Zupko I, Falkay G, Mathe I. Comparison of antioxidant activity in enzyme-independent system of six Stachys species. Fitoterapia. 2008;77:521–524. doi: 10.1016/j.fitote.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hollman PCH, Hertog MGL, Katan MB. Analysis and health effects of flavonoids. Food Chem. 1996;57:43–46. doi: 10.1016/0308-8146(96)00065-9. [DOI] [Google Scholar]
- Ivanova A, Nechev J, Tsvetkova I, Stefanov K, Popov S. Chemical composition of the halophyte plant Stachys maritima Gouan from the Bulgarian Black Sea coast. Nat Prod Res. 2009;23:448–454. doi: 10.1080/14786410802048027. [DOI] [PubMed] [Google Scholar]
- Karakaya S, Kavas A. Antimutagenic activities of some foods. J Sci Food Agric. 1999;79:237–242. doi: 10.1002/(SICI)1097-0010(199902)79:2<237::AID-JSFA178>3.0.CO;2-K. [DOI] [Google Scholar]
- Kayraldiz A, Yavuz-Kocaman A, Rencuzogullari E, Istifli ES, Ila HB, Topaktas M, Daglioglu YK. The genotoxic and antigenotoxic effects of Aloe vera leaf extract in vivo and in vitro. Turk J Biol. 2010;34:235–246. [Google Scholar]
- Khanum F, Anilkumar KR, Viswanathan KR. Anticarcinogenic properties of garlic: a review. Crit Rev Food Sci Nutr. 2004;44:479–488. doi: 10.1080/10408690490886700. [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M, Sofuni T, Aardemac M. Report from the in vitro micronucleus assay working group. Mutat Res. 2003;540:153–163. doi: 10.1016/j.mrgentox.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Kocaman AY, Rencuzogullari E, Topaktas M, Istifli ES, Buyukleyla M (2011) The effects of 4-thujanol on chromosome aberrations, sister chromatid exchanges and micronucleus in human peripheral blood lymphocytes. Cytotechnology doi:10.1007/s10616-011-9372-7 [DOI] [PMC free article] [PubMed]
- Kukic J, Petrovic S, Niketiv M. Antioxidant activity of four endemic Stachys taxa. Biol Pharm Bull. 2006;29:725–729. doi: 10.1248/bpb.29.725. [DOI] [PubMed] [Google Scholar]
- Lenherr A, Meier B, Sticher O. Modern HPLC as a tool for chemotaxonomical investigations: iridoid glucosides and acetylated flavonoids in the group of Stachys recta. Planta Med. 1984;50:403–409. doi: 10.1055/s-2007-969749. [DOI] [PubMed] [Google Scholar]
- Mace MLJ, Daskal Y, Wray W. Scanning electron microscopy of chromosome aberrations. Mutat Res. 1978;52:199–206. doi: 10.1016/0027-5107(78)90141-0. [DOI] [PubMed] [Google Scholar]
- Madhavi D, Devil KR, Rao KK, Reddy PP. Modulating effect of phyllanthus fruit extract against lead genotoxicity in germ cells of mice. Environ Biol. 2007;28:115–117. [PubMed] [Google Scholar]
- Madle S, Beek B, Nowak C. Zum Verstandnis von Chromosomenmutationstests an Somazellen. In: Fahrig R, editor. Mutationsforschung und Genetische Toxicologie. Germany: Wissenschaftliche Buchgesellschaft; 1993. pp. 224–242. [Google Scholar]
- Maron DM, Ames BN. Revised method for he Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ML. Antimutagenic effects in humans. Mutat Res. 1992;267:257–264. doi: 10.1016/0027-5107(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Milner JA. A historical perspective on garlic and cancer. J Nutr. 2001;131:1027–1031. doi: 10.1093/jn/131.3.1027S. [DOI] [PubMed] [Google Scholar]
- Murata T, Endo Y, Miyase T, Yoshizaki F. Iridoid glycoside constituents of Stachys lanata. J Nat Prod. 2008;71:1768–1770. doi: 10.1021/np8001805. [DOI] [PubMed] [Google Scholar]
- Ozturk M, Duru ME, Aydogmus-Ozturk F, Harmandar M, Mahlicli M, Kolak U, Ulubelen A. GC-MS analysis and antimicrobial activity of essential oil of Stachys cretica subsp Smyrnaea. Nat Prod Commun. 2009;4:109–114. [PubMed] [Google Scholar]
- Paz-y-Miño C, Bustamante G, Sánchez ME, Leone PE. Cytogenetic monitoring in a population occupationally exposed to pesticides in Ecuador. Environ Health Perspect. 2002;110:1077–1080. doi: 10.1289/ehp.021101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PE, Thompson EJ. The methodology of sister chromatid exchanges. In: Kilbey BJ, Legator M, Nichols W, Ramel C, editors. Handbook of mutagenicity test procedures. 2. Amsterdam: Elsevier Sciences; 1984. pp. 495–529. [Google Scholar]
- Qu YH, Xu GX, Zhou JZ, Chen TD, Zhu LF, Shields PG, Wang HW, Gao YT. Genotoxicity of heated cooking oil vapors. Mutat Res. 1992;298:105–111. doi: 10.1016/0165-1218(92)90035-X. [DOI] [PubMed] [Google Scholar]
- Rencuzoğullari E, Azirak S, Canimoglu S, Parlak S, Buyukleyla M. Effects of natamycin on sister chromatid exchanges, chromosome aberrations and micronucleus in human lymphocytes. Drug Chem Toxicol. 2009;32:47–52. doi: 10.1080/01480540802431371. [DOI] [PubMed] [Google Scholar]
- Roncada T, Vicentini VE, Mantovani MS. Possible modulating actions of plant extracts on the chromosome breaking activity of MMC and Ara-C in human lymphocytes in vitro. Toxicol Invit. 2004;18:617–622. doi: 10.1016/j.tiv.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Sajjadi MH, Amiri H. Chemical constituents of the essential oils of different stages of the growth of Stachys lavandulifolia Vahl. from Iran. Pak J Biol Sci. 2007;10:2784–2786. doi: 10.3923/pjbs.2007.2784.2786. [DOI] [PubMed] [Google Scholar]
- Savic MM, Kukic JM, Grayer RJ, Milinkovic MM, Marin PD, Divljakovic J, Van-Linn M, Cook JM, Petrovic SD. Behavioural characterization of four endemic Stachys taxa. Phytother Res. 2010;24:1309–1316. doi: 10.1002/ptr.3106. [DOI] [PubMed] [Google Scholar]
- Serbetci T, Demirci B, Guzel CB, Kultur S, Erguven M, Baser KH. Essential oil composition, antimicrobial and cytotoxic activities of two endemic Stachys cretica subspecies (Lamiaceae) from Turkey. Nat Prod Commun. 2010;5:1369–1374. [PubMed] [Google Scholar]
- Shin TY. Stachys riederi inhibits mast cell-mediated acute and chronic allergic reactions. Immunopharmacol Immunotoxicol. 2004;26:621–630. doi: 10.1081/IPH-200042365. [DOI] [PubMed] [Google Scholar]
- Sowjanya BL, Devi KR, Madhavi D. Modulatory effects of garlic extract against the cyclophosphamide induced genotoxicity in human lymphocytes in vitro. J Environ Biol. 2009;30:663–666. [PubMed] [Google Scholar]
- Speit G, Haupter S. On the mechanisms of differential Giemsa staining of bromodeoxyuridine-substituted chromosomes II. Differences between the demonstration of sister chromatid differentiation and replication patterns. Hum Genet. 1985;70:126–129. doi: 10.1007/BF00273070. [DOI] [PubMed] [Google Scholar]
- Tepe B, Degerli S, Arslan S, Malatyali E, Sarikurkcu C. Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia. 2011;82:237–246. doi: 10.1016/j.fitote.2010.10.006. [DOI] [PubMed] [Google Scholar]