Abstract
The skin of the amphibian Bombina maxima is rich in biologically active proteins and peptides, most of which have mammalian analogues. The physiological functions of most of the mammalian analogues are still unknown. Thus, Bombina maxima skin may be a promising model to reveal the physiological role of these proteins and peptides because of their large capacity for secretion. To investigate the physiological role of these proteins and peptides in vitro, a fibroblast cell line was successfully established from Bombina maxima tadpole skin. The cell line grew to form a monolayer with cells of a uniform shape and abundant rough endoplasmic reticulum, which are typical characteristics of fibroblasts. Further identification at a molecular level revealed that they strongly expressed the fibroblast marker protein vimentin. The chromosome number of these cells is 2n = 28, and most of them were diploid. Growth property analysis showed that they grew well for 14 passages. However, cells showed decreased proliferative ability after passage 15. Thus, we tried to immortalize the cells through the overexpression of SV40 T antigen. After selecting by G418, cells stably expressed SV40 large T antigen and showed enhanced proliferative ability and increased telomerase activity. Signal transduction analysis revealed functional p42 mitogen-activated protein (MAP) kinase in immortalized Bombina maxima dermal fibroblasts. Primary fibroblast cells and the immortalized fibroblast cells from Bombina maxima cultured in the present study can be used to investigate the physiological role of Bombina maxima skin-secreted proteins and peptides. In addition, the methods for primary cell culturing and cell immortalization will be useful for culturing and immortalizing cells from other types of amphibians.
Keywords: Bombina maxima, Fibroblast, Immortalization, Skin, Vimentin
Introduction
The red-bellied toad is an Anura amphibian with ecological significance in southwest China. Amphibians have long been used as animal models for scientific research. For example, Xenopus laevis and Xenopus tropicalis are useful in developmental studies. Salamanders are widely used for regeneration biology research. Amphibian skins contain both granular glands and mucous glands that can secrete a number of proteins and peptides with various activities. Many proteins and peptides have been cloned and purified from the skin of Bombina maxima in our previous studies (Lai et al. 2002b, c, 2003; Zhang et al. 2005; Liu et al. 2008; Gao et al. 2011b). Among them are low molecular weight peptides such as bradykinin (Lai et al. 2001b), proline-rich bombesin (Lai et al. 2002c), Bv-8-like peptides (Lai et al. 2003), and antimicrobial peptides (Lai et al. 2002a, d). In addition to these peptides, a trefoil protein containing a two trefoil domain termed Bm-TFF2 (Zhang et al. 2005, 2011), a βγ-crystalline and trefoil peptide complex termed βγ-CAT (Liu et al. 2008; Gao et al. 2011a) and a skin albumin (Zhang et al. 2006) have also been identified and characterized from the skin of Bombina maxima. These peptides and proteins may play an important role in maintaining skin function by killing pathogens, facilitating wound repair and promoting innate immunity. However, no continuous cell lines derived from the skin of Bombina maxima are currently available; thus, it is impossible to investigate the physiological function of these secreted proteins and peptides in vitro. Fibroblast cells synthesize the extracellular matrix, including collagen, which is the structural framework for animal tissues. Dermal fibroblasts play a vital role in maintaining skin function. They are also involved in the process of wound repair (Darby and Hewitson 2007) and can act as sentinel cells by synthesizing chemokines and regulating inflammation (Smith et al. 1997), at least in mammals and in bony fish (Ingerslev et al. 2010; Ossum et al. 2004). Thus, fibroblast cell lines derived from Bombina maxima skin may be very useful for studying the physiological roles of its skin secretions. In the present study, a dermal fibroblast cell line was established from the skin of Bombina maxima tadpoles. To prolong the lifespan of this cell line, a retroviral vector expressing SV40 large T antigen was constructed and introduced into primary Bombina maxima dermal fibroblasts, and the cells were successfully immortalized.
Materials and methods
Primary cell culture
Bombina maxima tadpoles were raised in our laboratory. Body skins, not including the tails, were dissected with forceps from one tadpole and then washed three times with 60% L15 medium (Gibco) supplemented with 200 IU/mL penicillin (Sigma), 200 μg/mL streptomycin (Sigma), and 240 μg/mL gentamicin (Sigma). The skins were chopped into 1 mm3 pieces and then incubated with type I collagenase (Gibco) at 25 °C for 4 h. The isolated cells and the remaining tissue pieces were then washed twice with 60% L15 medium and seeded in flasks (Corning). They were then cultured at 25 °C in 60% DMEM/F12 medium (Gibco) with 10% fetal bovine serum (Hyclone). Logarithmic phase cells were harvested and split into new flasks at a ratio of 1:2–1:3 and incubated at 25 °C with 5% CO2.
Cryopreservation and recovery
Cells were frozen and recovered in a manner similar to the methods used to preserve mammalian cells but with an osmotic pressure change in freezing medium (Li et al. 2010) to acquire the same osmotic pressure as the culturing medium (Ellinger et al. 1983). After three passages, the cells were centrifuged at 1,000 rpm for 5 min. The supernatant was discarded, and the cell pellet was re-suspended in a freezing medium containing 10% dimethyl sulfoxide (DMSO) (Sigma), 50% FBS and 40% distilled water. The final cell concentration of cells was about 3 × 106 per mL. The cell suspensions were then kept in cryovials (Corning) that were then put into a programmed cryopreservation system (Nalgene) for 20 h and then transferred to liquid nitrogen for long-term storage. When recovery was needed, the frozen vials were taken from the liquid nitrogen and thawed in a 30 °C water bath. Cell suspensions were then transferred into a flask containing medium and cultured at 25 °C.
Electron microscopy
Cells were collected by digestion with 0.125% trypsin and were then centrifuged. The cells were fixed and processed (Fan et al. 2010) as previously described. Ultrathin sections were stained with 2% uranyl acetate-lead citrate and observed with a transmission electron microscope (PHILIPS CM120).
Growth kinetics
Cells (4 × 104) were seeded on 12-well microplates (Corning) and cultured for 7 days. Cells were counted every 24 h until reaching the plateau phase. Mean values were used to plot a growth curve and to calculate the population doubling time.
Chromosome and karyotype analysis
Immortalized or non-immortalized cells were grown at 25 °C in medium containing 60% DMEM/F12, 10% FBS and 30% distilled water. After treating the cells with 50 μg/mL colchicine (Sigma) for 2 h, the cells were harvested by trypsinization. Chromosome preparations were made following standard procedures according to the method used by Nie et al. (2002). Approximately 100 spreads were sampled in order to count the chromosome number per initial spread.
Molecular cloning of vimentin and immunohistochemistry
The construction of a directional cDNA library of Bombina maxima skin and the random sequencing of cloned cDNAs were performed as described previously (Lee et al. 2005). DNA sequencing was performed on an ABI Prism 377 DNA sequencer (Applied Biosystems). The nucleotide sequence data reported in this paper are available from the GenBank database (accession number: HQ874648).
Logarithmic phase cells were cultured on glass coverslips for 24 h. After they were washed three times with ice-cold PBS, cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.05% NP-40 for 20 min at room temperature and blocked with 1% BSA in PBS. The cells were subsequently incubated with mouse monoclonal antibodies against vimentin (Santa Cruz Biotechnology, sc-58901) overnight at 4 °C. The cells were then washed three times with PBS and incubated with FITC-labeled goat anti-mouse IgG secondary antibodies for 30 min at room temperature. The cells were then washed three times, stained with PI, and observed under the confocal microscope. Images were acquired using a confocal system (LSM 510 Image Examiner, Zeiss).
Construction of retroviral vector containing SV40 LT and the production of retroviruses
To clone the open reading frame of SV40 LT antigen into the retroviral vector pDON5-neo, a polymerase chain reaction (PCR) was performed with pW2TT, which contains SV40 LT template cDNA. The primers used were as follows: 5′-gcg agatct ACCATGGATAAAGTTTTAAAC-3′ and 5′-ccg gtcgac TTATGTTTCAGGTTCAGGG-3′. PCR was performed with an initial denaturation at 95 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 3 min, and a final extension at 72 °C for 7 min. After digestion with BglII (Takara Biotechnology) and SalI (Takara Biotechnology), the products were cloned into pDON5-Neo (Takara Biotechnology). The HEK293T packaging cells were co-transfected with pDON5-neo-SV40LT and pCL-ampho by using lipofectamine 2000 (Invitrogen). The supernatants containing the recombinant retrovirus were harvested, filtered through a 0.45-μm filter, and frozen at −80 °C until use as the infectious source for immortalization.
Immortalization of dermal fibroblasts
After three passages, logarithmic phase cells were seeded on 35-mm dishes. After overnight incubation at 25 °C, cells were infected with a 100 μL viral stock per plate. Twenty-four hours later, the virus-containing medium was replaced with culture medium. After another 24 h, neomycin-resistant cells were selected by adding 300 μg/mL G418 (Invitrogen) into the medium. G418-resistant colonies were isolated and subcultured. After more than 20 passages, the growth properties and karyotypes of the immortalized cells were analyzed.
Western blotting of vimentin and SV40 LT
Immortalized or non-immortalized cells were grown on 35-mm dishes. After reaching confluency, cells were collected, washed, and then immediately lysed on ice with a lysis buffer containing 50 mM HEPES (pH 7.4), 5 mM EDTA, 50 mM NaCl, 1% Triton X-100, 50 mM NaF, 5 mg/mL aprotinin, 5 mg/mL leupeptin and 1 mM phenylmethylsulfonyl fluoride. Proteins (30 μg) were electrophoresed in SDS–polyacrylamide gels and transferred onto PVDF membranes. The membrane was subsequently blocked with 3% BSA and incubated with the appropriate primary and secondary antibodies. Protein bands were visualized with Super Signal chemiluminescence reagents (Pierce, Rockford, IL, USA), as previously described (Taupin and Podolsky 1999).
Measurement of telomerase activity
Telomerase activity was determined using the TRAPeze telomerase detection kit (Chemicon, Temecula, CA, USA). After washing in PBS, 106 viable cells were resuspended in 200 μL CHAPS lysis buffer (10 mM Tris–HCl, pH 7.5, 1 mM MgCl1, 1 mM EGTA, 0.1 mM Benzamidine, 5 mM β-mercaptoethanol, 0.5% CHAPS, 10% Glycerol), incubated on ice for 30 min, and then centrifuged at 13,000 rpm at 4 °C for 20 min. Supernatants were used for the detection of telomerase activity. The detection of telomerase activity in the supernatants was carried out as previously described (Ossum et al. 2004). After telomerase extension for 30 min at 30 °C, extension products were amplified by a two-step polymerase chain reaction (PCR) (94 °C for 30 s, 60 °C for 30 s) for 30 cycles. The products were separated by 10% polyacrylamide gel electrophoresis then exposed to X-ray film (Kodak) for varying periods for autoradiography.
Activation of p42 MAPK by FGF2
Confluent non-immortalized or immortalized Bombina maixma dermal fibroblasts in 6-well plate were treated with recombinant human FGF2 (Invitrogen) (10 ng/mL) for 0 min, 15 min and 30 min. Then the collected cells were washed, and immediately lysed on ice with the lysis buffer containing 50 mM HEPES (pH 7.4), 5 mM EDTA, 50 mM NaCl, 1% Triton X-100, 50 mM NaF, 5 mg/mL aprotinin, 5 mg/mL leupeptin, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride. Proteins (20 μg) were electrophoresed in SDS–polyacrylamide gels and transferred onto PVDF membranes. The membrane was subsequently blocked with 3% BSA and incubated with anti-pERK (Santa Cruz, sc7383) or anti-ERK2 (p42 MAPK) (Santa Cruz, sc154) primary and then secondary antibodies. Protein bands were visualized with super signal reagents (Pierce, Rockford, IL, USA) as described (Taupin and Podolsky 1999).
Results
Establishment and characterization of the Bombina maxima dermal fibroblast cell line
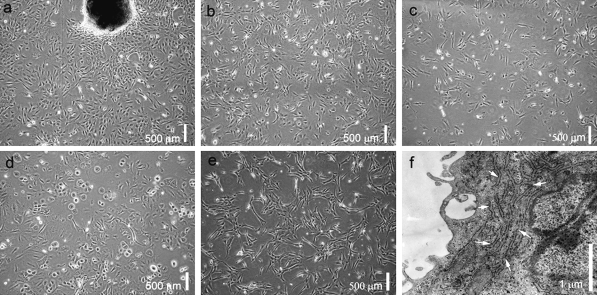
The partially digested small skin pieces attached to the flasks 6 h after seeding. Fibroblast-like cells and other types of cells migrated out from the skin pieces 5 days later (Fig. 1a). Cells continued to proliferate and became approximately 80% confluent in another 6 days. The cells were then subcultured. After three passages, purified fibroblast-like cells were obtained based on their faster growth rate relative to other types of cells (Fig. 1b). These cells were spindle shaped. Ultrastructural analysis of these cells showed that they possessed abundant rough endoplasmic reticulum (Fig. 1f), which is typical of fibroblasts. After thawing, more than 80% of the cells were viable and grew well (Fig. 1c). Unfortunately, after 15 passages, cells showed a decreased ability to proliferate (Fig. 1d).
Fig. 1.
Morphology and ultrastructure of Bombina maxima dermal fibroblasts. a Primary cultured cells from Bombina maxima tadpole skin pieces. b Subcultured Bombina maxima dermal fibroblasts (BMDF) at passage 5. c Thawed Bombina maxima dermal fibroblasts cultured for 24 h. dBombina maxima dermal fibroblasts at passage 15. e Immortalized Bombina maxima dermal fibroblasts. f Ultrastructure of Bombina maxima dermal fibroblasts, showing abundant rough endoplasmic reticulum (white arrows)
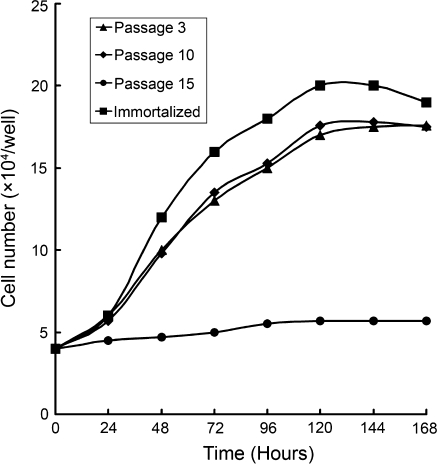
We generated growth curves of different passages of the Bombina maxima dermal fibroblast cell line. The growth properties of cells that were between passage 3 and passage 10 were not significantly different from one another. However, after passage 15, cells showed a decreased proliferative ability (Fig. 2).
Fig. 2.
Growth curve of Bombina maixma dermal fibroblasts. Cells at passage 3 (filled triangle) and passage 10 (filled diamond) showed almost the same proliferative ability. Cells at passage 15 (filled circle) showed decreased proliferative ability. Immortalized cells (filled square) showed much higher ability of proliferation
Growth curves were also generated for cryopreserved cells. We found that cryopreservation had no influence on cell growth properties. The appearance of the cells also remained the same as those cells that were not cryopreserved (Fig. 1c).
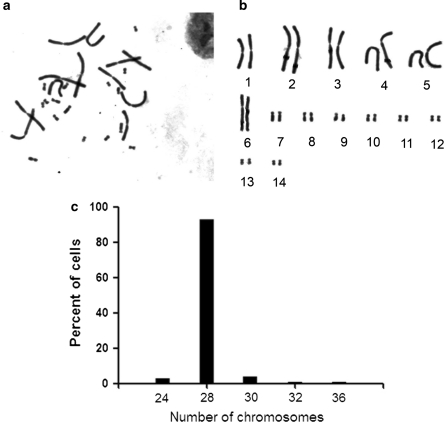
The chromosome number of Bombina maxima was 2n = 28. We observed six pairs of large chromosomes, one pair of medium chromosomes and seven pairs of small chromosomes (Fig. 3b). These results were consistent with those previously reported by Liu and Yang (1994). Chromosomes were counted at the third and fifth passage, and 93% of the analyzed cells were diploid in 100 cell spreads (Fig. 3c).
Fig. 3.
Karyotype of Bombina maxima dermal fibroblasts. a Cells were treated with 50 μg/mL of colcemid for two hours, and chromosomes were then prepared. b The diploid karyotype of Bombina maxima dermal fibroblasts, and the chromosome number was 2n = 28. c Chromosome number of Bombina maxima dermal fibroblasts at passage 5 ranged from 24 to 36, and 93% of cells have normal diploid karyotype
Identification of Bombina maxima dermal fibroblast cell line
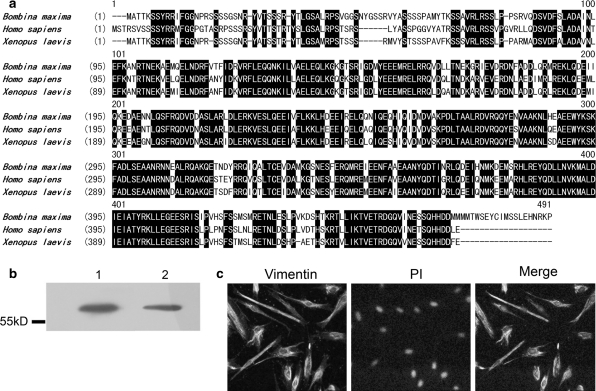
The intermediate filament vimentin has long been considered a reliable fibroblast marker (Sappino et al. 1990). Thus, the coding sequence of Bombina maxima vimentin mRNA was cloned in the present study. The deduced protein sequence of Bombina maxima vimentin was 84% identical (93% similar) to Xenopus laevis vimentin. The sequence was also 77% identical (90% similar) to human vimentin (Fig. 4a).
Fig. 4.
Amino acid sequence of Bombina maxima vimentin and identification of Bombina maxima dermal fibroblasts. a Multiple alignment of deduced amino acid sequence of Bombina maxima vimentin and vimentin from other species (Homo sapiens and Xenopus laevis). The identical amino acids were indicated by white letters on a black background. b Immunoblot detection of vimentin in Bombina maxima dermal fibroblast (lane 2) lysate and in human dermal fibroblast (lane 1) lysate. Position of molecular weight marker is shown on the left. c Immunostaining of vimentin in Bombina maxima dermal fibroblasts. The green color represents vimentin immunoreactivity stained with anti-vimentin antibody, while the red color shows nuclei stained with propidium iodide
To identify the Bombina maxima dermal fibroblasts, lysates were probed with a vimentin antibody. As shown in Fig. 4b, a 57 kD band was detected that corresponds to the calculated molecular weight of Bombina maxima vimentin. Bombina maxima vimentin has a molecular weight similar to human vimentin. Indirect immunofluorescence demonstrated that Bombina maxima dermal fibroblasts were positive for vimentin and that the vimentin is mainly located in the cytosol (Fig. 4c).
Immortalization of Bombina maxima dermal fibroblasts
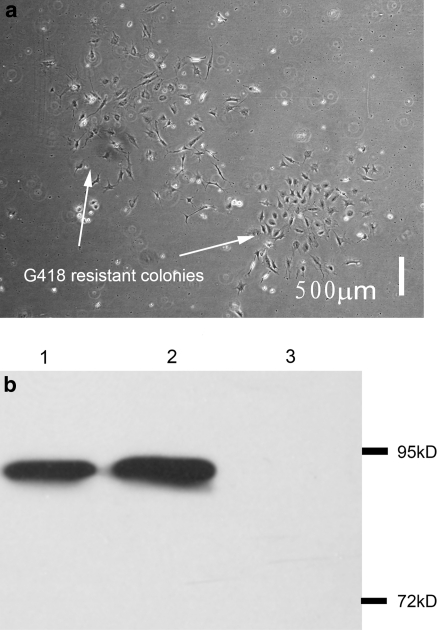
To construct the retroviral vector expressing the SV40 T antigen, a fragment containing the SV40 T coding sequence was cloned into the pDON5-neo vector. After verification of the construct by DNA sequencing, the pDON5-neo vector expressing the SV40 T antigen was co-transfected with the packaging vector pCL-ampho into HEK293T packaging cells. The recombinant retroviruses were then successfully obtained from the supernatants of transfected 293T cells. After incubating with recombinant retroviruses for 24 h in medium containing G418 for 13 days, G418-resistant cell colonies were observed (Fig. 5a). These colonies were then subcultured.
Fig. 5.
Selection of SV40 LT expressing colonies and immunoblot detection of SV40 LT expression in immortalized Bombina maxima dermal fibroblast. a G418-resistant colonies were obtained after selection by G418 (300 μg/mL) for 13 days from retrovirus-transduced Bombina maxima dermal fibroblasts. b Immunoblot detection of SV40 LT in immortalized Bombina maxima dermal fibroblasts. Non-immortalized Bombina maxima dermal fibroblasts (lane 3) do not express SV40 LT while immortalized Bombina maxima dermal fibroblasts strongly express SV40 LT (lane 2). The lysate of HEK293T cells which stably express SV40 Tag was used as positive control (lane 1). Positions of molecular weight markers are shown on the right
To confirm the expression of SV40 large T antigen in the G418 resistant colonies, cell lysates were probed with an SV40 T antigen antibody. As shown in Fig. 5, there was a clear 94 kD band that corresponds with the molecular weight of SV40 large T antigen. The growth curve of the SV40 large T antigen-positive cells demonstrates that these cells grew much faster than the non-transfected cells (Fig. 2). These cells were successfully propagated for more than 50 passages without morphological changes (Fig. 1e).
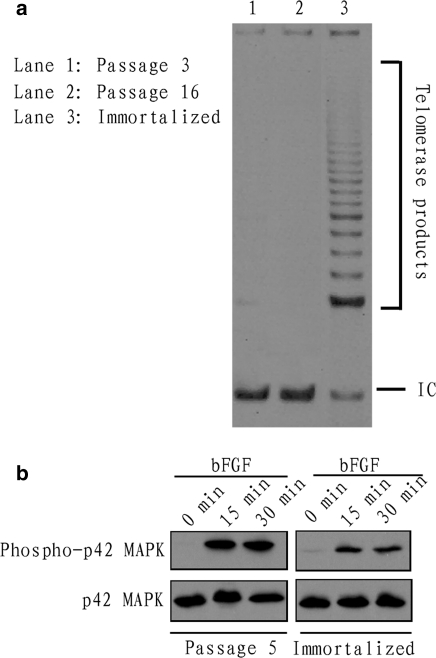
To reveal the mechanism involved in the immortalization of Bombina maxima dermal fibroblasts by SV40 T antigen, telomerase activities on low passage primary cells (Fig. 6a, lane 1), immortal cells (Fig. 6a, lane 3) and on cells in passage >15 (Fig. 6a, lane 2) were assessed. Bombina maxima dermal fibroblasts at low passage (passage 3) showed low telomerase activity. And the cells at passage >15 (passage 16) showed almost no telomerase activity. However, immortalized cells stably expressing SV40 T antigen showed much higher telomerase activity than non-immortalized cells.
Fig. 6.
Telomerase activity assay and p42 MAPK activation. a Detection of telomerase activity in the Bombina maxima fibroblasts. Telomerase activities of low passage (passage 3) primary cells (lane 1), immortal cells (lane 3) and of cells in passage >15 (lane 2) were assessed using the telomeric repeat amplification protocol (TRAP) assay. The presence of active telomerase is visualized by a characteristic 6 bp ladder after electrophoresis of the PCR-amplified telomerase products. IC, the 36 bp internal control, is useful for determining PCR efficiency. b p42 MAPK activation induced by recombinant human FGF2 (10 ng/mL). Both immortalized (right) and non-immortalized (left) Bombina maxima dermal fibroblasts displayed increased phophorylation level of p42 MAPK
Activation of p42 MAP kinase by basic fibroblast growth factor (FGF)
p42 MAPK phosphorylation level in immortalized or non-immortalized Bombina maxima dermal fibroblasts were assessed by western blotting at different times after stimulation with basic FGF. The results showed that after stimulation by basic FGF (10 ng/mL) for 15 min and 30 min, both immortalized (Fig. 6b, right) and non-immortalized (Fig. 6b, left) Bombina maxima dermal fibroblasts displayed increased phophorylation level of p42 MAPK. After the same time and dose of stimulation by basic FGF, the immortalized and the non-immortalized Bombina maxima dermal fibroblasts showed almost the same level of p42 MAPK phophorylation level. The results indicated that the immortalized Bombina maxima dermal fibroblasts could be efficiently used as in vitro tools for study of signal transduction, at least for research on p42 MAPK, which is a intracellular regulator implicated in many important cellular events such as cell proliferation (Zhang and Liu 2002), cell migration (Han et al. 2007) and cell differentiation (Lai et al. 2001a).
Discussion
Amphibian skin secretes a large amount of biologically active peptides and proteins, most of which have mammalian analogues. The amount of peptides and proteins secreted by the skin of amphibians is always much greater than the skin of mammals (Bevins and Zasloff 1990). Amphibian secretions are often used as defensive chemicals against predators. However, some secreted proteins show neither toxic nor antimicrobial activities, which indicates that they may target their own tissues and cells in an endocrine or paracrine manner. To uncover the physiological roles of these proteins, the ability to culture amphibian skin cells such as keratinocytes and fibroblasts seems indispensable. In the present study, a fibroblast cell line was established from Bombina maxima tadpole skins. The successful establishment of these cells was confirmed by our observation of cell morphology and vimentin immunostaining, which is a well-known fibroblast marker. In addition, these fibroblasts were immortalized by the overexpression of SV40 large T antigen to enable long-term culturing.
For the primary culturing of Bombina maxima dermal fibroblasts, lengthy tissue digestion by enzymes would be hazardous to cells; however, tissue pieces that had not been enzymatically digested were not easy to attach to the culture flasks. In addition, it was difficult for the cells inside the tissue to migrate out due to the tight extracellular matrix structure of the undigested tissue. Thus, the tissue pieces of tadpole skin were partially digested by type I collagenase in the present study. The results showed that partial digestion may be useful for facilitating the migration of cells from the tissue pieces with little harm to the cells.
Appropriate osmotic pressure and temperature conditions are important for cellular survival and proliferation (van der Merwe et al. 2010; Ellinger et al. 1983; Nishikawa et al. 1990). The osmotic pressure of most amphibian body fluids is lower than that of mammals (Balls and Worley 1973), which means that the traditional medium used for mammalian cell cultures may not be appropriate for culturing Bombina maxima dermal fibroblasts. Thus, DMEM/F12 was diluted with ultrapure water to produce the same osmotic pressure as Bombina maxima body fluids. The average temperature of the Bombina maxima habitat is about 25 °C, so we carried out all culturing procedures of Bombina maxima cells at 25 °C. Our results showed that the temperature of 25 °C was appropriate for the growth of Bombina maxima dermal fibroblasts.
Normal cells possess a stable chromosome number; thus, we analyzed the karyotypes of cultured cells. The results, on one hand, confirmed that the cells were indeed derived from the species Bombina maxima without contamination of other species of amphibian cells, and on the other hand, demonstrated that most cells were normal.
Although the cell morphology and ultrastructural images indicated that the cultured cells were fibroblasts, identification at the molecular level was performed for further confirmation. The intermediate filament vimentin is used as a marker for mesoderm-derived cells, including smooth muscle cells, cardiac muscle cells and fibroblasts. Within the skin, fibroblasts are the main cell type that is derived from the mesoderm. In fact, vimentin is one of the most commonly used fibroblast markers (Richards et al. 1995). Both western blotting and immunostaining showed that these cells strongly expressed vimentin, which further proved that the cells we isolated and cultured from the skin of Bombina maxima were indeed fibroblasts.
Growth curve analysis of cells at different passages indicated that the cells grew well, but unfortunately, after passage 14, their proliferative ability decreased. Thus, we then attempted to immortalize the cells for long-term culture. Several methods have been used for cell immortalization. Viral genes such as simian virus (SV40) T antigen (Zhu et al. 1991), Epstein-Barr virus (EBV) (Tosato et al. 1986), human papillomavirus (HPV) E6 and E7 (Halbert et al. 1992), and adenovirus E1A and E1B (Hurwitz and Chinnadurai 1985) can induce immortalization in many cell types. Among them, the SV40 T antigen may be the most simple and reliable agent for the immortalization of many different cell types in culture. The SV40 T antigen induces cell immortalization by inactivating tumor suppressor genes such as p53, Rb and others. Researchers have also found that SV40 T antigen can induce telomerase activity in the infected cells.
Many methods can be utilized for the overexpression of target genes. These methods include the transfection of non-viral-based vectors and infection with viral-based vectors. Viral-based vectors are always more efficient than non-viral-based vectors. Retroviral vectors have been successfully used to overexpress genes in amphibian cell lines (Burns et al. 1996). In the present study, the viral-based expression vector pDON5-neo was used to overexpress SV40 T antigen. This method resulted in both a high infection efficiency and a high level of SV40 large T antigen expression. Bombina maxima dermal fibroblasts were successfully immortalized as a result of the overexpression of SV40 large T antigen.
SV40 infection induces telomerase activity in human mesothelial cells (Foddis et al. 2002). Human primary mammary epithelial cells gradually acquired telomerase activity after transfection of SV40 large T antigen (Li et al. 2008). Thus, we assessed the telomerase activities on low passage primary cells, immortal cells and on cells in passage >15 and the results indicated that SV40 T antigen induced telomerase activity in Bombina maxima dermal fibroblasts, which may contribute to the maintenance of telomeric DNA.
Signal transduction analysis revealed functional p42 MAPK in immortalized Bombina maxima dermal fibroblasts. Thus, the immortalized Bombina maxima dermal fibroblasts may be useful in research on signal transduction in Bombina maxima physiology and pathology. However, to become a powerful in vitro tool, more details about the signal transduction system in Bombina maxima dermal fibroblasts need to be further elucidated.
In conclusion, we successfully cultured and immortalized the dermal fibroblasts of Bombina maxima. The cell line will be useful for further studies of the physiological role of Bombina maxima skin secreting proteins and peptides.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 Program, 2010CB529800), and the Chinese National Natural Science Foundation (30870304).
References
- Balls M, Worley RS. Amphibian cells in vitro II. Effects of variations in medium osmolarity on a permanent cells line isolated from Xenopus. Exp Cell Res. 1973;76:333–336. doi: 10.1016/0014-4827(73)90384-4. [DOI] [PubMed] [Google Scholar]
- Bevins CL, Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- Burns JC, McNeill L, Shimizu C, Matsubara T, Yee J-K, Friedmann T, Kurdi-Haidar B, Maliwat E, Holt CE (1996) Retroviral gene transfer in Xenopus cell lines and embryos. In Vitro Cell Dev Biol 32: 78–84. doi:10.1007/BF02723038 [DOI] [PubMed]
- Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- Ellinger MS, Sharif S, BeMiller PM. Amphibian cell culture: established fibroblastic line from embryos of the discoglossid frog, Bombina orientalis. In Vitro. 1983;19:429–434. doi: 10.1007/BF02619560. [DOI] [PubMed] [Google Scholar]
- Fan TJ, Ren BX, Geng XF, Yu QT, Wang LY. Establishment of a turbot fin cell line and its susceptibility to turbot reddish body iridovirus. Cytotechnology. 2010;62:217–223. doi: 10.1007/s10616-010-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foddis R, Rienzo A, Broccoli D, Bocchetta M, Stekala E, Rizzo P, Tosolini A, Grobelny JV, Jhanwar SC, Pass HI, Testa JR, Carbone M. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene. 2002;21:1434–1442. doi: 10.1038/sj.onc.1205203. [DOI] [PubMed] [Google Scholar]
- Gao Q, Xiang Y, Chen Z, Zeng L, Ma X, Zhang Y. betagamma-CAT, a non-lens betagamma-crystallin and trefoil factor complex, induces calcium-dependent platelet apoptosis. Thromb Haemost. 2011;105:846–854. doi: 10.1160/TH10-10-0690. [DOI] [PubMed] [Google Scholar]
- Gao Q, Xiang Y, Zeng L, Ma XT, Lee WH, Zhang Y (2011b) Characterization of the betagamma-crystallin domains of betagamma-CAT, a non-lens betagamma-crystallin and trefoil factor complex, from the skin of the toad Bombina maxima. Biochimie 93(10):1865–1872. doi:10.1016/j.biochi.2011.07.013 [DOI] [PubMed]
- Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MY, Kosako H, Watanabe T, Hattori S. Extracellular signal-regulated kinase/mitogen-activated protein kinase regulates actin organization and cell motility by phosphorylating the actin cross-linking protein EPLIN. Mol Cell Biol. 2007;27:8190–8204. doi: 10.1128/MCB.00661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz DR, Chinnadurai G. Immortalization of rat embryo fibroblasts by an adenovirus 2 mutant expressing a single functional E1a protein. J Virol. 1985;54:358–363. doi: 10.1128/jvi.54.2.358-363.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerslev HC, Ossum CG, Lindenstrom T, Nielsen ME (2010) Fibroblasts express immune relevant genes and are important sentinel cells during tissue damage in rainbow trout (Oncorhynchus mykiss). PLoS One 5:e9304. doi:10.1371/journal.pone.0009304 [DOI] [PMC free article] [PubMed]
- Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- Lai R, Liu H, Hui Lee W, Zhang Y. A novel bradykinin-related peptide from skin secretions of toad Bombina maxima and its precursor containing six identical copies of the final product. Biochem Biophys Res Commun. 2001;286:259–263. doi: 10.1006/bbrc.2001.5359. [DOI] [PubMed] [Google Scholar]
- Lai R, Liu H, Hui Lee W, Zhang Y. An anionic antimicrobial peptide from toad Bombina maxima. Biochem Biophys Res Commun. 2002;295:796–799. doi: 10.1016/S0006-291X(02)00762-3. [DOI] [PubMed] [Google Scholar]
- Lai R, Liu H, Lee WH, Zhang Y. Identification and cloning of a trypsin inhibitor from skin secretions of Chinese red-belly toad Bombina maxima. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:47–53. doi: 10.1016/S1096-4959(01)00479-1. [DOI] [PubMed] [Google Scholar]
- Lai R, Liu H, Lee WH, Zhang Y. A novel proline rich bombesin-related peptide (PR-bombesin) from toad Bombina maxima. Peptides. 2002;23:437–442. doi: 10.1016/S0196-9781(01)00642-8. [DOI] [PubMed] [Google Scholar]
- Lai R, Zheng YT, Shen JH, Liu GJ, Liu H, Lee WH, Tang SZ, Zhang Y. Antimicrobial peptides from skin secretions of Chinese red belly toad Bombina maxima. Peptides. 2002;23:427–435. doi: 10.1016/S0196-9781(01)00641-6. [DOI] [PubMed] [Google Scholar]
- Lai R, Liu H, Lee WH, Zhang Y. Two novel Bv8-like peptides from skin secretions of the toad Bombina maxima. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:509–514. doi: 10.1016/S1096-4959(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Lee WH, Liu SB, Shen JH, Jin Y, Zhang Y. Cloning of bradykinin precursor cDNAs from skin of Bombina maxima reveals novel bombinakinin M antagonists and a bradykinin potential peptide. Regul Pept. 2005;127:207–215. doi: 10.1016/j.regpep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Li L, Willimsky G, Seitz S, Xu Y, Li Y, Schwarz LE, Schlag PM, Blankenstein T. SV40 large T antigen-transformed human primary normal and cancerous mammary epithelial cells are phenotypically similar but can be distinguished in 3D culture with selection medium. Int J Cancer. 2008;123:1516–1525. doi: 10.1002/ijc.23657. [DOI] [PubMed] [Google Scholar]
- Li P, Li ZH, Dzyuba B, Hulak M, Rodina M, Linhart O. Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques. Biol Reprod. 2010;83:852–858. doi: 10.1095/biolreprod.110.085852. [DOI] [PubMed] [Google Scholar]
- Liu W, Yang D. On systematics and phylogeny of toads of genus Bombina in China. Acta Metallurgica Sinica. 1994;15:131–141. [Google Scholar]
- Liu SB, He YY, Zhang Y, Lee WH, Qian JQ, Lai R, Jin Y. A novel non-lens betagamma-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS One. 2008;3:e1770. doi: 10.1371/journal.pone.0001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W, Wang J, O’Brien PC, Fu B, Ying T, Ferguson-Smith MA, Yang F. The genome phylogeny of domestic cat, red panda and five mustelid species revealed by comparative chromosome painting and G-banding. Chromosome Res. 2002;10:209–222. doi: 10.1023/A:1015292005631. [DOI] [PubMed] [Google Scholar]
- Nishikawa A, Shimizu-Nishikawa K, Miller L. Isolation, characterization, and in vitro culture of larval and adult epidermal cells of the frog Xenopus laevis. In Vitro Cell Dev Biol. 1990;26:1128–1134. doi: 10.1007/BF02623689. [DOI] [PubMed] [Google Scholar]
- Ossum CG, Hoffmann EK, Vijayan MM, Holt SE, Bols NC. Isolation and characterization of a novel fibroblast-like cell line from the rainbow trout (Oncorhynchus mykiss) and a study of p38MAPK activation and induction of HSP70 in response to chemically induced ischemia. J Fish Biol. 2004;64:1103–1116. doi: 10.1111/j.1095-8649.2004.0378.x. [DOI] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod. 1995;52:609–615. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- Taupin D, Podolsky DK. Mitogen-activated protein kinase activation regulates intestinal epithelial differentiation. Gastroenterology. 1999;116:1072–1080. doi: 10.1016/S0016-5085(99)70010-7. [DOI] [PubMed] [Google Scholar]
- Tosato G, Marti GE, Yarchoan R, Heilman CA, Wang F, Pike SE, Korsmeyer SJ, Siminovitch K. Epstein-Barr virus immortalization of normal cells of B cell lineage with nonproductive, rearranged immunoglobulin genes. J Immunol. 1986;137:2037–2042. [PubMed] [Google Scholar]
- Merwe M, Auzoux-Bordenave S, Niesler C, Roodt-Wilding R. Investigating the establishment of primary cell culture from different abalone (Haliotis midae) tissues. Cytotechnology. 2010;62:265–277. doi: 10.1007/s10616-010-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Wan SG, Wei SS, Lee WH. Bm-TFF2, a trefoil factor protein with platelet activation activity from frog Bombina maxima skin secretions. Biochem Biophys Res Commun. 2005;330:1027–1033. doi: 10.1016/j.bbrc.2005.03.077. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Wang YY, Lee WH, Zheng YT, Zhang Y. Apoptotic activity of frog Bombina maxima skin albumin. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:153–159. doi: 10.1016/j.cbpb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu G, Wang Y, Xiang Y, Gao Q, Jiang P, Zhang J, Lee W (2011) Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cell Mol Life Sci. doi:10.1007/s00018-011-0678-6 [DOI] [PMC free article] [PubMed]
- Zhu JY, Abate M, Rice PW, Cole CN. The ability of simian virus 40 large T antigen to immortalize primary mouse embryo fibroblasts cosegregates with its ability to bind to p53. J Virol. 1991;65:6872–6880. doi: 10.1128/jvi.65.12.6872-6880.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]