Abstract
Recent evidence suggests adults selectively attend to features of action, such as how a hand contacts an object, and less to configural properties of action, such as spatial trajectory, when observing human actions. The current research investigated whether this bias develops in infancy. We utilized a habituation paradigm to assess 4-month-old and 10-month-old infants’ discrimination of action based on featural, configural, and temporal sources of action information. Younger infants were able to discriminate changes to all three sources of information, but older infants were only able to reliably discriminate changes to featural information. These results highlight a previously unknown aspect of early action processing, and suggest that action perception may undergo a developmental process akin to perceptual narrowing.
Effective management of one’s daily affairs requires efficient processing of the actions of others. Processing human action is critical for understanding others’ goals and intentions, which are in turn crucial for navigating social situations. Since human action is inherently complex and dynamic in nature, a powerful cognitive system that utilizes both top-down and bottom-up processes likely underlies action analysis (Baldwin, 2005). While adults can make use of top-down information to guide their action processing and goal inference, infants lack much of this information, and must rely more heavily on bottom-up processes to analyze others’ behavior. Thus, investigating how infants process the actions of others provides a window into the development of early social cognition.
One aspect of infant action perception that has yet to be directly examined is action discrimination – that is, infants’ ability to perceive differences among actions. We use the term action to refer to the intentional movements of a human agent in the execution of a goal, including the initiation, unfolding, and completion of the movements. Characterized as such, there are numerous properties of action that a human agent can vary: the spatial trajectory of their actions, the speed of their actions, the particular limb used, the kind of grasp used, etc. Exploring action discrimination in infancy can reveal what properties of action infants attend to and encode during their observation of other’s behavior. For instance, do infants readily discriminate between actions that vary according to a single property, such as speed? Are they better able to discriminate changes in certain properties over other properties? The goal of the current research was to investigate what properties of action infants attend to when observing actions, and whether their attention during action observation changes across development.
What is known about infants’ action discrimination comes from research exploring infants’ goal understanding. Infants’ ability to discriminate actions on the basis of goal information changes across development. Woodward (1998) tested whether infants understand that actions are directed toward particular goals. In her study, infants were habituated to an actor seated in front of two toys repeatedly selecting a particular toy on one side over another toy on the opposite side. Following habituation, the location of the toys were switched, and the actor now reached for the other toy in the old location (new goal) or the previously selected toy in the new location (new path). Thus, in the new goal event, the spatial trajectory and the reach location were maintained while the goal was altered, and in the new path event, the spatial trajectory and the reach location were altered but the goal was maintained. By 5–6 months, infants selectively encode the goal of a reach and grasp action – that is, grasping a particular toy – over the spatial trajectory of the reach (as evidenced by longer looking at the new goal event). In contrast, infants younger than 5 months of age fail to selectively encode the goal of the reach (Sommerville, Woodward, & Needham, 2005). Findings such as these, and findings with older infants and more complex actions, indicate that infants discriminate between actions that differ in terms of overall goal structure (Sommerville & Woodward, 2005; Woodward, 2009; Woodward & Guajardo, 2002).
The paradigm used in Woodward (1998) is optimal for testing whether infants appreciate the goal-directed nature of reaching actions. However, it provides much less information about what properties of action infants attend to in their online processing of action. Even for a simple action like reaching for and grasping a toy, infants could attend to multiple different properties: which toy the actor selects, the particular grasp used, the spatial trajectory of the reach, how fast the reach is executed, etc.1 In Woodward’s paradigm, infants’ looking was timed to the static outcome of the reach, and thus it did not measure what properties of the action infants register as they observe action unfold. A more direct investigation of the properties of action infants are sensitive to over the course of development would shed light on these issues, and would also enrich our understanding of early action processing.
Thus, the aim of the present research was to identify properties of action that infants attend to while they observe action, and to investigate whether there are changes in infants’ sensitivity to these properties over the course of development. The paradigm involved changing properties of a simple action – grasping and moving a toy – without changing the outcome or goal of the action. This approach allowed us to explore infants’ action discrimination abilities without requiring infants to understand goals. Note, however, that since goal understanding is a central aspect of mature action processing, infants’ sensitivity to different properties of action likely supports this understanding.
Recently, Loucks and Baldwin (2009) investigated action discrimination in adults, borrowing ideas from research on face discrimination. They identified two sources of perceptual information adults could attend to for discriminating actions: featural and configural action information. Featural action information was defined as information regarding relatively local, fine-motor information, such as how a hand contacts an object. Configural action information was defined as information regarding more global, spatial-relational properties of action, such as head, body, and arm trajectory. Loucks and Baldwin tested adults’ ability to detect changes in featural and configural action information. The data revealed that adults do attend to both sources of information, and also indicated that these two sources are processed distinctly: 1) processing of configural information was disrupted by inversion, while processing of featural information was not, 2) processing of featural and configural information relied on relatively distinct spatial frequencies, and 3) featural action information was selectively attended to over configural action information.
The selective attention to featural information was intriguing given that the changes in configural information were objectively larger changes on the screen relative to the changes in featural information. Loucks and Baldwin (2009) analyzed each of their change videos using an algorithm that measured the degree of pixel change between the standard and change video, and designed their configural change videos to alter significantly more pixels compared to the featural change videos. However, even when the changes in configural action information had an objective advantage, adults were more sensitive to the relatively small changes in featural action information.
Thus, adults are biased toward processing the features of action, and although they are able to process the configural aspects of action, they are somewhat less skilled in this regard. If these results highlight the end point of action perception in the mature action processing system, what is the nature of its development? Is this bias innate, or does it develop with experience processing action? If action perception changes with experience, what is the nature of change?
In the current experiments, we investigated whether developmental change occurs in infants’ attention to different properties of human action, as evidenced by their action discrimination. One possibility is that infants become increasingly sensitive to both sources of information, but continue to gain sensitivity to featural information when sensitivity to configural information levels off. Another possibility is that infants begin with relatively broad sensitivity to both sources, but lose sensitivity to configural information while maintaining sensitivity to featural information. In any case, we might expect young infants to be differentially sensitive to these sources of information compared to older infants. Especially considering that infants prior to the age of 5–6 months lack goal understanding (Sommerville et al., 2005), they may process action differently from adults. Older infants, who can reliably infer the goal of reaching actions, may process action more like adults and selectively attend to featural action information. Thus, the selective attention effects documented in adulthood may have their roots in infancy.
Although Loucks and Baldwin (2009) only examined featural and configural sources of information in their published work, there is a third source of perceptual information that is specific to action as a dynamic stimulus: temporal action information. Temporal action information can be defined as information regarding the speed at which an action is carried out. In unpublished work, Loucks and Baldwin have found that adults are relatively poor at detecting changes in this property of action, as they are with configural action information. Thus, in the current research we also examined infants’ discrimination of temporal action information, as they may attend to this information more than adults.
The younger infants in our study were 4 months of age, and the older infants were 10 months of age. Prior work has demonstrated a broad transition between these ages in infants’ ability to process the actions of others (Baldwin, Baird, Saylor, & Clark, 2001; Falck-Ytter, Gredebäck, & von Hofsten, 2006; Sommerville & Woodward, 2005; Sommerville et al., 2005; Woodward, 1998, 2009), and thus these ages may be maximally distinct from one another in terms of potential developmental change. In order to examine infants’ action discrimination, infants were habituated to a simple video of an actor moving a toy across a table. Following habituation, infants saw videos with changes to featural, configural, and temporal properties of the action. Our hypothesis was that younger infants would attend differently to these sources of information in comparison to older infants, and that older infants, like adults, would show stronger sensitivity to changes in featural action information.
Method
Participants
Participants included 24 four-month-old infants (10 females, 14 males, Mage = 4 months 1 day, range = 3 months 23 days to 4 months 11 days) and 24 ten-month-old infants (12 females, 13 males, Mage = 9 months 23 days, range = 9 months 17 days to 10 months 6 days). All infants were full term (at least 37 weeks gestation), typically developing, and from a large metropolitan area. Participants were recruited from a database maintained by the university at which the research was conducted. Based on parental report of ethnicity, 43 infants were classified as White, 1 as Asian/Pacific Islander, and 4 as mixed or unlisted ethnicity. An additional 24 infants (10 ten-month-olds and 14 four-month-olds) were tested but excluded from the final sample due to: failure to habituate (N = 11), excessive fussiness or lack of attention (N = 8), having a test trial looking time beyond three standard deviations of the mean (N = 1), or experimental error (N = 2). Infants received a small toy for their participation.
Stimuli
Stimuli included four videos: the standard video, the featural change video, the configural change video, and the temporal change video. All videos were recorded digitally at 30 frames per second, imported and edited on a computer, and exported as Quicktime files without sound. Each video was the same length (7.5 seconds).
Still frames from each of the videos can be found in Figure 1. In the standard video, a smiling actor sat at a table with a toy cup to the actor’s right. In the standard video, the actor turned to look at the toy, reached for and grasped the toy with a whole hand grasp around the outside of the toy, moved it in a straight path across the table to his left, and the released the toy and withdrew his hand. The actor moved in sync with a metronome while recording to control the timing of each action segment. In each of the change videos, care was taken to ensure that each of the changes only affected the relevant property, without altering the other two properties. In the featural change video, the actor performed nearly identical actions with identical timing, except grasped the toy on the inside of the toy cup instead of the outside. In the configural change video, the actor performed nearly identical actions with identical timing, except moved the toy in an arcing trajectory across the table, instead of a straight path. In the temporal change video, the actor performed identical actions, but moved the toy across the table at twice the speed of the standard: traversing the distance in one second instead of two seconds, the speed of the standard video. The reaching, grasping, and withdrawing actions were also made to be slightly slower in the temporal change video (approximately a half second slower on each end of the movement portion of the action), in order for it to match the standard video in overall length. Infants saw the actions in each video multiple times per trial, as the videos looped during playback.
Figure 1.
Still frames depicting the standard, featural change, and configural change videos. The temporal change video is not depicted.
The same algorithm previously described by Loucks and Baldwin (2009) was used to measure the amount of objective physical change between the change videos and the standard video. This algorithm compared the amount of pixel change between each frame in a change video and each frame in the standard video. Individual frames from a change video were temporally aligned with the individual frames of the standard video (e.g., the first frame of the standard aligned with the first frame of the change video, the second standard frame aligned with the second change frame, etc.), and the amount of pixel change between these aligned frames was calculated.2
Comparing the first frames of each of the change videos revealed that the change videos were unequal in their initial pixel change values. Since the actor remained still for the first second of the video, these differences were likely due to imperceptible changes in the position of actor, the toy, or the actor’s hands. Because such initial differences were essentially noise, we subtracted out this initial pixel change value from each of the successive pixel changes values to provide a baseline-corrected comparison. From these baseline corrected values, the average physical change across a video was then calculated for each change video/standard video comparison.
The pixel-change test demonstrated that, although somewhat larger, the amount of change in the configural change video (M = 677,255, SD = 908,915) was not significantly larger than in the featural change video (M = 626,446, SD = 708,550), t(214) = 1.59, p = .12, or in the temporal change video (M = 632,113, SD = 819,536), t(214) = 1.55, p = .11. The featural change video was also not significantly different from the temporal change video in this respect, t(214) = 0.15, p = .88. Thus, all three change videos were approximately equal in the amount of objective, physical change.
Design and Procedure
Infants were seated in their parent’s lap approximately 75 centimeters from a computer monitor. The monitor rested on a black table (61 × 98 cm), and infants sat at approximately the height of the monitor. From where infants were seated, the video stimuli subtended approximately 20.6 × 16.4 degrees of visual angle. The table and the infant and parent were surrounded on three sides by black curtains that reached to the ceiling and to the wall behind the infant and parent. A camera was mounted behind the curtains and above the screen such that only the lens was visible, in order to record infants’ looking. Parents were instructed to remain quiet and neutral throughout the testing session, and were also asked not to look at the screen.
Video stimuli and looking time calculations were controlled by a program utilizing Psychtoolbox (Brainard, 1997). Once the program was initiated, the looking task was entirely controlled by infants’ looking behavior. At the beginning of each trial, an attention getting stimulus was displayed on the screen (a flashing red and white checkerboard accompanied by a chime) in order to elicit infants to look at the screen. Once they were looking, the video for the trial began playing. Videos looped continuously, with a 0.5 second black screen inserted between successive presentations. The video played until two criteria were met: 1) the infant had looked for a cumulative total of at least four seconds, and 2) the infant looked away continuously for two or more seconds. If neither of these criteria were met, the video played for a maximum of 10 presentations (80 seconds). At the end of a trial, the next trial began immediately, again with the attention getting stimulus.
Infants first began with a habituation phase, in which the standard video was shown each trial. Infants viewed the habituation trials until the average looking on the last three consecutive trials was at or below half of the average looking time across the first three trials. Thus, infants could view a minimum of 6 and a maximum of 10 habituation trials. Following the habituation phase, the test phase began immediately. In the test phase, each of the change videos was shown twice, in alternating order, for a total of six test trials. The order of alternation of test trials was fully counterbalanced across infants.
A trained observer, unaware of the particular events shown on each trial, coded infants’ looking times online, by pressing a button on the keyboard when the infant was looking at the screen. A second observer recoded infants’ looking times offline from video. Trials in which both observers identified the same look away as ending the trial were considered agreements. Agreement was high (87.4%).
Results
For ease of interpretation, looking time data are reported as means. However, because this data was significantly positively skewed, all significance tests and effect sizes are based on natural log transforms of the looking time data. Preliminary analyses revealed no significant effects of gender or test trial order on looking times, so these variables were not considered further.
Habituation Trials
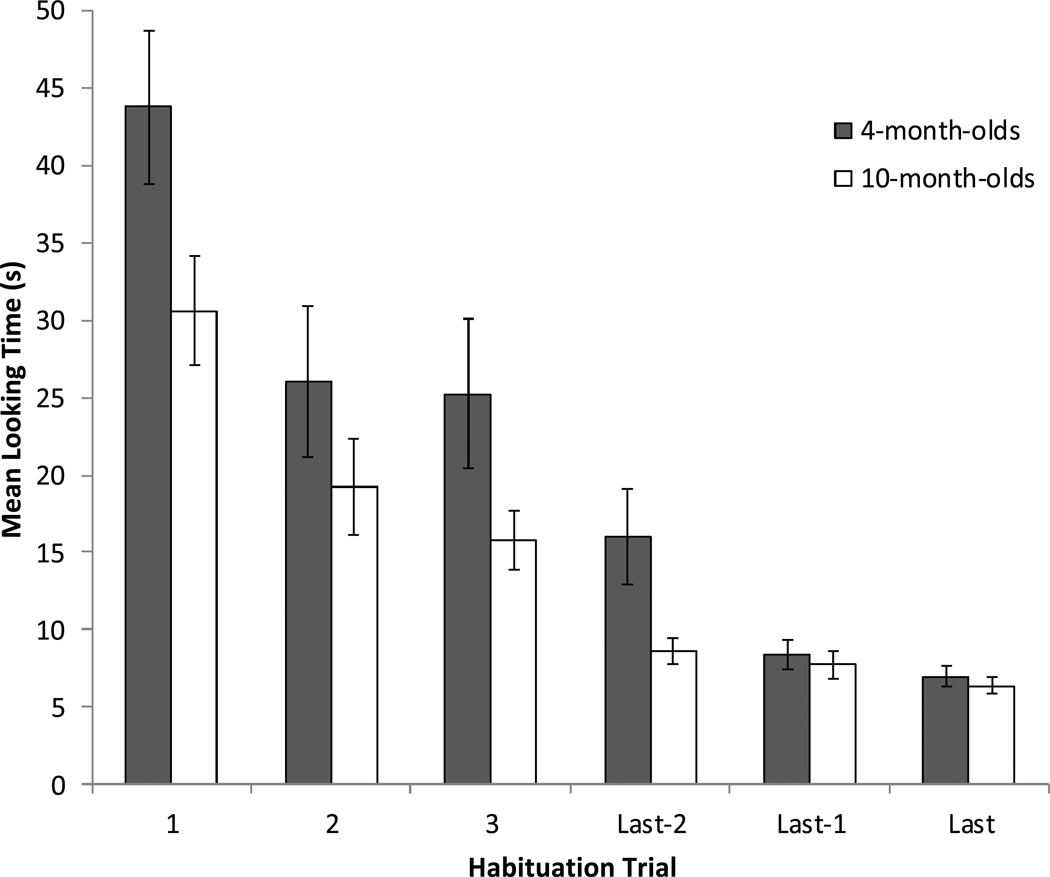
Looking data from the first three and last three habituation trials can be found in Figure 2. These data were entered into a 2 (age) × 6 (trial) mixed ANOVA. This revealed a significant main effect of trial, F(5,46) = 58.66, p < .001, η2 = .56. Orthogonal contrasts indicated that the effect was a significant curvilinear decline in looking, F(1,46) = 5.10, p = .029, η2 = .15. There was also a marginally significant main effect of age, F(1,46), = 3.60, p = .064, η2 = .07, indicating that 4-month-olds (M = 21.07, SD = 21.83) tended to look longer overall during habituation trials compared to 10-month-olds (M = 14.72, SD = 13.34). Importantly, however, there was no significant interaction between age and trial, F(5,46) = 0.63, p = .68, indicating that each age group showed similar rates of habituation to the stimuli. Similarly, the number of trials needed to reach the habituation criterion did not differ between 4-month-olds (M = 7.04, SD = 1.20) and 10-month-olds (M = 6.96, SD = 1.43), t(46) = 0.22, p = .83.
Figure 2.
Mean looking times during the first three and last three habituation trials as a function of age. Error bars represent standard error.
Test Trials
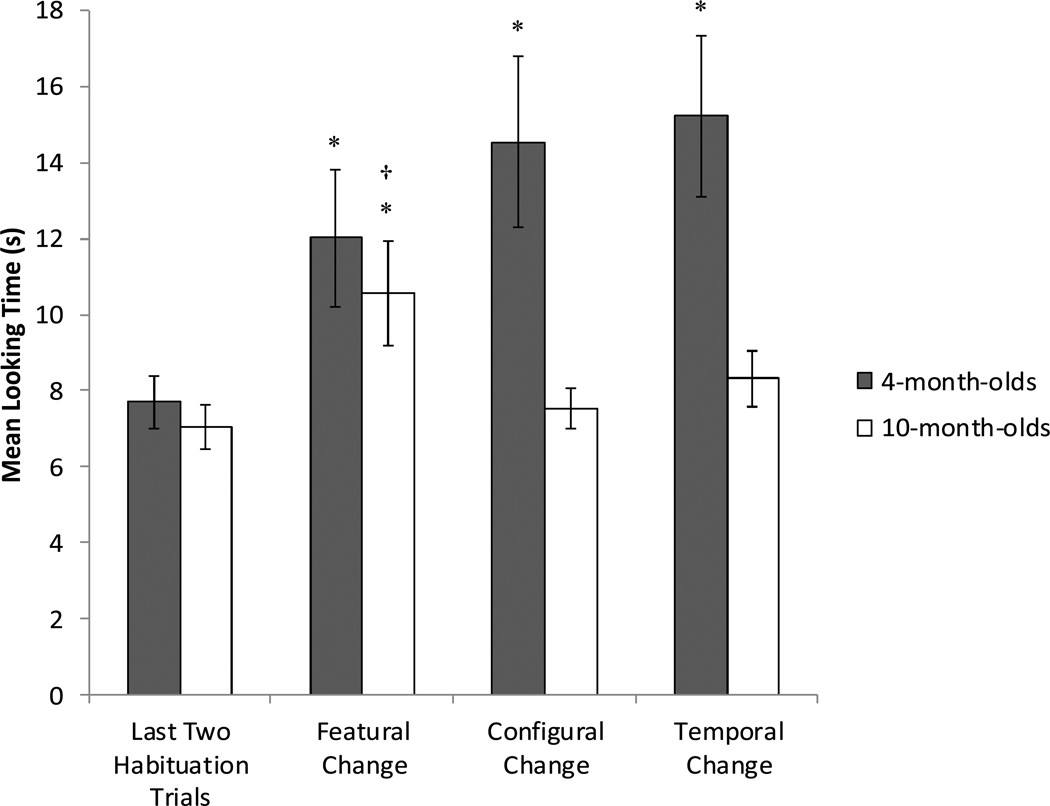
Total looking times to each of the change videos were averaged across pairs of test trials, and the total looking time to the last two habituation trials was also averaged. Mean looking times to the average of the last two habituation trials and the average of each of the test trials can be found in Figure 3.
Figure 3.
Mean looking times as a function of age and trial type. Error bars represent standard error. The asterisk indicates significant recovery from habituation, while the cross indicates significantly longer looking compared to the other test trials.
From these means, recovery scores for each change type were created by subtracting the mean looking time to each test trial type from the mean of the last two habituation trials.3 Recovery scores were submitted to a 2 (age) × 3 (trial) mixed ANOVA. This revealed a significant main effect of age, F(1,46) = 4.39, p = .042, η2 = .09. Younger infants (M = 6.23, SD = 10.49) recovered significantly more overall than did older infants (M = 1.76, SD = 4.59). There was no significant main effect of trial, F(2,92) = 0.55, p = .58. However, there was a significant interaction between age and trial, F(2,92) = 3.38, p = .038, η2 = .07
Follow-up analyses indicated that 4-month-olds recovered significantly more than the 10-month-olds to both the configural change, t(46) = 2.31, p = .026, Cohen’s d = 0.68, and the temporal change, t(46) = 2.49, p = .012, Cohen’s d = 0.73. The two age groups did not differ in the amount of recovery to the featural change, however, t(46) = 0.10, p = .93.
Additional planned t-tests within each age group were also conducted. For the 4-month-olds, looking to each of the changes was significant recovery from habituation: to the featural change, t(23) = 2.81, p = .01, Cohen’s d = 0.57, to the configural change, t(23) = 3.25, p = .004, Cohen’s d = 0.66, and to the temporal change, t(23) = 3.90, p = .001, Cohen’s d = 0.80. There were no significant differences in the amount of recovery between the featural and configural change, t(23) = 0.79, p = .44, the featural and temporal change, t(23) = 1.39, p = .18, or the configural and temporal change, t(23) = 0.62, p = .54. For the 10-month-olds, only looking to the featural change was significant recovery from habituation, t(23) = 3.62, p = .001, Cohen’s d = 0.74. Recovery to the temporal change approached significance, t(23) = 1.97, p = .061, Cohen’s d = 0.17, while looking to the configural change was not statistically distinguishable from habituation, t(23) = 0.86, p = .40. Furthermore, the recovery to the featural change in 10-month-olds was significantly larger than the recovery to the configural change, t(23) = 3.21, p = .004, Cohen’s d = 0.66, and the temporal change, t(23) = 2.21, p = .037, Cohen’s d = 0.45. The recovery difference between the configural and temporal changes was not significant, t(23) = 0.96, p = .35.
Finally, the data were also analyzed categorically, to see whether infants at each age group preferred a particular change type over others. The 4-month-olds appeared to show no particular preference: 9 looked longest at the featural change, 7 looked longest at the configural change, and 6 looked longest at the temporal change, and this pattern was not statistically different from chance, χ2(2, N = 24) = 0.25, p = .88. However, the pattern for the 10-month-olds was different: 15 looked longest at the featural change, only 3 looked longest at the configural change, and 6 looked longest at the temporal change. This pattern was significantly different from chance, χ2(2, N = 24) = 9.75, p = .008. Thus, at the very least, 10-month-olds preferred the featural change event over the configural change event.
General Discussion
The ability to discriminate changes in actions is a key component of people’s ability to process the goals and intentions of others. The current findings indicate that young infants are better able to discriminate a wider range of action properties than adults, and even older infants. When habituated to an actor moving a toy, 4-month-old infants were sensitive to changes in the way the actor’s hand contacted the toy (featural information), the path the toy and the actor’s hand took through space (configural information), and the speed at which the actor moved the toy (temporal information). The discrimination abilities of 10-month-old infants were much narrower: 10-month-olds only reliably discriminated the change in featural information. Thus, by 10 months of age, infants perceive action in a manner that is very similar to adults (Loucks & Baldwin, 2009).
Since the primary purpose of processing others’ actions is goal inference (Baldwin & Baird, 2001; Woodward, 2009), adults and older infants may selectively attend to featural information over configural and temporal information for the purposes of goal inference. Why is featural information more important for goal inference? One possible reason is that it provides functional information relevant for predicting future actions. For instance, picking up a coffee mug by the handle is functional for taking a sip from the mug, and depending on the circumstances an observer might predict that action will occur next. However, grasping a coffee mug over the open top of the mug is not functional for taking a sip – using this grasp has functional consequences in that it constrains the possible future actions that can be taken with the mug. Recent research suggests that by 10 months of age, infants are sensitive to changes in the type of grasp used on objects as it relates to the functional consequences of action (Loucks & Sommerville, in press).
It appears as if infants are initially prepared to process multiple sources of information that could potentially be relevant to goal inference, and then learn to identify which sources of information are more and less relevant for this purpose. Note, however, that configural and temporal information are still important sources of information in action to attend to in this regard. Information about the spatial trajectory of reaches is the basis for inferring the target of an individual’s reach, which is central to subsequent goal processing. In addition, some action categories are fully realized as changes in configural or temporal information: for example, pushing versus pulling, or placing versus slamming. Adults can discriminate actions based on these two sources of information (Loucks & Baldwin, 2009), and accordingly we do not believe infants lose the ability to discriminate changes to these properties. The current research only indicates that infants’ attention to these sources of information undergoes a change between 4 and 10 months of age.
In addition, the current research only examined discrimination based on one kind of action: a simple grasp and move event. It is not clear from the present results whether infants’ sensitivity to featural, configural and temporal information is similar for other types of actions, and whether similar developmental changes would occur between 4 and 10 months for other actions. Thus, future research should explore how generalizable the current findings are to other types of actions.
However, even if one assumes that featural information is more relevant for goal inference, why should sensitivity to configural and temporal information decrease over infancy? One possibility is that infants’ perceptual systems are able to dedicate more processing to featural information at the cost of processing the other two sources of information. By reducing cognitive processing of irrelevant sources of information, processing may be enhanced for relevant sources of information. If this were true, infants may be able to make more fine discriminations to changes in featural information at 10 months than younger infants can. Future research should explore this intriguing possibility.
Action discrimination is an aspect of action processing which supports goal inference at any age, and thus developmental change in action discrimination abilities inform developmental theories of early goal understanding. For example, to the extent that infants’ early goal understanding is accomplished using innate, modular, abstract principles (Gergely, Nádasdy, Csibra, & Bíró, 1995; Király, Jovanovic, Prinz, Aschersleben, & Gergely, 2003; Premack, 1990), the current findings clearly indicate that such principles are supported by underlying perceptual processes that may not be innate, and may be experience dependent. In addition, to the extent that changes in infants’ ability to understand the minds of others is a gradual, constructive process (Baldwin, 2005; Meltzoff, 2007; Tomasello, 1999; Woodward, 2009), changes in action perception appear to go hand in hand with broad social-cognitive developments that manifest around 9 months of age (Corkum & Moore, 1998; Tomasello, 1999). The developmental changes revealed in the present research may be another correlate of infants’ emerging intentional inference skills.
Woodward has shown that infants understand the goal structure of simple reach and grasp actions by 5–6 months (Woodward, 1998). The current research may help to explain why infants below 5 months of age are unable to demonstrate goal understanding in Woodward’s paradigm. Goal understanding in this paradigm is indicated by relatively less looking at the test trial in which the actor reaches for the old toy with a new spatial trajectory (consistency in object selection). The present results suggest that 4-month-old infants would find such a change in spatial trajectory salient, and potentially as salient as the change in object selection.4 Indeed, before the age of 5 months, infants look equally long at both test events, suggesting that spatial trajectory information weighs heavily in their representation (Sommerville et al., 2005).
Overall, the current results bear a striking resemblance to the phenomenon of perceptual narrowing that has been observed across several other domains of perception. In phoneme perception (Kuhl et al., 2006; Werker & Tees, 1984), face perception (Kelly et al., 2007; Pascalis, de Haan, & Nelson, 2002), music perception (Hannon & Trehub, 2005a, 2005b), and visual system development (Dobkins & Anderson, 2002), research has shown that infants begin with relatively broad skills for discriminating multiple properties or classes of stimuli, and with experience lose sensitivity to some properties while maintaining or enhancing sensitivity to other properties. Because this phenomenon has been seen across many domains, Scott, Pascalis, and Nelson (2007) raised the possibility that perceptual narrowing is a domain-general process in development that acts upon domain-specific areas of perception. At the neural level, the mechanism underlying the process may be a retraction of exuberant synaptic connections within the first-year of life (Dobkins, 2009; Huttenlocher, 1990). The present findings may in fact be another instantiation of this domain-general process of narrowing in a new perceptual domain.
If experience is the relevant mediating factor in this narrowing of perception between 4 and 10 months, what is the relevant experience? One potential candidate is infants’ first-person motor experience. Research supports the idea that infants’ motor representations are linked to their understanding of goal structure in others’ behavior (Sommerville & Woodward, 2005; Woodward & Guajardo, 2002), and that motor experience is causal in this role (Sommerville, Hildebrand, & Crane, 2008; Sommerville et al., 2005). As infants begin to reach and grasp for objects, they may come to learn that the configural and temporal aspects of their reaches are less relevant to the goal of obtaining an object. What path the infant takes to get an object, or how quickly the object is reached for and moved toward the infant, are less relevant than simply getting the object. However, using an appropriate grip that matches the properties of the object is critical to securely holding the object. As infants gain experience with these principles in their own behavior, they may map them on to their processing of others’ behavior. Recent evidence partially supports this idea: 10-month-olds’ understanding of the functional consequences of the precision grasp is correlated with their ability to perform precision grasps themselves (Loucks & Sommerville, in press). Similarly, Daum, Prinz, and Aschersleben (2011) found that 6-month-old infants’ ability to perform grasps influenced their understanding of the relation between different grasps and object shape. However, although we believe that active experience is the relevant input for these changes, it is an open question as to when the changes actually occur: they may be concomitant with the onset of grasping, or they may be tightly related to age-related cortical changes that occur around 9 to 12 months (Huttenlocher, 1990; Scott et al., 2007). We are currently conducting studies to explore these possibilities.
In conclusion, the present experiments shed light on the development of action discrimination skills in infancy, and provide the first evidence of developmental change in infants’ attention to action properties. Infants begin with undifferentiated action discrimination abilities, which gradually narrow over time to focus on processing the features of others’ actions. Infants early processing of action is different from older infants’ processing of action. This research opens the door to explorations of the potential benefits of this change in discrimination, and what mechanisms ultimately account for this specialization in perception.
Acknowledgements
This research was made possible by an NRSA postdoctoral fellowship F32HD058445 awarded to the first author, and an NICHD grant (1R03HD053616-01A1) to the second author. We are grateful to the members of the Early Childhood Cognition Lab for their assistance with data collection and coding for this study. We would also like to thank all of the families who volunteered to participate in the research.
Footnotes
Although the findings of Woodward (1998) seem to suggest that infants do not attend to spatial trajectory, they are ambiguous in this respect. Because infants’ looking was timed to the static outcome of the reach, infants may have ignored the spatial trajectory and only focused on the hand/toy relation.
Subtractions were performed after the log transformation.
While 4-month-olds found the configural change as salient as the featural change in this experiment, we cannot say that they would find such a change as salient as a change in object selection. Although object selection seems very similar to featural information, the way featural information has been defined here and in Loucks and Baldwin (2009), it is not identical.
References
- Baldwin D. Discerning intentions: Characterizing the cognitive system at play. In: Homer BD, Tamis-LeMonda CS, editors. The development of social cognition and communication. Mahwah: Lawrence Erlbaum Associates Publishers; 2005. pp. 117–144. [Google Scholar]
- Baldwin D, Baird JA. Discerning intentions in dynamic human action. Trends in Cognitive Sciences. 2001;5(4):171–178. doi: 10.1016/s1364-6613(00)01615-6. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Baird JA, Saylor MM, Clark MA. Infants parse dynamic action. Child Development. 2001;72(3):708–717. doi: 10.1111/1467-8624.00310. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Corkum V, Moore C. Origins of joint visual attention in infants. Developmental Psychology. 1998;34(1):28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- Daum MM, Prinz W, Aschersleben G. Perception and production of object-related grasping in 6-month-olds. Journal of Experimental Child Psychology. 2011;108(4):810–818. doi: 10.1016/j.jecp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Dobkins KR. Does Visual Modularity Increase Over the Course of Development? Optometry & Vision Science. 2009;86(6):E583–E588. doi: 10.1097/OPX.0b013e3181a72854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM. Color-based motion processing is stronger in infants than in adults. Psychological Science. 2002;13(1):76–80. doi: 10.1111/1467-9280.00414. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, von Hofsten C. Infants predict other people’s action goals. Nature Neuroscience. 2006;9(7):878–879. doi: 10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Gergely G, Nádasdy Z, Csibra G, Bíró S. Taking the intentional stance at 12 months of age. Cognition. 1995;56(2):165–193. doi: 10.1016/0010-0277(95)00661-h. [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences. 2005a;102:12639–12643. doi: 10.1073/pnas.0504254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Metrical Categories in Infancy and Adulthood. Psychological Science. 2005b;16(1):48–55. doi: 10.1111/j.0956-7976.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia, Developmental plasticity and recovery of function. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király I, Jovanovic B, Prinz W, Aschersleben G, Gergely G. The early origins of goal attribution in infancy. Consciousness and Cognition: An International Journal. 2003;12(4):752–769. doi: 10.1016/s1053-8100(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9(2):F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Loucks J, Baldwin D. Sources of information for discriminating dynamic human actions. Cognition. 2009;111(1):84–97. doi: 10.1016/j.cognition.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Loucks J, Sommerville D. The role of motor experience in understanding action function: The case of the precision grasp. Child Development. doi: 10.1111/j.1467-8624.2012.01735.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. The “like me” framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007;124(1):26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Premack D. The infant’s theory of self-propelled objects. Cognition. 1990;36(1):1–16. doi: 10.1016/0010-0277(90)90051-k. [DOI] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain-general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16(4):197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL. Pulling out the intentional structure of action: the relation between action processing and action production in infancy. Cognition. 2005;95(1):1–30. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96(1):B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H-joo, Baillargeon R. Can 9.5-month-old infants attribute to an agent a disposition to perform a particular action on objects? Acta Psychologica, Becoming an intentional agent. 2007;124(1):79–105. doi: 10.1016/j.actpsy.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Cambridge: Harvard University Press; 1999. [Google Scholar]
- Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior & Development. 1984;7(1):49–63. [Google Scholar]
- Woodward AL. Infants selectively encode the goal object of an actor’s reach. Cognition. 1998;69(1):1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants’ grasp of others’ intentions. Current Directions in Psychological Science. 2009;18(1):53–57. doi: 10.1111/j.1467-8721.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AL, Guajardo JJ. Infants’ understanding of the point gesture as an object-directed action. Cognitive Development. 2002;17(1):1061–1084. [Google Scholar]