Abstract
Introduction
p38γ kinase is highly enriched in skeletal muscle and is implicated in myotube formation. However, the activation status of p38γ in muscle is unclear.
Methods
p38γ activity in slow and fast adult mouse skeletal muscle tissue was examined as well as the impact of p38γ deficiency on muscle development and gene expression.
Results
p38γ is preferentially activated in slow muscle, but it is inactive in fast muscle types. Furthermore, the loss of p38γ in mice led to decreased muscle mass associated with a smaller myofiber diameter in slow muscle, but there was no impact on fast muscle in either mass or myofiber diameter. Finally, p38γ deficient muscle showed selective changes in genes related to muscle growth in slow muscle fibers.
Conclusion
Our study provides evidence that p38γ is selectively activated in slow skeletal muscle and is involved in normal growth and development of a subset of skeletal muscle.
Keywords: p38γ, mice, skeletal muscle, development, phosphorylation
Introduction
Muscular dystrophies and muscle wasting from AIDS, cancer and aging all cause deficits in muscle function. Understanding the developmental signaling pathways of skeletal muscle is crucial for development of future therapies aiming to ameliorate these disease states. Several previous studies have indicated that the p38 mitogen activated protein kinase (MAPK) family is involved in muscle development.1 p38 MAPKs are stress activated protein kinases that are activated in a cascade from MAP3K (or MAP kinase kinase kinase) to MAP2K kinase (i.e. MKK6) to MAPK (i.e. p38), which then activate downstream effectors,2 although alternative mechanisms of activation have also been identified recently.3,4 The p38 MAP kinase family includes 4 isoforms: p38α, β, γ and δ. The p38α isoform is ubiquitously expressed and is critical for skeletal muscle development by affecting myoblast proliferation based on both in vitro and in vivo loss of function studies.5-9
Although p38γ shares 66% homology with p38α, it has a unique PDZ binding domain and is insensitive to the inhibitor SB203580 due to a substitution of Met 109 in the ATP binding region.10 Unlike p38α, p38γ is almost exclusively expressed in skeletal muscle.11-14 This specific expression pattern in skeletal muscle led us to hypothesize that p38γ also plays a pivotal role in skeletal muscle development and pathophysiology. Indeed, earlier studies found that p38γ expression was increased during myoblast fusion.11,12 Myoblast differentiation could also be enhanced by p38γ overexpression, whereas loss of p38γ activity reduced the ability of myoblasts to form multinucleated myofibers.9,12 In this report, we found that p38γ was constitutively activated in slow skeletal muscle, while in fast muscle p38γ was not activated under basal condition. In p38γ−/− mice, myofiber and overall muscle size were reduced only in slow muscle, while fast muscle remained unaffected. The abnormal growth of slow skeletal muscle in p38γ−/− mice was also associated with changes in the expression of genes associated with muscle growth and atrophy, including myostatin, IGF-1R, atrogin and Murf1. Therefore, our study provides in vivo evidence that p38γ is constitutively activated in slow twitch muscle. This selective activation of p38γ appears to play a role in the normal development and growth of slow muscle.
Materials and Methods
Immunoblot
Immunoblotting was performed on protein samples prepared from muscle tissues isolated from 2 month old wild type (C57BL6) and p38γ−/− mice. The muscles were lysed by glass on glass homogenizers in lysis buffer containing protease and phosphatase inhibitors (50mM Tris HCl pH7.4, EDTA 1mM, EGTA 1mM, Triton X100 1%, 2.5mM Sodium Pyrophosphate, 1mM β–glycophosphate, 20mM Sodium Fluoride, 1mM Sodium Orthovanadate, 1mM PMSF and protease inhibitor tablet (Roche)). The total lysates from soleus, lateral gastrocnemius and diaphragm muscles were separated on an SDS-PAGE gel and blotted with antibodies specific for phospho-p38 (PT180/Y182), p-HSP27, p-MK2 (Cell Signaling), β-actin (Santa Cruz) and p38γ (provided by Dr. Jiahuai. Han). Quantitation of phospho-p38γ was performed by the Odyssey imaging software (li-cor).
p38γ mice
The p38γ−/− mice used in this study were generously provided by Boehringer Ingelheim Pharmaceuticals, Inc. under an Material Transfer Agreement with UCLA. The mice were originally generated by Boehringer Ingelheim Pharmaceuticals, Inc. in collaboration with Lexicon (Lexicon Pharmaceuticals, Inc., The Woodlands, TX) and bred in the C57B6 background. Adult male p38γ −/− and C57mice from 2 to 3 months of age were used in this study.
Histology and Immunohistochemistry
Standard hematoxylin and eosin (H&E) staining was performed on soleus and gastrocnemius muscles from p38γ and wild type mice. Briefly, anesthetized mice were perfused via the apex of the heart with 10% formalin to fixate for paraffin embedding and sectioning followed by H&E staining. All sections were imaged on a Nikon eclipse microscope and acquired with SPOT software and camera. As the gastrocnemius is multipennate, we measured fiber size through the smallest diameter of the fiber. For immunohistochemistry, (minimum of 3 mice per group) the soleus was cryosectioned and immunostained with antibodies specific for fMHC and sMHC (Novocastra, United Kingdom).
mRNA analysis
Total RNA was isolated from either the lateral gastrocnemius or the soleus muscles using the Trizol RNA purification system (Invitrogen). 1μg of total RNA was then reverse transcribed with OligoDT (SuperscriptII kit, Invitrogen) and used as a template for quantitative real-time polyermase chain reaction (qRT-PCR) using the MyiQ Real Time PCR detection system (Bio-rad) according to manufacturer’s specifications. For each mRNA examined, the cycle threshold (2cycle number) was normalized by glyceradehyde 3-phosphate dehydrogenase, and then as a fold change from wild type mice. See Table 1 for primer sequence information.
Table 1.
Sequences of primer pairs used for quantitative RT-PCR
| Gapdh-f TCCTGCACCACCAACTGCT | Gapdh -r GATGACCTTGCCCACAGCC |
| Tnni1-f GGACCTGAAGCTGAAGGTGC | Tnni1-r GACTTAGCAGCGTCGAACAT |
| Myod1-f TCCGCTACATCGAAGGTCTG | Myod1-r CCGCCTCACTGTAGTAGGCG |
| My5-f AGGAAAAGAAGCCCTGAAGC | My5-r CGAAAGCAAAAAGAACAGGC |
| Ppara-f TTGTGGCCAAGATGGTGGCCAA | Ppara-r CAGTTCTAAGGCATTGAACTTC |
| Ppargc1a-f AACTCAGCAAGTCCTCAGGGCT | Ppargc1a -r TTAGTTCACTGGTCTTGTCTGA |
| Slc2a4-f TCCAGCCATGAGCTATGTCTCC | Slc2a4-r CTCCTGCTCTAAAAGGGAAGGT |
| Rcan1-f GAGGAGGAAGAGGAAGAGAT | Rcan1-r TTCAGTGAATGGCCCAAGAC |
| Myh7-f CCTGGAGAAACCTGCCAAGTATGATGACA |
Myh7-r CTGCTTCCACCTAAAGGGCTG |
| Myh2-F AAGCGAAGAGTAAGGCTGTC | Myh2-R CTTGCAAAGGAACTTGGGCTC |
| Myh4-f GAAGAGCCGAGAGGTTCACAC | Myh4-r CAGGACAGTGACAAAGAACGTC |
| Myh1-f GAAGAGTGATTGATCCAAGTG | Myh1-r TATCTCCCAAAGTTATGAGTACA |
| Mstn-f ACCCATGAAAGACGGTACAAG | Mstn-r TCATCACAGTCAAGCCCAAAG |
| Trim63-f AAGCAGCTCATCAAGAGCATT | Trim63-r GTCTCTGCGTCCAGAGCG |
| Igf1r-f GAGATCGCCACGCTGGCT | Igf1r-r TAGTAGAAGGAGACCTCCTG |
| Atn1-f CTCAGAGAGGCAGATTCGC | Atn1-r CCGACTCCCAGCCATCCA |
| Igf1-f CTTTTACTTCAACAAGCCCAc | Igf1-r AGAGCGGGCTGCTTTTGTAG |
Statistical Analysis
For comparisons between p38γ−/− and wild type mice, the student t-test was employed.
Results
Selective activation of p38γ activity in slow skeletal muscles but not in fast muscle
We characterized the expression and activity of p38 MAP kinase isoforms by immunoblotting in different muscles, including the soleus, diaphragm and the lateral portion of the gastrocnemius. The soleus and diaphragm are considered slow muscles (predominantly type I and IIa fibers), while the lateral portion of the gastrocnemius is fast (predominantly type IIb, IIx). As shown in Figure 1A,B, phospho-p38α and total p38α were readily detected in all tissue samples at similar levels. In addition, the phosphorylation levels of canonical p38α downstream targets, including heat shock protein 27 (P-HSP27) and \MAPKAP2 (P-MK2) remained the same among different muscle types. In contrast, phospho-p38γ was only detected in the soleus and diaphragm but not in the lateral gastrocnemius, although total p38γ protein was expressed at the same level among different muscle tissues. This finding was replicated in muscles from 8 different mice (data not shown). This is thus an in vivo observation that p38γ is selectively activated in slow skeletal muscle tissues at a basal state.
Figure 1. Activation of p38γ in different muscle types.
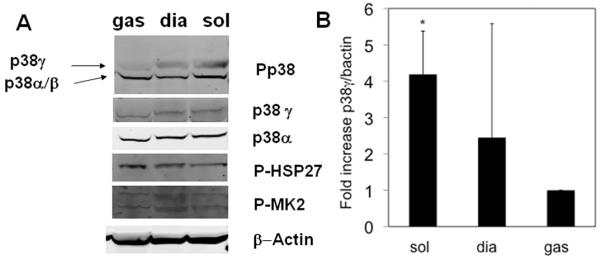
A). Representative immunoblots for phosphor-p38, total p38γ and total p38α, phosphor-Hsp27 and phosphor-MK2 as indicated in the protein extract prepared from adult mouse gastrocnemius (gas), soleus (sol), and the diaphragm (dia) tissues. actin was used for loading control. B). Quantitation of phospho-p38γ was from 3 separate muscles per muscle type and were subsequently normalized to β-actin. *p= 0.05
Genetic inactivation of p38γ in mice
p38γ−/− mice were generated via homologous recombination with LoxP sites by floxing Exon 1 and 2 of p38γ−/− (see Figure 2A).15 These mice were then crossed with a germline Cre, thereby creating a complete p38γ knockout mouse. As shown in Figure 2B, p38γ−/− solei have a complete loss of p38γ protein and a loss of p38 activity without any significant increase in p38α phosphorylation. (Figure 2B). These mice were viable and had no gross changes in appearance or behavior (data not shown) and no discernable change in total body weight (Figure 2C).
Figure 2. Characterization of p38γ−/− mice.
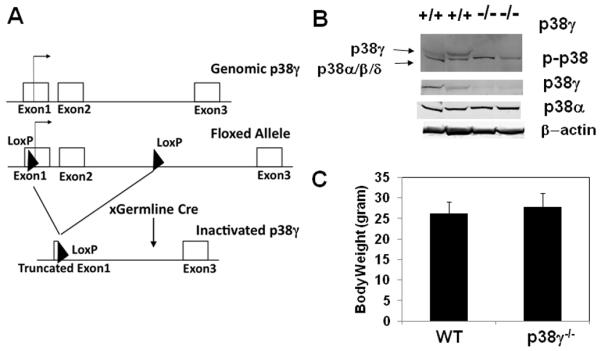
A). Illustration of p38γ−/− allele created by deletion of exon 1 and 2 of p38γ. B). Immuno-bolt of samples prepared from skeletal muscle for phosphor-p38, p38γ, p38α as indicated C).Total body weight of 2 month old male wild type (n=11) and p38γ−/− (n=14) mice.
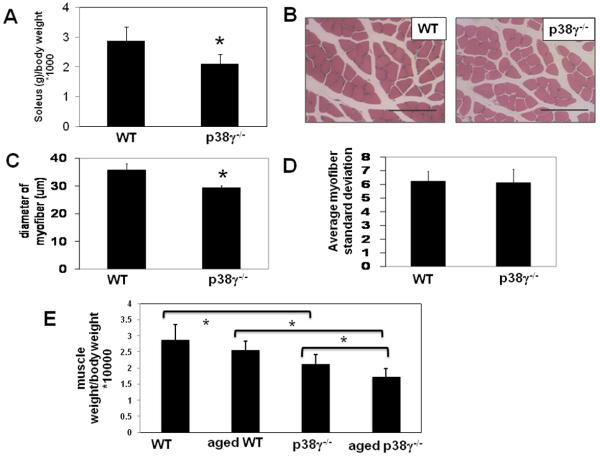
p38γ−/− mice have a selective decrease in muscle weight and myofiber size in the soleus
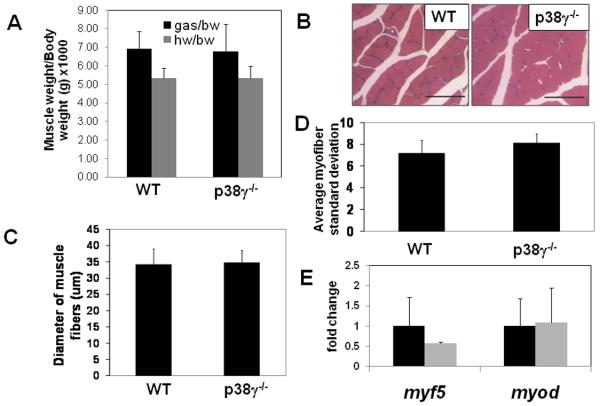
At 2 months of age, the solei of p38γ−/− mice were smaller in mass than wild type when they were normalized to body weight. This reduction in mass was restricted to the soleus, as the normalized masses of gastrocnemius and heart remained the same in both groups. (Figure 3A, 4A)
Figure 3. Histology of soleus muscle.
A). Body weight normalized soleus mass from wild type (n=11) and p38γ−/− (n=14) mice. B).Representative H&E section of wild type and p38γ−/− soleus muscle. C). soleus fiber diameter and D). standard deviation of diameter of widltype and p38γ−/− mice. E). Soleus muscle mass normalized to body weight from young and aged mice (n=4). *p= 0.05. Bar= 100μm
Figure 4. Histology of gastrocnemius muscle.
A). Body weight normalized heart mass and gastrocnemius mass (n=6 each). B). Representative H&E section of wild type and p38γ−/− gastrocnemius muscle. C). Quantification of gastrocnemius fiber diameter and D). standard deviation of diameter. Bar= 100μm. E). Relative mRNA levels of myf5 and myod in soleus measured by quantitative RT-PCR.
Based on histological analysis using H&E staining of muscle, the average p38γ−/− soleus myofiber diameter was significantly smaller than wild type controls (Figure 3B-C), while in the gastrocnemius, there was no difference between p38γ−/− and wild type controls (Figure 4B-C). Therefore, the selective reduction in soleus muscle mass appears to be caused by the smaller myofiber diameter in p38γ−/− mice.
This reduction in soleus muscle mass and myofiber size did not cause a change in total soleus fiber number at the widest soleus cross-section (773.3 ± 140.60 vs. p38γ−/− 631.0 ± 103.20, p=0.23) although there was a trend toward decreased total muscle area in p38γ−/− solei (941.5 ± 151.71) compared to wild type mice (1512.0 ± 411.17 μm2, p=0.08).
To determine whether there is an effect on muscle mass during aging, we compared older (5-8 months) and young (2 month old) solei. We found that wild type solei were identical to young solei in mass, however aged p38γ−/− solei were significantly smaller than young p38γ−/− solei. This indicates that p38γ is involved in the maintenance of muscle mass over time (Figure 3E).
Despite their smaller size, there was no induction in fiber size variability in p38γ−/− fibers when compared to wild type controls. (Figure 3D, 4D). Also there was no increase in centronucleation in any of these muscles from p38γ−/− mice. Additionally, quantitative RT-PCR showed no change in soleus mRNA levels for myoD or myf5, which are induced during muscle regeneration (Figure 4E). As all of these parameters of regeneration were unchanged in p38γ−/− muscle, we speculate that the reduced soleus muscle size observed in p38γ−/− mice was not due to defective regeneration or ongoing degeneration.
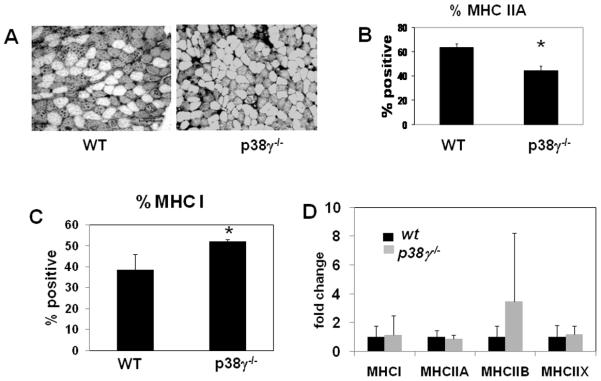
p38γ−/− mice have an increased percentage of slow myosin heavy fibers
As basal p38γ activity was detected selectively in the soleus, and selective reduction of soleus muscle size was observed in p38γ−/− mice, we further determined whether and how p38γ inactivation could have an impact on fiber type. By immunohistochemistry with antibodies that recognize fast myosin heavy chains (type IIa) or slow myosin heavy chain (type I), we found the percentage of fast twitch fibers in the soleus was significantly decreased in p38γ−/− animals compared to age-matched wild type controls (Figure 5A-B), while the slow twitch fibers was reciprocally increased (Figure 5C). However, the total soleus mRNA levels of different myosins (type I, IIa, IIb or IIx) were not changed as measured by qRT-PCR (Figure 5D).
Figure 5. Fiber type composition of soleus.
A). Representative image of wild type and p38γ−/− soleus muscles immunostained for fast MHC. B). Quantitative analysis of fast fiber (fast MHC positive) composition in wild type and p38γ−/− soleus muscle. C). Quantitative analysis of slow fiber (slow MHC positive) in wild type and p38γ−/− soleus muscle. D). mRNA levels of myosin isoforms in wild type and p38γ−/− soleus muscles analyzed by quantitative RT-PCR * p < 0.05. Bar= 100μm
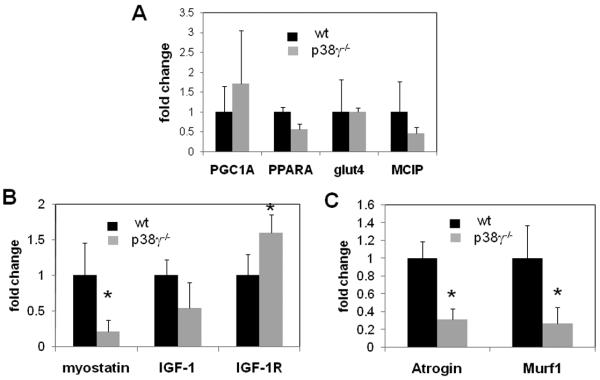
p38γ−/− mice have an altered expression of growth and atrophy related genes
Despite the significant changes in fiber types in p38γ−/− soleus muscle, we did not observe significant changes in the soleus mRNA levels of key metabolic regulators (PPARα, PGC1α and Glut4) or calcineurin downstream gene (MCIP) mRNA (Figure 6A). In contrast, a number of key regulators of muscle growth and atrophy were significantly altered, including a significant induction of IGF-1R and reduction of myostatin, atrogin and Murf1 (Figure 6 B, C). Therefore, the altered muscle mass, fiber size and fiber type in p38γ−/− soleus is associated with significant changes in genes related to myocyte growth and atrophy.
Figure 6. Gene expression analysis.
A). Relative mRNA levels of a subset of metabolic and calcineurin genes in soleus, B). muscle hypertrophy related genes and C). muscle atrophic genes. A minimum of 3 samples from each group was used and results are presented as arbitrary units after normalization against GAPDH.
Discussion
p38γ expression is highly enriched in adult skeletal muscle, and the p38 signaling pathway was shown to be involved in skeletal muscle differentiation and development in vitro. Yet, the specific in vivo role of p38γ activation across different muscle types had not previously been established. In this report, we found that p38γ was selectively activated in slow muscles but not in fast muscles. In p38γ−/− mice there was a decrease in soleus myofiber diameter and muscle mass. However, there was no significant change in fiber size variability, or induction of centronucleation or regeneration genes in the p38γ−/− soleus. In contrast, loss of p38γ did not change myofiber diameter or mass in the gastrocnemius, where p38γ activity was not observed basally in wild type animals. These observations imply that selective activation of basal p38γ activity has an important role in slow skeletal muscle development.
The relatively mild muscle phenotype observed in the p38γ−/− animals suggests that p38γ is not an essential factor for general skeletal muscle differentiation, and a redundant function of other p38 isoforms may have compensated its loss. However, the specific defects observed in the p38γ−/− soleus muscle and its selective activation pattern in slow muscle indicates that p38γ activity have a role in muscle growth and development of this subset of skeletal muscle.
It remains to be determined why p38γ is preferentially activated in slow muscles and not in fast muscles, as well as the molecular identity of the physiologically relevant upstream activators and downstream targets. The decrease in soleus fiber size and loss of fiber mass in p38γ−/− animals was not associated with significant changes in fiber size variability, or induction of centronucleation or early regeneration genes, thus suggesting that loss of p38γ in the soleus did not induce degeneration or injury. Furthermore, our gene expression data also suggest that changes observed in p38γ−/− are unlikely to result from defects in the calcineurin pathway, a critical player in muscle fiber type regulation16-20 or from metabolic genes involved in fiber type changes including PGC-1α.21,22 The underlying molecular mechanism involved in p38γ mediated regulation of muscle fiber switch in soleus requires further investigation.
In a recent study by Pogozelski et al, it was reported that p38γ−/− mice lost motor nerve stimulation-induced mitochondrial biogenesis and angiogenesis.15 However, in p38γ−/− mice, they also did not observe any changes in PGC-1α expression in association with the increase in type I fibers at a basal state. Interestingly, the soleus and diaphragm are more vascularized and contain more mitochondria than the lateral gastrocnemius. As the level of p38γ is activated in these slow muscles, it appears that, at rest p38γ activity may maintain this higher mitochondrial content in a basal state, similar to the need for p38γ to increase mitochondrial content as an adaption to exercise.
While the exact activation mechanism of p38γ is unclear in the diaphragm and soleus, it is plausible that the higher metabolic activity of slow muscle and higher mitochondrial content lead to higher oxidative stress in these muscles, which in turn activates p38γ. Previous studies found that p38γ is a stress activated protein kinase that was activated by MKK6 in an ATM-dependent manner during DNA damage.23 As p38γ was reported to interact with the mitochondrial proteins Sab and OMP25, it is possible that perturbations to p38γ could result in decreased mitochondrial function, which can have preferential impact on slow muscle.24,25 In a previous study by Carlson et al, p38 activity in the soleus and plantaris was increased at 24 hrs during compensatory hypertrophy. In their report, the phospho-p38 immunoblot detected two p38 bands from the soleus that correlated with p38α as well as a slower migrating band at the position of p38γ. Although the authors did not confirm this with a p38γ specific antibody, this slower migrating band was present only in the soleus but not in the more fast plantaris,26 in agreement with our observation. In overload surgeries that remove synergist muscles, the proportion of slow twitch fibers in plantaris increases at 9 weeks.27 It is possible that p38γ could become active in the plantaris as it becomes slow type muscle. In another study using a clenbuterol-mediated hypertrophy model, hypertrophy was found in the gastrocnemius but not in the soleus, and this was accompanied by a restricted increase in ERK in fast muscles (including gastrocnemius).28 Therefore, it is possible that the p38γ pathway is a slow muscle specific pathway for growth, while β-adrenergic stimulation of ERK is more important for fast muscle hypertrophy. Interestingly, we found a decrease in atrophic gene expression and an increase in IGF-1 receptor in p38γ−/− soleus tissue, supporting the conclusion from the histological analysis that the loss of soleus muscle mass was unlikely to be the result of enhanced muscle atrophy. Rather, it is possible that a loss of p38γ mediated growth signaling triggers these compensatory changes to enhance IGF-1 signaling and to suppress muscle atrophy. Future studies should investigate how p38γ affects the function of slow skeletal muscle. As p38γ loss modifies soleus muscle mass, it will be very interesting to see if these decreases also lead to a corresponding decrease in muscle strength and glucose uptake.
In summary, this study implicates p38γ as a specific regulator of selective muscle types under basal conditions. The establishment of the p38γ knockout mouse model will enable us to further investigate its role in muscle biology and diseases, and ultimately contributes to our better understanding of signal transduction in muscle development and regulation.
Acknowledgement
We would like to thank Dr. Jiahuai Han (The Scripps Research Institute, La Jolla, CA) for the p38γ antibody. The authors would also like to acknowledge the excellent technical assistance from Haiying Pu (UCLA) and the generation of the p38γ−/− mice by Lynn Pantages, Mary McFarland and Huiping Jiang (BIPI). This study is in part supported by NIH grants HL62311, HL088640 and HL080111 (YW). William Foster is a recipient of the UCLA Chancellor’s Fellowship and Yibin Wang was an American Heart Association Established Investigator
Reference
- 1.Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252(1-2):224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 3.Lu G, Kang YJ, Han J, Herschman HR, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281(9):6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- 4.Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ, Jr., Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6(4):390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 5.de Angelis L, Zhao J, Andreucci JJ, Olson EN, Cossu G, McDermott JC. Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev Biol. 2005;283(1):171–179. doi: 10.1016/j.ydbio.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Keren A, Bengal E, Frank D. p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev Biol. 2005;288(1):73–86. doi: 10.1016/j.ydbio.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102(2):221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 8.Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6(1):109–116. [PubMed] [Google Scholar]
- 9.Perdiguero E, Ruiz-Bonilla V, Gresh L, Hui L, Ballestar E, Sousa-Victor P, Baeza-Raja B, Jardi M, Bosch-Comas A, Esteller M, Caelles C, Serrano AL, Wagner EF, Munoz-Canoves P. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26(5):1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellon S, Fitzgibbon MJ, Fox T, Hsiao HM, Wilson KP. The structure of phosphorylated p38gamma is monomeric and reveals a conserved activation-loop conformation. Structure. 1999;7(9):1057–1065. doi: 10.1016/s0969-2126(99)80173-7. [DOI] [PubMed] [Google Scholar]
- 11.Tortorella LL, Lin CB, Pilch PF. ERK6 is expressed in a developmentally regulated manner in rodent skeletal muscle. Biochem Biophys Res Commun. 2003;306(1):163–168. doi: 10.1016/s0006-291x(03)00936-7. [DOI] [PubMed] [Google Scholar]
- 12.Lechner C, Zahalka MA, Giot JF, Moller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci U S A. 1996;93(9):4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens S, Craxton M, Goedert M. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 1996;383(3):273–276. doi: 10.1016/0014-5793(96)00255-4. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun. 1996;228(2):334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 15.Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4(11):e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. Embo J. 2000;19(9):1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275(7):4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 18.Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274(31):21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- 19.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12(16):2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest. 2007;117(9):2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 22.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282(41):30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, McGowan CH, Zhao M, He L, Downey JS, Fearns C, Wang Y, Huang S, Han J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20(13):4543–4552. doi: 10.1128/mcb.20.13.4543-4552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Court NW, Kuo I, Quigley O, Bogoyevitch MA. Phosphorylation of the mitochondrial protein Sab by stress-activated protein kinase 3. Biochem Biophys Res Commun. 2004;319(1):130–137. doi: 10.1016/j.bbrc.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 25.Court NW, Ingley E, Klinken SP, Bogoyevitch MA. Outer membrane protein 25-a mitochondrial anchor and inhibitor of stress-activated protein kinase-3. Biochim Biophys Acta. 2005;1744(1):68–75. doi: 10.1016/j.bbamcr.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Carlson CJ, Fan Z, Gordon SE, Booth FW. Time course of the MAPK and PI3-kinase response within 24 h of skeletal muscle overload. J Appl Physiol. 2001;91(5):2079–2087. doi: 10.1152/jappl.2001.91.5.2079. [DOI] [PubMed] [Google Scholar]
- 27.Tsika RW, Herrick RE, Baldwin KM. Subunit composition of rodent isomyosins and their distribution in hindlimb skeletal muscles. J Appl Physiol. 1987;63(5):2101–2110. doi: 10.1152/jappl.1987.63.5.2101. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, Zeng C, Ricome A, Hannon KM, Grant AL, Gerrard DE. Extracellular signal-regulated kinase pathway is differentially involved in beta-agonist-induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol. 2007;292(5):C1681–1689. doi: 10.1152/ajpcell.00466.2006. [DOI] [PubMed] [Google Scholar]