Abstract
Recent work implicates a link between action control systems and action understanding. In this study, we investigated the role of the motor system in the development of visual anticipation of others’ actions. 12-month-olds engaged in behavioral and observation tasks. Containment activity, infants’ spontaneous engagement in producing containment actions; and gaze latency, how quickly they shifted gaze to the goal object of another’s containment actions, were measured. Findings revealed a positive relationship: infants who received the behavior task first evidenced a strong correlation between their own actions and their subsequent gaze latency of anothers’ actions. Learning over the course of trials was not evident. These findings demonstrate a direct influence of the motor system on online visual attention to others’ actions early in development.
Social life presents a suite of information processing challenges. Interacting successfully with others requires swift and selective encoding of information about their actions, inferences about their motives, and predictions about their future actions. Research from several perspectives implicates the involvement of action control systems in these perceptual processes: neural and cognitive systems contribute to action production and are active during the observation of others’ actions (Beilock & Carr, 2001; Chartrand & Bargh, 1999; Decety, Chaminade, Grezes & Meltzoff, 2002; DiPelligrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992; Iacoboni, 2005; Knoblich & Sebanz, 2006; Rizzolatti & Craighero, 2004). These findings have fostered wide-ranging proposals concerning the functional role of “mirror” systems in action perception, both in adults (Flanagan & Johansson, 2003; Gallese & Goldman, 1998; Kilner, Friston, & Frith, 2007; Wilson & Knoblich, 2005) and in development (Csibra, 2007; Gerson & Woodward, 2010; Longo & Bertenthal, 2006; Meltzoff, 2007; von Hofsten, 2004). In the current study, we investigated the possibility that action production systems support the emergence of action anticipation.
Skilled action production requires prospective control, and this is manifest in the coordination of perception and action: actors visually anticipate the endpoints of their own actions (Johansson, Westling, Bäckstrom, & Flanagan, 2001). Adults show similar patterns of visual anticipation when observing others’ actions, suggesting that the same system is recruited in both kinds of responses (Flanagan & Johansson, 2003; Falck-Ytter, Gredebäck, & von Hofsten, 2006). Cannon and Woodward (2008) found adults’ anticipation of others’ goal-directed actions was disrupted by concurrent motor load, but not by concurrent non-motor tasks, suggesting a functional relation between action production and action anticipation. Kilner, Vargas, Duval, Blakemore, & Sirigu (2004) documented this relation at the neural level; activation associated with motor preparation was found when adults viewed the first step of an action that could be predicted based on prior experience. Thus, in adults, anticipation of others’ actions seems to recruit action production systems.
Although it has been shown that infants visually anticipate nonsocial events based on both learned regularities (Canfield, Smith, Bresznyak, & Snow, 1997; Wentworth & Haith, 1998) and a priori expectations (Johnson et al., 2003; Rosander & von Hofsten, 2004), less is known about the mechanisms that support the development of action anticipation. In the first study to investigate this issue, Falck-Ytter et al. (2006) utilized eye-tracking methods to investigate infants’ and adults’ anticipation of human actions as compared to nonsocial events. Participants viewed events in which a person grasped and placed three balls into a bucket sequentially or events in which the balls moved on their own and landed in the bucket. In both events, the movement of the balls was highly regular and thus predictable. However, adults and 12-month-old infants anticipated the ball’s arrival at the bucket robustly only for the events involving the human action. Furthermore, Falck-Ytter and colleagues reported that both 12-month-olds and adults responded in this way from the early trials onward, suggesting that this response did not depend on learning over the course of the experiment.
If action production systems support the development of action anticipation, then developments that occur in infants’ action control during the first years of life should correspond to developments in their action anticipation. Falck-Ytter and colleagues’ findings suggest this may be the case: Six-month-old infants tested in their procedure did not anticipate the human action. They speculated that 6-month-olds’ lack of anticipation might reflect their lack of motor representation for that particular containment action. Two recent studies provide more direct evidence for a relation between action experience and action anticipation in infants. Gredebäck and Melinder (2010) found that 12-month-old infants’ propensity to visually anticipate the goal of a feeding action correlated strongly with their level of lifetime experience with feeding interactions. However, because infants’ feeding actions were not directly assessed, it is not clear whether the relation involves infants’ actions rather than other aspects of feeding experience. In a second study, Gredebäck and Kochukhova (2010) assessed 2-year-olds’ skills at placing pieces into a puzzle and their visual anticipation of events in which an adult placed pieces into a puzzle. Children’s success at placing puzzle pieces was correlated with their tendency to visually anticipate the adult’s actions. This finding provided the first direct evidence for a relation between action production and action anticipation in young children. It raises the question of whether this relation exists in younger infants, when action anticipation is first evident.
To address this question, we assessed 12-month-old infants’ anticipation of and spontaneous engagement in containment actions like those used by Falck-Ytter and colleagues. Although 12-month-old infants are able to place objects in containers, there is variation in the extent to which they spontaneously do so. We assessed this individual variation by giving infants the opportunity to engage in containment activities either before or after we assessed their action anticipation. We predicted that infants who engaged in high levels of containment actions would also be more likely to visually anticipate these actions when produced by others.
Method
Participants
Thirty infants were tested (mean age: 12 months; 12 days, range 12;2–13;0). Fifteen infants were randomly assigned to each order: behavior task first (9 females), or observation task first (9 females). From this sample, infants were non-Hispanic and 70% white, 17% African-American, 7% Asian, and 7% multiracial. Infants had a minimum 37 weeks gestation. An additional five were excluded from analyses due to an insufficient number of data points in the observation task (n = 3), or technical/equipment errors (n = 2).
Procedure
Behavioral task
Infants sat on a parent’s lap in front of a small table and were presented with a container holding four toys. The experimenter removed each toy, drew the infant’s attention to it, and then placed it on the table within the infant’s reach. Then the infant was given two minutes to play with the toys and container. Parents were asked to not to intervene. If the infant did not place a toy into the container after 45 seconds, the experimenter prompted the infant by placing an object into the container, and she repeated this with two more objects if the infant did not respond. If the infant placed all four toys into the container, the experimenter took them out and placed them back on the table. The trial ended after the infant placed a total of 12 objects into the container (i.e., all four objects put in, three times) or after two minutes. Each infant received three trials with unique sets of objects and containers.1
Session videos were coded offline by an observer who was unaware of the experimental hypothesis, using digital coding software (Interact, Mangold) to record all instances of the infant making manual contact with the container and toys as well as each time the infant placed an object into the container. This coding was used to compute two measures of engagement in containment actions: containment latency (the latency to the first placement of a toy into a container) and containment activity (the total number of toys placed into the container), as well as a measure of overall activity (the total amount of time spent touching the toys and/or container). A second observer coded 33% of the infants tested. Agreement was high for containment activity (93% of the coded trials). Containment latency and overall activity judgments were highly correlated (r > .97).
Observation task
Data were collected via corneal reflection using a Tobii 1750 eye-tracker with 17” monitor, from a viewing distance of approximately 60 cm. Clearview 2.5.1 software (Tobii Technology) was used for calibration and collection of time-integrated gaze data with the viewed images. Infants sat on their parent’s lap. Parents were asked not to talk or direct the infant’s attention. Each infant received a 9-point calibration to Clearview’s pre-set locations. The stimulus was a 13.5 s movie of a person iteratively placing three balls into a bucket. The first, second and third events in this sequence took 1.10, 1.08, and 1.33 s, respectively, from the start time of ball pick-up until entry into the bucket region of interest. Infants were shown the movie nine times, with 2–4 s of attention-getting animations before each presentation.
Start and End areas of interest (AOIs) were defined in the film clips (See Figure 1). For each of the 27 placement events, time of ball arrival to the End AOI was subtracted from the time of gaze arrival to the End AOI (gaze latency). Thus, a gaze latency of zero indicates gaze arrival at the same time as the ball, negative as gaze arrival before the ball, and positive after the ball’s arrival.
Figure 1.
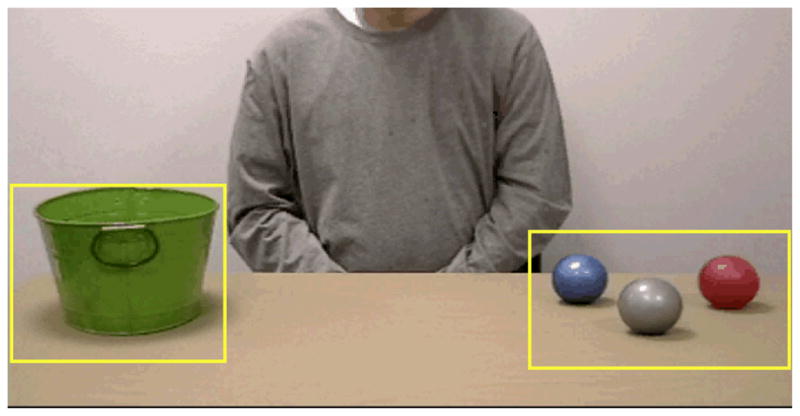
Areas of interest (AOI) used for data analysis: Start AOI, the region surrounding the balls (right), and End AOI, the region surrounding the bucket (left).
Data were collected within a time window surrounding each event, three times the length of the ball’s movement to the End AOI, with 1/3 preceding the pickup, and 1/3 after the ball’s arrival into the End AOI. For a data point to be calculated, the infant’s gaze must have fallen within the Start AOI prior to the ball’s arrival into the End AOI, followed by a gaze shift to the End AOI after the ball’s movement was initiated. Because this criterion was not obtained for every event from every infant, these data points were aggregated into one median score for some of the analyses reported here. 2 Infants with fewer than 9 of the possible 27 data points were excluded to reduce the variance commonly found in developmental data (n = 3 infants, as reported above). This inclusion criterion was slightly more conservative than the minimum of 7 used by Falck-Ytter and colleagues. An average of 18.70 (SD = 4.90) of the 27 data points per infant was obtained (69%, range = 10–27).
Results
First, we assessed the behavioral and observation tasks separately, with focus on potential effects of task order. No reliable effects of sex or age (as a covariate) were found, thus were not included in subsequent analyses.
Behavioral Task
Table 1 summarizes infants’ responses during the behavioral task. Twenty-eight of the 30 infants put at least one toy into the container (range = 0–12). Infants in the two task orders, behavior first and observation first, did not differ in their latency to begin placing toys into the container (t(28) = .31, p > .75), the number of toys placed into the container (t(28) = .08, p > .93), their overall levels of engagement with the toys and containers (t(28) = 1.27, p > .21), or in the number of prompts received from the experimenter (t(28) = .65, p > .52). As might be expected, infants with smaller containment latencies also had more containment activity, r = −.82, p < .001. Overall activity, the amount of time engaging with the containers and toys, was not correlated with containment activity, r = .19, p > .32, nor containment latency, r = 0.00, p > .99.
Table 1.
Means (and Standard Deviations) of behavioral measures for each task order.
| Task Order
|
||
|---|---|---|
| Behavior First | Observation First | |
|
M (SD) |
M (SD) |
|
| Number of Instances: | ||
| Containment Activity | 6.51 (3.86) | 6.99 (3.55) |
| Experimenter Prompts | 1.18 (1.25) | 0.92 (.89) |
| Amount of Time: | ||
| Containment Latency (seconds) | 37 (40) | 33 (26) |
| Overall Activity (seconds) | 71 (28) | 82 (17) |
|
|
||
Note. n = 15 in each group
Observation Task
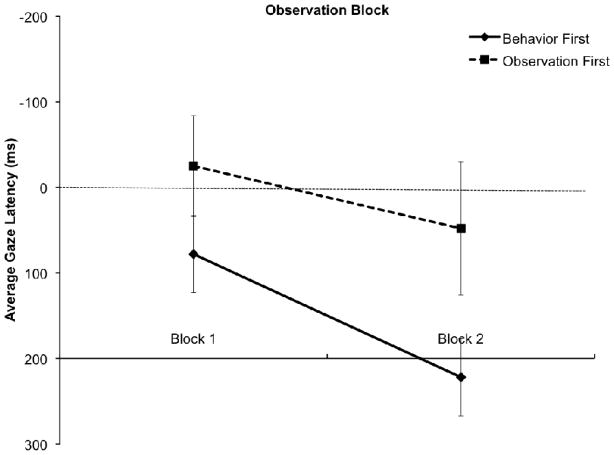
Figure 2 summarizes gaze latency scores across trials in the two orders. A analysis of variance with task order (behavior first, observation first) as the between subjects factor and trial block (trials 1–5 vs. trials 6–9) revealed effects of both block, F(1, 27) =4.34, p < .05, ηp2 = .14, and order, F(1, 27) = 4.34, p < .05, ηp2 =.14. Gaze latencies became larger over trials for infants in both orders, and infants in the observation first condition showed shorter latencies overall than did infants in the behavior first condition. These results suggest that infants’ anticipatory responses fatigued over the course of the testing session. Because gaze latency in the earlier trials (block 1) did not differ by task order, t(28) = 1.38, p > .18, the correlational analyses reported below were conducted both for gaze latencies overall, and for the gaze latencies on block 1.
Figure 2.
Average gaze latencies based on task order group (behavior first and observation first) and observation block (trials 1–5 and trials 6–9). The median gaze latency score for events in block 1 and block 2 was calculated for each infant, and averaged across the group. Points above 0 indicate gaze arrival before the ball arrival, and above 200 ms accounts for saccades launched prior to the ball arrival.
We next asked whether infants reliably anticipated the arrival of the hand at the container. Two criteria have been employed in past studies to address this question. Gredebäck and colleagues have classified gaze shifts that arrive at the target within 200ms of the event’s completion as anticipatory (e.g., for object representations: Gredebäck & von Hofsten, 2007, and actions: Gredebäck, Stasiewicz, Falck-Ytter, Rosander, & von Hofsten, 2009), based on estimates of occulomotor processing time in infants (see Gredebäck, Johnson, & von Hofsten, 2010 for a discussion). This criterion is applicable when the subject’s preparedness for a certain physical event is evaluated, for instance when shifting gaze to the reappearance of an occluded object (von Hofsten, Kochukhova, & Rosander, 2007). In contrast, in an action observation task there is no single distinct event, rather the whole action has to be considered. Gaze has to arrive at the goal ahead of the hand to guide it there. This is also the case when performing a manual action (Flanagan & Johansson, 2003). If gaze is also shifted to the goal proactively in the case of an observed manual action, this is taken as evidence that the action is projected onto the observer’s action system. There is evidence that infants perform such anticipatory gaze shifts (Falck-Ytter et al. 2006; Kochukhova & Gredebäck 2010; Rosander & von Hofsten, submitted). In line with this we classify saccades as anticipatory if they arrived before the hand at the container (< 0 ms). Using this criterion, overall, only 46% of the gaze shifts were anticipatory in the observation first condition and 36% in the behavior first condition. However, recall that within each trial, infants saw three balls placed into the container. Majority of gaze shifts by the third event to the container were anticipatory (56% in the observation first condition and 53% in the behavior first condition). Moreover, the gaze shift latencies overall were not just a reaction (200 ms or greater) to seeing the hand arriving at the goal (M = 71 ms; t(29) = 3.92, p < .001). They were slightly better than a pure reaction time when behavior was first: M = 142 ms; SD = 108 ms, t(14) = 2.10, p = .05; and much faster than a reaction time, on average when the observation was first: M = 0 ms; SD = 212 ms, t(14) = 3.65, p < .01).
Gaze latencies on the very first trial were also analyzed. We evaluated anticipatory saccades for each of the three events during the first trial. Although not all infants contributed usable data for each event, 96% of the possible data points were obtained. Data were “missing completely at random” as indicated by a non-significant Little’s MCAR test, χ2 (6) = 6.65, p > .35. Thus, a generalized estimating equations (GEE) model was used to test task order (behavior first, observation first) and repeated factor event (first, second or third ball to the bucket) on gaze latency in the very first movie presentation. There was a main effect of event, χ2 (2) = 46.35, p < .01, and no reliable effects of order (p > .32). The estimated marginal means (EMM) along with the 95% confidence intervals (CI) were as follows: event 1: EMM = 213 ms, CI = 130 to 296 ms; event 2: EMM = −71 ms, CI = −153 to 10 ms; event 3: EMM = −267 ms, CI = −394 to −139 ms. In the very first event, the CI ranged around 200 ms, not anticipatory by either criterion. However, by the time the person picked up the third ball, the gaze shifts were clearly anticipatory on this first trial. Therefore, infants’ anticipatory responses emerged very quickly, after just one or two passes of the event.
The overall gaze latencies we observed differed from those of Falck-Ytter and colleagues (2006), who found that 12-month-old infants, on average, anticipated the ball’s arrival at the container at the 0 ms criterion. One procedural difference that could account for this difference in findings is that, unlike Falck-Ytter et al., we did not use artificial cues to mark the end of each event. In their events, when each ball landed in the bucket an artificial sound was played and a face pattern imposed on the bucket made a small movement. Our findings are similar to findings from both infants (Gredeback et al., 2009) and adults (Eshuis, Coventry, & Vulchanova, 2009) for events which do not contain these cues. Across these experiments, when these end cues were present, adults and infants showed gaze latencies significantly below zero. In the absence of these cues, however, adults and infants failed to meet this criterion. Although our findings do not provide unambiguous evidence that infants, as a group, robustly anticipated the events, they do provide a measure of infants’ relative propensity to do so that can be used to ask our central question, that is, whether individual variation in infants’ prospective attention to actions correlates with their own action experience. The next analyses examined this issue.
Relations between action and observation
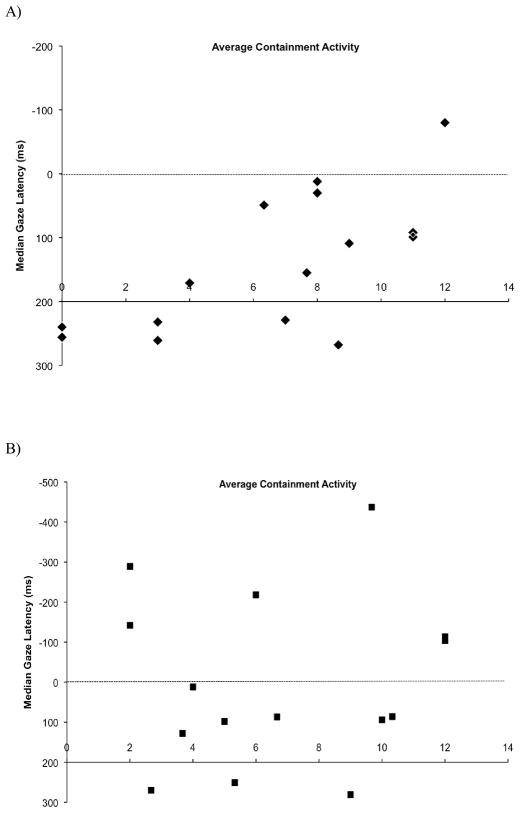
The question of interest was whether containment activity was related to gaze latency in the observation task. A regression conducted for containment activity on median gaze latency scores across all events and orders trended positive, though was not significant, F(1,29) = 2.18, p >.15, r = .27. However, because task order influenced gaze latency, the groups were split for separate regressions. As plotted in Figure 3, the behavior first group revealed a strong relationship between containment activity and median gaze latency, F(1,14) = 14.03, p <.01, r = .68, whereas the observation first group did not, F(1,14) = .12, p >.73, r = .10. Critically, a follow-up analysis of median gaze latency and containment activity controlling for age confirmed the relationship was particular to containment activity for the behavior first group, r(12) = .66, p = .01, but not the observation first group, r(12) = −.03, p > .91. Additional analyses of covariance of containment activity x order on gaze latency indicated the relationship was strong in block 1 for the behavior first group, F(1,14) = 6.93, p < .05, r = .59. All other analyses involving containment activity on gaze latency (observation first group and both groups in block 2) were not significant (all ps > .16). Similar patterns emerged for behavioral measure of containment latency. The behavior first group showed a significantly negative relation between containment latency and median gaze latency, in that shorter latencies to put objects into containers were related to more anticipatory gaze latencies, F(1,14) = 5.07, p < .05, r = −.53, but this relation was not strongly evident in the observation first group, F(1,14) = .46, p > .51, r = .18.
Figure 3.
Individuals’ median gaze latency as a function of containment activity for each task order, a) behavior task first, r = .68, and b) observation task first, r = .10.
Follow-up analyses evaluated whether more general aspects of task familiarity and attentiveness drove the relations found between action anticipation and containment activity. Overall activity (reported in Table 1) during the behavioral task was not reliably correlated with gaze latency for either task order group, ps > .53, ruling out the possibility that simple contact with and exposure to toys and containers prior to observation were responsible for the relations reported above. Rather, engagement in containment actions per se seemed to be critical. We next asked whether the relation between containment activity and gaze latency could be explained by differential attentional engagement. For example, infants who were high in containment activity may have been more likely to pay attention to the videos. As a measure of attention in the observation task, we used the number of usable data points obtained during the session, because these were contingent on infants’ attention at the beginning and end of each event. The two groups did not vary: the behavior first group averaged 17.53 (SD = 5.68) and the observation first group averaged 19.33 (SD = 3.90) data points, t(28) = 1.35, p > .18. Moreover, no relations were found between containment activity and number of data points in either group, (behavior first: r = .27, p > .33; observation first: r = −.37, p > .17). Thus, the correlation between gaze latency and infants’ prior actions seemed to derive from a specific relation between these two measures rather than from infants’ general activity level and attention to the events.
General Discussion
These findings reveal a relation between infants’ production of actions and their prospective attention to those same actions in others. Infants were variable in the extent to which they attended prospectively to the observed actions. Indeed, consistent with prior findings using similar methods (Gredeback et al., 2009), it is not clear whether infants’ overall gaze latencies in the present experiment can be considered anticipatory. Even so, gaze shifts at the very start of the session were unambiguously anticipatory. Similarly, although nearly all infants produced containment actions, they varied greatly in the extent to which they did so. Critically, individual variation on these two measures was correlated. When infants were given the opportunity to engage in containment actions prior to the observation task, their spontaneous level of activity placing objects into containers predicted their subsequent tendency to anticipate the observed containment actions. These findings are consistent with Gredebäck and Kochukhova (2010), who found a relationship between motor behaviors performed and anticipatory gaze shifts at two years. Thus, regardless of whether infants in this study consistently anticipated the goal of other people’s actions in their gaze shifts, infants’ tendency to attend prospectively to others’ actions was related to their own actions.
This correlation was not a product of differences in overall attention. Infants who were relatively inactive with the containers attended to the subsequent observation events to the same extent as those who were more active. However, their gaze latency to the end point of the action they viewed was longer than those who were active. Further, it was not simply the case that overall activity predicted stronger anticipation. Rather, it was engagement in containment action specifically that predicted infants’ subsequent anticipation. This relation was strongest when infants’ own actions came first. Thus, we conclude that infants’ actions prior to observation entrained their subsequent action anticipation.
Our findings did not provide strong evidence that the relation holds in the other direction: When the observation task was conducted first, infants’ gaze latencies did not strongly predict their subsequent actions with the containers. This asymmetry is consistent with the hypothesis that motor representations provide structure for and thus influence action perception (Falck-Ytter et al., 2006; Flanagan & Johansson, 2003; Gredebäck & Melinder, 2010). However it is also possible that such a correlation existed, but was not strongly reflected in our findings. Perhaps attention to observed actions primes infants’ own actions in ways that we did not assess, for example persistence in the activity over longer time periods. Further research is needed to investigate this issue.
Because our study targeted an age when most infants are able to engage in containment actions, and because anticipation of the observed events in this task was not contingent on immediate prior experience, we cannot conclude from these findings whether motor experience with containment actions is a pre-requisite for visual anticipation of these sequences. Because infants in both task orders showed a tendency to anticipate the goal of the observed actions during the very first trial, it seems likely that they brought with them knowledge about the structure of containment actions that supported action anticipation. It is not clear, from the current findings, whether this knowledge derived uniquely from prior active experience rather than from previous observational experience. The work here found no evidence that observation experience immediately influenced subsequent performance on the behavior task. Studies of younger infants or over longer developmental time spans are needed to evaluate the long-term effects of both active and observational experience on social perception. One potentially informative route for future work could be to investigate potential effects of a delay period between the two tasks.
These issues, aside, our findings show a clear relation between action perception and production. This finding is consistent with adults’ visual anticipation of actions (Cannon & Woodward, 2008). When adults engaged in concurrent manual activity while viewing an action, anticipatory responses declined. In contrast, concurrent tasks that did not involve overt actions did not interfere with adults’ action anticipation. Thus, findings from both adults and infants indicate that motor activity influences the visual anticipation of others’ actions. This influence of acting on action anticipation suggests overlap in the systems that subserve each of these processes.
The events used in this study, like the ones developed by Falck-Ytter and colleagues (2006), presented infants with information about the visible movements of a human arm and information about a familiar goal-directed action (placing objects into a container). Thus, infants’ responses could have been based in an analysis of the movements of arm or the goal-directed action it undertook. Although the current findings do not distinguish between these alternatives, other recent findings do. Gredebäck and colleagues (2009) presented 14-month-old infants with actions that culminated in a salient goal (putting an object into a container), similar actions for which the goal was less salient (transporting an object to an unmarked location), or arm movements that followed the same path but did not involve a clear goal. Infants’ propensity to anticipate the endpoints varied as a function of goal salience, with anticipation for containment events but not arm movements (for similar results with adults, see also Eshuis et al., 2009). Moreover, Gredebäck and Melinder (2010) found that 12-month-old infants anticipated the goal of a feeding event (looking at the recipient’s mouth) even when the feeding action was carried out in a novel and apparently irrational manner. Based on these findings, we believe it is likely the current findings reflect priming of goal-based anticipation rather than anticipation of the arm’s movement through space.
Although it has been hypothesized that the mirror neuron system is the basis for interactions between action production and action perception, as yet it is not known whether this is the case during infancy. Our findings suggest a functional relation between acting and prospective attention, but do not implicate the neural mechanisms involved. Neural activity associated with the motor system has been detected in paradigms in which infants observe and are assumed to anticipate another’s action (Nyström, Ljunghammar, Rosander, & von Hofsten, in press; Southgate, Johnson, El Karoui, & Csibra, 2010; Southgate, Johnson, Osborne, & Csibra, 2009). However, because these studies did not include direct measures of visual action anticipation or infants’ motor behavior, further work is needed to elucidate this issue.
The current findings add to the evidence linking action production to action perception in infancy, including neonatal and infant imitation (Meltzoff & Moore, 1977; Meltzoff, 2007), and the effects of action production on their sensitivity to the goals of observed actions (Gerson & Woodward, under revision; Sommerville, Hildebrand, & Crane, 2008; Sommerville, Woodward, & Needham, 2005). Understanding and imitating others’ actions is a critical component in the development of social cognition. Beyond this, however, anticipating others’ actions is critical to engaging and collaborating with others in everyday life. The current findings support the conclusion that action production also influences this aspect of infant social cognition.
Acknowledgments
This research was supported in part by an Office of Naval Research grant (ONR N000140910126) and by NICHD (P01 HD064653) to ALW.
Footnotes
This work was conducted when E. Cannon and A. Woodward were in the Psychology Department at the University of Maryland.
Some infants (n = 6 in the behavior first condition, and n = 7 in the observation first condition) completed only two of the three sets due to inattention or distress. For this reason, measures were averaged across the number of trials completed.
Of 810 possible data points, 248 were excluded because of failure to meet these criteria. Of these, 123 began with gaze falling within the Start AOI (balls) with no subsequent gaze to the End AOI, 109 did not include any gaze data in the Start AOI, and 10 were cases in which the infant looked to both the start and goal AOIs before the hand started to move. These trials were eliminated not only because of the difficulty in calculating a latency score, but also because they were cases in which infants failed to attend fully to the stimulus action, neither following nor anticipating it.
References
- Beilock SL, Carr TH. On the fragility of skilled performance: What governs choking under pressure? Journal of Experimental Psychology: General. 2001;130:701–725. [PubMed] [Google Scholar]
- Canfield RL, Smith EG, Bresznyak MP, Snow MP. Informational processing through the first year of life: A longitudinal study using the visual expectation paradigm. Monographs of the Society for Research in Child Development. 1997:62. [PubMed] [Google Scholar]
- Cannon EN, Woodward AL. Action anticipation and interference: A test of prospective gaze. In: Love BC, McRae K, Sloutsky VM, editors. Proceedings of the 30th Annual Conference of the Cognitive Science Society. Austin, TX: Cognitive Science Society; 2008. pp. 981–984. [PMC free article] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: The perception-behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Csibra G. Action mirroring and action interpretation: An alternative account. In: Haggard P, Rosetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition. Attention and Performance XXII. Oxford: Oxford University Press; 2007. pp. 435–459. [Google Scholar]
- Decety J, Chaminade T, Grézes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. NeuroImage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- DiPelligrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Eshuis R, Coventry KR, Vulchanova M. Predictive eye movements are driven by goals, not by the mirror neuron system. Psychological Science. 2009;20:438–440. doi: 10.1111/j.1467-9280.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, von Hofsten C. Infants predict other peoples’ action goals. Nature Neuroscience. 2006;9:878–879. doi: 10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gerson S, Woodward AL. Building intentional action knowledge with one’s hands. In: Johnson SP, editor. Neo-constructivism. Oxford University Press; 2010. [Google Scholar]
- Gerson S, Woodward A. The effects of active versus passive experience on infants’ attribution of goals. What’s in a mitten? (under revision) [Google Scholar]
- Gredebäck G, Kochukhova O. Goal anticipation during action observation is influenced by synonymous action capabilities, a puzzling developmental study. Experimental Brain Research. 2010;202:493–497. doi: 10.1007/s00221-009-2138-1. [DOI] [PubMed] [Google Scholar]
- Gredebäck G, Melinder A. Infants understanding of everyday social interactions: a dual process account. Cognition. 2010;114:197–206. doi: 10.1016/j.cognition.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gredebäck &, von Hofsten C. Taking an action perspective on infant’s object representations. Progress in Brain Research. 2007;164:265–282. doi: 10.1016/S0079-6123(07)64015-1. [DOI] [PubMed] [Google Scholar]
- Gredebäck G, Stasiewicz D, Falck-Ytter T, Rosander K, von Hofsten C. Action type and goal type modulate goal-directed gaze shifts in 14-month-old infants. Developmental Psychology. 2009;45:1190–1194. doi: 10.1037/a0015667. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Current Opinion in Neurobiology. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckstrom A, Flanagan JR. Eye-hand coordination in object manipulation. The Journal of Neuroscience. 2001;21:6917–6932. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SP, Amso D, Slemmer JA. Development of object concepts in infancy: Evidence for early learning in an eye tracking paradigm. Proceedings of the National Academy of Sciences (USA) 2003;100:10568–10573. doi: 10.1073/pnas.1630655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore SJ, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7:1299–1301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: An account of the mirror neuron system. Cognitive Processing. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich G, Sebanz N. The social nature of perception and action. Current Directions in Psychological Science. 2006;15:99–104. [Google Scholar]
- Longo MR, Bertenthal BI. Common coding of observation and execution of action in 9-month-old infants. Infancy. 2006;10:43–59. doi: 10.1207/s15327078in1001_3. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. The ‘like me’ framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Nyström P, Ljunghammar T, Rosander K, von Hofsten C. Using mu rhythm perturbations to measure mirror neuron activity in infants. Developmental Science. doi: 10.1111/j.1467-7687.2010.00979.x. (in press) [DOI] [PubMed] [Google Scholar]
- Rosander K, von Hofsten C. Infants’ emerging ability to represent occluded object motion. Cognition. 2004;91:1–22. doi: 10.1016/s0010-0277(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Hildebrand EA, Crane CC. Experience matters: The impact of doing versus watching on infants’ subsequent perception of tool use events. Developmental Psychology. 2008;44:1249–1256. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C. An action perspective on motor development. Trends in Cognitive Science. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- von Hofsten C, Kochukhova O, Rosander K. Predictive tracking over occlusion by 4-Month-old Infants. Developmental Science. 2007;10:625–640. doi: 10.1111/j.1467-7687.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Wentworth N, Haith MM. Infants’ acquisition of spatiotemporal expectations. Developmental Psychology. 1998;34:247–257. doi: 10.1037//0012-1649.34.2.247. [DOI] [PubMed] [Google Scholar]
- Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychological Bulletin. 2005;131:460–473. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]