Abstract
Bone is an organic-inorganic composite which has hierarchical structuring that leads to high strength and toughness. The nanostructure of bone consists of nanocrystals of hydroxyapatite embedded and aligned within the interstices of collagen fibrils. This unique nanostructure leads to exceptional properties, both mechanical and biological, making it difficult to emulate bone properties without having a bone-like nanostructured material. A primary goal of our group’s work is to use biomimetic processing techniques that lead to bone-like structures.
In our prior studies, we demonstrated that intrafibrillar mineralization of porous collagen sponges, leading to a bone-like nanostructure, can be achieved using a polymer-induced liquid-precursor (PILP) mineralization process. The objective of this study was to investigate the use of this polymer-directed crystallization process to mineralize dense collagen substrates. To examine collagen scaffolds that truly represent the dense-packed matrix of bone, manatee bone was demineralized to isolate its collagen matrix, consisting of a dense, lamellar osteonal microstructure. This biogenic collagen scaffold was then remineralized using polyaspartate to direct the mineralization process through an amorphous precursor pathway.
Various conditions investigated included polymer molecular weight, substrate dimension and mineralization time. Mineral penetration depths of up to 100 μms were achieved using this PILP process, compared to no penetration with only surface precipitates observed for the conventional crystallization process. Electron microscopy, wide-angle X-ray diffraction, and thermal analysis were used to characterize the resulting hydroxyapatite/collagen composites. These studies demonstrate that the original interpenetrating bone nanostructure and osteonal microstructure could be recovered in a biogenic matrix using the PILP process.
Keywords: Biomineralization, bone, hydroxyapatite, amorphous calcium phosphate, biomimetic, collagen
1. Introduction
Bone is a multifunctional organ composed of organic and inorganic materials that are interconnected in an intimate and complex arrangement to accomplish desired functions. Bone is not only the major framework of vertebrate species which supports the body and protects the internal organs [1–3], but it also plays the crucial role of maintaining the concentration of inorganic ions (i.e. calcium and phosphate) through its continuous resorption and remodeling [4, 5]. Its major components can be categorized into three categories: i) inorganic mineral (ca. 65%) consisting of nanocrystals of carbonated hydroxyapatite (HA); ii) organic phases (ca. 25%), including extracellular matrix (collagen), non-collagenous proteins (NCPs) and cells; and iii) water that is associated with the collagen (ca. 10%) [6–10]. The main organic portion of bone is constituted of type-I collagen, which has a triple-helical molecular structure (known as tropocollagen) that self-assembles into fibrils [11]. In electron microscopy, a periodic banding pattern is observed in type-1 collagen fibrils due to a quarter-staggered arrangement that leads to hole and overlap zones that are thought to play a vital role in the intrafibrillar mineralization of collagen [11–13].
The basic building block of bone is the mineralized collagen fibril [1]. Collagen fibrils are mineralized with carbonated hydroxyapatite platelets which are embedded within the interstices of the fibril and roughly [001] aligned parallel to the long axis of the fibril [12, 14, 15]. The fibrils self-assemble into higher levels of structure, such as in parallel arrays that rotate across the concentric lamellae of osteons [1, 16], with further hierarchy directed by osteoblasts as they lay down a trabecular and cortical bone macrostructure. All levels of hierarchy contribute to the unique mechanical and chemical properties of bone [4], including the interpenetrating arrangement of the collagen and mineral phase at the nanostructural level. By embedding the brittle mineral phase throughout the ductile collagen matrix, with energy dissipation occurring through a multiplicity of sacrificial bonds, bone has remarkable fracture toughness [17, 18]. This nanostructured architecture is also valuable for allowing bone to be resorbed by acidic secretions of osteoclasts [19], while HA in monolithic form is very slow to dissolve under physiological conditions [20].
Numerous research groups are working on the development of bone-like collagen-hydroxyapatite composites, typically using one of the following routes: 1) direct blending of collagen and mineral crystals; 2) co-precipitation of mineral during collagen fibrillogenesis; or 3) a “biomimetic” method of immersion of collagen scaffolds in simulated body fluid [21]. The latter method typically uses the conventional crystallization reaction, where nucleation of HA occurs heterogeneously on a collagen substrate placed in a mineralizing solution, such as simulated body fluid (SBF) or modified SBF [22–29]. While the conventional methods have been successful at fabricating simple composites with a surface layer of random clusters of HA, they have failed to achieve intrafibrillar mineralization. The use of SBF types of reaction conditions are often considered “biomimetic” even though this approach alone does not accurately mimic how bone is formed or its nanostructure. Recently, alternative approaches using acidic polypeptides to emulate the important role of the NCPs have demonstrated this as a feasible means for achievement of intrafibrillar mineralization [12, 30–33].
One of the research thrusts in our group focuses on biomineralization, and more specifically, on the use of in vitro model systems to examine the function of acidic soluble polypeptides in mimicking the role of the acidic NCPs in bone formation. Gower and coworkers discovered that the addition of acidic polypeptides to the mineralization solution induces or stabilizes an amorphous precursor to the mineral which is so highly hydrated that it has liquid-like character [34, 35]. It was proposed that this polymer-induced liquid-precursor (PILP) process could lie at the foundation of biomineral morphogenesis, both in vertebrates and invertebrates [12, 36–42]. As to mimicking bone formation, it was shown that intrafibrillar mineralization of collagen can be achieved in vitro via this PILP process, wherein nano-sized hydroxyapatite crystals are embedded and [001] aligned within collagen fibrils [12, 40, 43–45]. The presence of acidic polypeptides produced an amorphous precursor that infiltrated the interstices of the collagen fibrils, which upon crystallization enabled the fundamental nanostructure of bone to be reproduced. It was therefore suggested that the old debate of bone formation occurring via an amorphous precursor needed to be revisited [12], and indeed, this has now been shown to be the case in the continuously forming fin bone of zebra fish [46, 47].
Since this fundamental breakthrough in mimicking intrafibrillar mineralization, recent studies using acidic polymer-mediated mineralization of collagen have focused on the fibrillar level, where individual collagen fibrils have been mineralized and characterized [12, 31, 33], or randomly-oriented bundles of fibrils in the form of porous collagen scaffolds have been investigated [12, 33, 48–50]. In these cases, the nanostructured architecture of the hydroxyapatite/collagen fibrils has been shown to resemble the fundamental nanostructure of bone (i.e., intrafibrillar mineral). However, at the microstructural level, bone consists of a densely-packed collagen matrix, which is necessary to achieve high strength and toughness. Therefore, the work reported herein is focused on using this same polymer-directed process to mineralize densely-packed collagen substrates, and more specifically, collagen that is organized in a native bone matrix. To do this, demineralized bone specimens from manatee ribs served as the collagen substrates of investigation.
Manatee rib bones are high density cortical bone, with a high degree of mineralization (69 wt.%) as an adaptation to hydrostasis [51]. Demineralized bone was chosen primarily because of the retained presence of multiple hierarchical levels of collagen organization (fibrils, lamellae, osteons), which one might anticipate would be more difficult to mineralize than a porous collagen sponge. There is precedent though that a biogenic scaffold can be remineralized. Gu et al. showed that the collagen matrix of acid etched dentin could be remineralized using a dual matrix analogue system, which included both an acidic polymer (polyacrylic acid) as the sequestering agent to generate the amorphous nanoprecursors, as well as a templating analogue of polyphosphate [52]. In their system, the acid etch created a 5 to 8 μm thick layer of completely demineralized collagen matrix over a mineralized dentin base. In our study, the bone sections are fully demineralized and are considerably thicker (200 and 500 μms); yet retain the hierarchical structure of the collagen matrix that defines osteonal bone.
Manatee bone specimens were sectioned, demineralized and subsequently remineralized with calcium phosphate using various reaction parameters (polymer molecular weight, substrate dimension and reaction time) in an attempt to maximize mineral precursor penetration into the dense substrate. Electron microscopy, wide-angle X-ray diffraction, and thermogravimetric analysis were used to characterize the resulting hydroxyapatite/collagen structure, mineral content, and penetration depth.
2. Materials and Methods
2.1. Demineralization of Manatee Bone
Manatee bone samples were kindly donated by J. Mecholsky from the Department of Materials Science and Engineering Department at the University of Florida. The samples used were rib bones composed of solid cortical bone [53]. To determine if the degree of mineral penetration was dependent on the specimen measurements, two sample dimensions (large and small) were prepared. Prior to demineralization, bone samples were cut into rectangular strips of 40 × 3 × 0.5 mm using a wet diamond saw (Exakt Technologies, Hamburg, Germany) or 20 × 0.4 × 0.2 mm using saw microtome (Leica SP1600, performed in Dr. Fratzl’s lab at the Max Planck Institute of Colloids and Interfaces in Potsdam, Germany). Demineralization of bone pieces was carried out in a 0.5 M EDTA solution (Acros Organics, Morris Plains, NJ, pH adjusted to 8.0 with NaOH) containing 0.02% (w/v) sodium azide (Sigma, St. Louis, MO) to avoid bacterial contamination. Bone pieces were incubated in the demineralization medium while stirring at room temperature for different time points (between 72 and 96 hours). After demineralization, bone samples were taken out from the demineralization solution, washed six times with large amounts of ultrapure water to remove all traces of EDTA, lyophilized and stored at −20°C until use.
2.2. Remineralization of Demineralized Manatee Bone In Vitro
Demineralized bone samples were remineralized with calcium phosphate (CaP) via the polymer-induced liquid-precursor (PILP) process. In this study, the mineralization solution was prepared by mixing equal volumes of 9 mM CaCl2·2H2O (Sigma, St. Louis, MO) and 4.2 mM K2HPO4 (Sigma, St. Louis, MO) solutions. To maintain the pH of the mineralization solution at 7.4, calcium and potassium solutions were made in Tris-buffered saline (TBS) supplemented with 0.02% (w/v) sodium azide. Poly-aspartates with different molecular weights were used as the PILP process-directing agent at a 100-μg/mL concentration. Poly-L-aspartic acid sodium salt (Mw: 10,500 or 27,000 Da; Sigma or Alamanda Polymers, Huntsville, AL) was added to the calcium solution before mixing an equal volume of the phosphate counterion solution (to avoid solution precipitates). The mineralization solution had a final concentration of 4.5 mM calcium and 2.1 mM phosphate in TBS buffer containing 0.9% (w/v) NaCl and 0.02% (w/v) sodium azide.
Demineralized bone pieces were incubated in the mineralization solution under vacuum conditions for 30 minutes to remove any air bubbles potentially trapped in the pores of the specimens (e.g., osteonal canals). After degassing the samples, the mineralization solution was kept in a 37°C oven to emulate physiological conditions. A control reaction, with no polyaspartate additive, was run for each set of experiments. At predetermined reaction times, mineralized samples were removed from the solution, copiously washed with de-ionized water, lyophilized and stored at −20°C until further use. Three samples of demineralized bone were used for each set of experiments.
2.3. Scanning Electron Microscopy (SEM) and Electron Beam Analysis
Surface- and cross-sections of samples were prepared by the freeze-fracture technique. Some of the cross-sections were obtained from epoxy-embedded samples which had a 500-μm thickness and were polished prior to coating. The sections were mounted on aluminum stubs covered in double-sided copper tape, and then sputter coated twice with amorphous carbon. Surface morphology of mineralized samples was then analyzed using a 6400 JEOL SEM instrument equipped with an energy dispersive spectrometer, at an accelerating voltage of 15 kV. For elemental analysis of mineralized samples, energy dispersive X-ray spectroscopy (EDS) analysis was done during SEM examination. To determine calcium and phosphate distribution within the bulk of the remineralized bone specimens, line scans were analyzed on each of the cross-sections, each completely within the interior of sample, with a 15 KeV accelerating voltage. For quantitative analysis, measurement of mineral penetration was done using the software ImageJ (NIH). In addition to line scan analysis, backscattered electron mode images and elemental mapping were obtained for further analysis of the mineral distribution.
2.4. Wide Angle X-ray Diffraction (WAXD) Analysis
WAXD analysis was used to determine the crystallographic structure of the samples (untreated and mineralized). To determine if the major hydroxyapatite peaks were present, which develop at around 26° (002) and 32° (combination of (211), (112) and (300)), the samples were scanned with Cu-Kα X-ray radiation from a Philips XRD ADP 3720 Diffractometer at 40 KV and 20 mA, using a step size of 0.01° mrad/s with a time of 1.25 sec/step, over a 2θ range of 10 – 50°.
2.5. Transmission Electron Microscopy (TEM) Analysis
Nano-structural analysis of mineralized samples was performed on a 200CX JEOL TEM instrument with an accelerating voltage of 200 kV. To determine the crystallographic orientation of the embedded hydroxyapatite nanocrystals, bright-field (BF), dark-field (DF), and selected area electron diffraction (SAED) modes were used. For TEM analysis, samples were pulverized in liquid nitrogen, dispersed in ethanol and added dropwise onto a copper TEM grid. To prevent electron charging, samples were lightly coated with amorphous carbon.
2.6. Thermogravimetric and Differential Thermal Analysis (TG/DTA)
To determine the degree of mineralization of the samples, TG/DTA analyses were conducted using a TG/DTA 320 (Seiko, Thermo Haake, Germany) instrument. A heating rate of 5°C/min was applied in the temperature range of 30 – 800°C under air at a flow rate of 100 mL/min. The amount of sample examined was between 5 – 10 mg, and alumina powder was used as the standard. To compare the degree of mineralization, the material remaining at 600°C was interpreted as the mineral content since the organic portion of the samples should be totally combusted by 600°C in an oxygen-containing environment [54].
3. Results and Discussion
3.1. In Vitro Remineralization of Demineralized Manatee Bone via the PILP Process
Manatee rib bone samples composed of cortical bone were demineralized with a calcium-chelating solution (EDTA) commonly used for the removal of mineral in biological samples. The use of a calcium-chelating agent as opposed to an acidic process was preferred for the demineralization of the samples to avoid any damage the acidic process might cause to the collagen matrix. When samples were collected after the demineralization process, they first went through a visual inspection for initial diagnosis of the mineral removal success.
After demineralization, the appearance of the manatee bone specimens changed. Even in the wet state, changes in the color of the samples were evident at the macroscopic level. The demineralized bone appeared less white and it was translucent as opposed to the native bone, which was white and opaque. These physical changes were even more evident when the samples were dried, as shown in Figure 1-(a) versus (b). In addition to change in pigmentation, demineralized samples lost their rigidity. In fact, they were easily bent with minimum force applied. Prior to remineralization of the samples, EDS and thermogravimetric analyses of the demineralized specimens were performed to confirm total demineralization of bone (results shown later).
Figure 1.
Photograph of thin sections of manatee bone (40 × 3 × 0.5 mm) treated under different conditions: (A) untreated, native bone, (B) EDTA demineralized bone, (C) remineralized bone treated with 4.5 mMCaCl2/2.1 mM K2HPO4 PILP solution under static conditions for 6 days, and (D) remineralized bone treated with 5.3 mMCaCl2/2.5 mM K2HPO4 PILP solution under dynamic (stirring) conditions for 6 days.
Demineralized bone specimens were remineralized either by the conventional crystallization process (i.e., nucleation and growth) without polymer additive, or by the polymer-induced liquid-precursor (PILP) process [12, 49, 50]. Figure 1 shows images of native bone, demineralized bone, and specimens remineralized with polyaspartate (10KDa) to induce the PILP process. After remineralization, physical changes of the samples were observed, reversing the differences caused by the initial mineral removal. PILP-remineralized samples showed a marked increase in their white pigmentation and opacity, as well as an increased stiffness (Fig. 1). This effect was particularly pronounced in sample (d), which was prepared using a higher supersaturated mineral solution in the presence of polyaspartate. The physical changes were so evident that even the degree of mineralization could be correlated to the physical alterations, as seen in Figure 1, where specimen (d) contains 20 wt.% more mineral than specimen (c) (mineral content obtained from TGA analysis). The remineralized specimens also exhibited a more pure white color relative to the native bone, which must have contained various “impurities” that apparently became removed during the EDTA treatment.
When looking at the samples’ topography via SEM (Figures 2A- surface & 2B-interior), the morphology of manatee rib bone appears very rough, and with highly mineralized collagen fibrils showing some degree of orientation, depending on the sample area. This initial morphology changed after the demineralization process with the calcium-chelating agent, where a smoother surface was observed, as seen in Figures 2D–E. When the demineralized specimen was placed in a mineralization solution in the absence of polyaspartate additive, it only provided heterogeneous nucleation sites for HA on the surface of the scaffold. Figure 2G shows the surface of demineralized bone treated with the conventional process (no polymer additive) where only a few spherulitic clusters of HA crystals deposited on the surface of the collagen substrate were observed. There was no detectable mineral on the interior (Figure 2H). The results for the control samples were anticipated since this is the common morphology of HA precipitated by the conventional crystallization process.
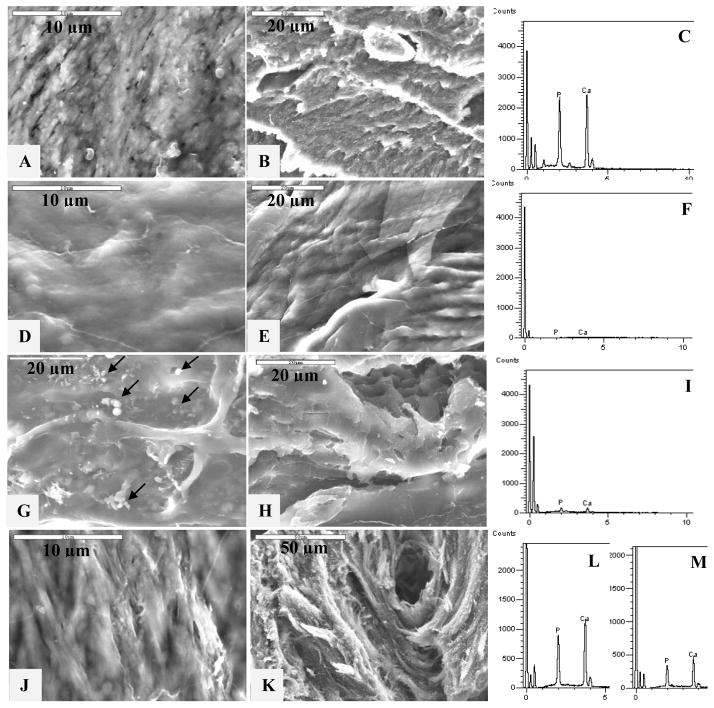
Figure 2.
High-magnification SEM and EDS of the surface and cross-sections of manatee bone samples. SEM showing the (A) surface and (B) cross-section of native bone. (C) EDS plot of the surface of natural bone. SEM of the (D) surface and (E) cross-section of demineralized bone, and (F) corresponding EDS of the surface of demineralized bone. SEM showing the (G) surface and (H) cross-section of demineralized bone treated with the conventional process (no polymer additive) showing only surface deposition of a few apatite clusters on the top of the demineralized collagen substrate (arrows). (I) EDS plot of demineralized bone treated with the conventional process. SEM showing the (J) surface and (K) cross-section of remineralized bone treated with a PILP solution containing 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days. Corresponding EDS of the (L) surface and (M) cross-section of PILP-remineralized bone.
In contrast to the spherulitic clusters produced with no polymer additive, the morphology of the PILP-remineralized samples (Fig. 2J–K) appears very similar to that of the original bone (Figs. 2A–B) after 7 days of treatment. None of the PILP-remineralized bone specimens showed evidence of surface deposits of the spherulitic type, as was the case for the control samples (Fig. 2G). Instead, the collagen fibrils appeared to be covered by a smooth extrafibrillar mineral coating, likely caused by hypermineralization, as has been observed in our prior studies [50]. This extrafibrillar mineral was more like a continuous film-like coating, as is often seen with PILP-formed mineral films [48, 55]. The anisotropic structure of the underlying collagen fibrils could still be seen under this extrafibrillar coating, as was the case for the native bone. Interestingly, the demineralized bone specimen showed a less distinct fibrillar structure (Fig. 2D), which returned upon PILP-remineralization (Fig. 2J–K), but not after conventional mineralization (Fig. 2G–H). We have seen this to be the case in some reconstituted collagen scaffolds as well, where the fibrillar texture is not readily apparent until after polymer-directed mineralization [49, 50].
To verify the presence or absence of mineral, energy dispersive spectroscopy (EDS) was used during the SEM examination (Fig. 2, spectra on the right). EDS analysis of the samples’ surfaces determined that the rough collagen fibrils seen in native and PILP-remineralized bone specimens contained a high amount of calcium and phosphorous when compared to the carbon peak at around 0 KeV (Figs. 2C and 2L, respectively). EDS of the demineralized specimen confirmed the absence of detectable mineral elements in the sample (Fig. 2F) while very low amounts of mineral (probably from the HA clusters) were detected for the specimen mineralized with the conventional crystallization process (Fig. 2I). EDS analysis of the cross-sectional areas of native and demineralized bone specimens did not show any difference with respect to the EDS results for the surface area of the samples (data not shown), while the cross-sectional areas of the PILP-remineralized bone specimens showed a lower amount of mineral relative to the surface (Fig. 2M).
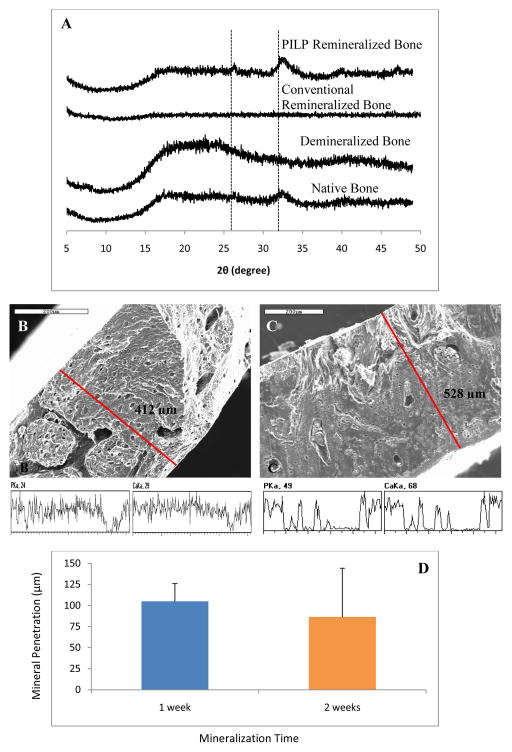
A comparison study of the XRD patterns of native, demineralized conventional remineralized, and PILP-remineralized bone is shown in Figure 3A. After 7 days of reaction, broad peaks of hydroxyapatite were observed in PILP-remineralized bone at around 26° and 32°, corresponding to the (002) and the combination of overlapping (211), (112), (300) peaks, respectively [55]. This XRD pattern of PILP-remineralized bone exhibits similar peak widths to the XRD patterns of native bone. The (211), (112), (300) peaks of carbonated hydroxyapatite in bone are normally overlapped into one broad peak due to the extremely small crystal size and defects such as carbonate, impurities and calcium vacancies [56–61]. In the case of demineralized bone and conventional remineralized bone specimens, no peaks were observed.
Figure 3.
XRD and line scan spectroscopy analysis of manatee bone samples. XRD curves of native, demineralized, conventional remineralized (no polymer additive) and PILP remineralized manatee bone (A). SEM with corresponding line-scan analysis of the cross-sectional area of (B) natural bone, and (C) PILP-remineralized bone. PILP remineralized bone was treated with a solution containing 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days. Scale Bars = 200 μm. (D) Mineral penetration of PILP-remineralized bone cross-sections (n = 3). No significant differences between the two time points were observed.
The surface topology, combined with EDS and XRD analyses, correlate to the presence of HA in the PILP-mineralized bone samples after 7 days of mineralization. Furthermore, given that hydroxyapatite clusters were not observed on the surface of PILP-remineralized specimens, as it is the case of samples treated without the polymer additive, the EDS and XRD readings of calcium and phosphorous suggest that hydroxyapatite crystals are localized within (intra) and around (inter) the collagen fibrils. The overall morphology of the PILP-remineralized specimen bears a striking resemblance to native bone (Fig. 2A), which also consists of intra- and extra-fibrillar mineral.
Cross-sectional analysis found that the PILP-remineralization of bone was not uniform across the sample thickness (~ 500 μm). As can be seen in the EDS line-scan in Figures 3B and 3C, only the edges of the 7-day remineralized sample showed mineral levels comparable to native bone. The interior of the specimen showed some areas where mineral was present, as well as areas with no mineral. Just taking into account the regions that were uniformly mineralized (near the two surfaces), it seems that the remineralization process only achieved about a 100-μm depth of mineral penetration across the sample on both surfaces. Aiming to increase the mineral penetration, demineralized bone samples were treated with our PILP-mineralization solution for up to four weeks. To determine calcium and phosphate distribution, line scans were analyzed on each of the cross-sections and ImageJ software was used to measure mineral penetration. However, no improvement in mineral penetration was observed beyond seven days of treatment, as shown in Figure 3D (data for 3 and 4 weeks not shown). Conversely, variability in the degree of mineral penetration among samples seemed to increase with longer incubation times. This may have been caused by regions of solidified mineral blocking further infiltration. The lack of further mineralization may have been due to a deficiency of calcium and phosphate in the solution since no extra ions were added while increasing the incubation time.
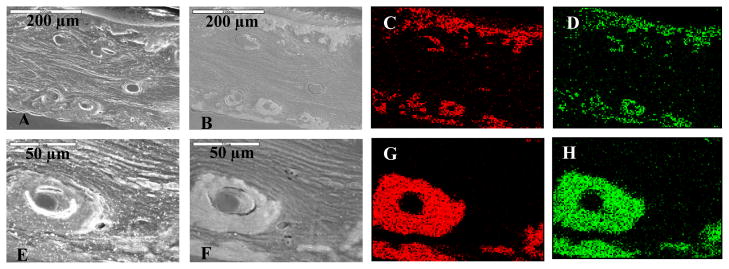
To further analyze mineral distribution across the bulk specimen, SEM in backscattered electron (BSE) mode and elemental mapping analysis of the PILP-remineralized samples was performed (Fig. 4). BSE mode was used because it gives an enhanced signal for higher atomic number elements, thereby enhancing the brightness of the mineralized regions. Regions of electron-dense material were found at the edges, as expected from the line scan analysis in Figure 4. But surprisingly, a pronounced enhancement of osteonal structures was observed in the BSE images of PILP-remineralized bone cross-sections (Figs. 4B and 4F). This was particularly apparent in the elemental mapping results. High levels of calcium and phosphorous were localized at the surfaces of the bulk sample (Fig. 4C–D), and in the oval structures of osteons (Fig. 4G–H).
Figure 4.
SEM and elemental mapping of PILP-remineralized manatee bone treated with 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days. (Top row) Cross-section of PILP-remineralized bone sample. (Bottom row) A closer look at an osteon found at the cross-section of the PILP-remineralized bone sample. (A, E) SEM showing the cross-sectional morphology of the sample. (B, F) Backscattered electron mode images of the same region. These images display more electron-dense areas at the edges and surrounding the osteonal structures of the specimen. Elemental mapping showing calcium (red) and phosphorus (green) distribution across the sample (C, G) and (D, H) respectively.
It is interesting that within the well-mineralized area of the specimen, calcium and phosphate ions show this preference for the osteonal structures (Figs. 4G–H). This could simply be due to the accessibility of the precursor to travel through the osteonal channels. However, on close inspection, these osteons do not appear to have a hollow channel interior, and yet are not filled with mineral (judging from the backscattered image and elemental mapping). Whatever was there (presumably vasculature), apparently does not readily mineralize. In addition, there appears to be a marked delineation between the osteons and surrounding layers, suggesting there may be more to this preferential mineralization than simply accessibility. The mineral’s preference for these particular structures could be related to the difference in structure of the collagen fibrils in the osteons. In nature, osteons are a result of cellular bone remodeling; therefore their structure is naturally designed to facilitate the remineralization process. This could be due to a lower degree of stable collagen crosslinks within the osteonal regions of bone compared to the interstitial regions (inter-osteonal regions are more mature bone) [62]. Thus, the less mature osteonal collagen fibrils might favor the amorphous precursor infiltration within the fibrils during the biomimetic remineralization of bone. This might also be one reason why it was difficult to mineralize these scaffolds to a greater extent, and why they are so heterogeneous; in addition to the high matrix density, the crosslinking of the biogenic collagen could impart some limitations. Perhaps our in vitro mineralization process is similar in the materials chemistry aspect to the native bone remodeling process, where both may require less stable crosslinked collagen fibrils to allow for infiltration of a precursor.
The similarities found in native and PILP-remineralized bone were very promising, but it was not immediately obvious that intrafibrillar mineralization of the PILP samples had taken place because of the extrafibrillar mineral on the surface (shown in Fig. 2J). In the case of PILP-mineralized collagen sponges in our prior studies, the individual fibrils were smooth and more distinct when they contained high levels of mineral, which occurred after only 3 days of mineralization [49, 50]. In previous studies [48–50, 55], we have also observed that interfibrillar mineralization starts after a certain degree of intrafibrillar mineral is achieved. At that time, the precursor begins to deposit on and around the fibrils, cementing them together with an external mineral coating. We believe the same process had occurred here, but intrafibrillar mineralization is more difficult to detect at the microstructural level due to the high density of the material. Therefore, TEM analysis was used to verify that these hyper- remineralized collagen fibrils also contain a high degree of intrafibrillar mineral.
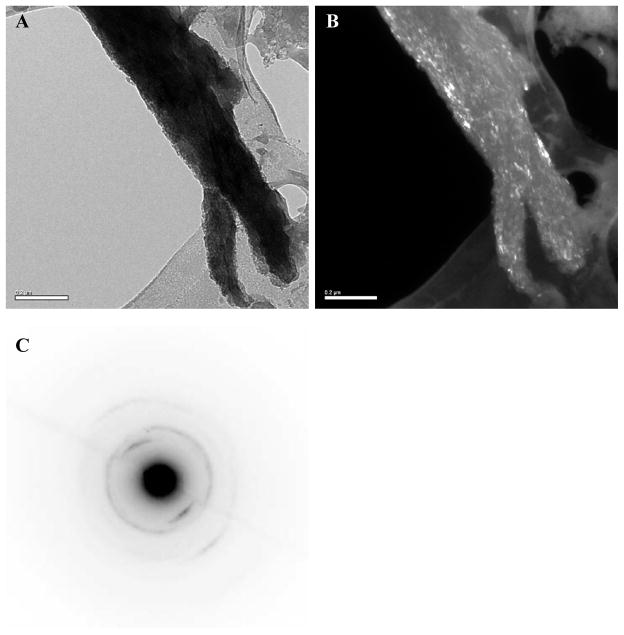
Bright-, dark-field TEM and selected area electron diffraction (SAED) modes were used to determine the orientation and phase of the calcium phosphate crystals present in the PILP-remineralized bone specimens. TEM sample preparation consisted of crushing remineralized bone in liquid nitrogen to isolate small fragments. As in bone, it is difficult to isolate separate mineralized fibrils in well mineralized samples, thus it becomes difficult to recognize the collagen-mineral relationship (one has to rely more on electron diffraction analysis). Figure 5A shows the TEM bright-field image of a mineralized collagen fibril. Interestingly, literature on hypermineralized whale bone shows fragments that appear very similar, where collagen fibrils are sometimes difficult to discern, as well as any banding pattern [63]. It should be noted that the TEM samples were not stained with phosphotungstic acid or any other electron-dense substance. The fibrils in Fig. 5A showed dark contrast and looked highly mineralized, which is further corroborated by the dark-field TEM mode of the same fibrils (Fig. 5B).
Figure 5.
TEM of PILP-remineralized manatee bone treated with 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days. (A) Bright- and (B) dark-field TEM micrographs of a PILP-remineralized bone sample. (C) SAED pattern of the remineralized fibril shown in (A).
To assess the crystallographic orientation of the intrafibrillar HA crystals, SAED was performed on individual fibrils. The SAED pattern of the remineralized bone fibrils illustrated in Figure 5C demonstrates that HA crystals are oriented in the [001] direction parallel to the long axis of the collagen fibrils (Fig 5A). This SAED pattern is very similar to that of bone [15, 64], with tilting and rotational disorder creating arcs for most of the planes [12]. As reported in previous studies [12, 33], certain domains in the collagen molecules that stimulate nucleation from specific crystallographic planes might be responsible for this preferential orientation. However, this favored orientation only seems to happen when there is an amorphous precursor phase involved in the crystallization process, which is not the case for conventional mineralization reactions (even though these are often considered biomimetic).
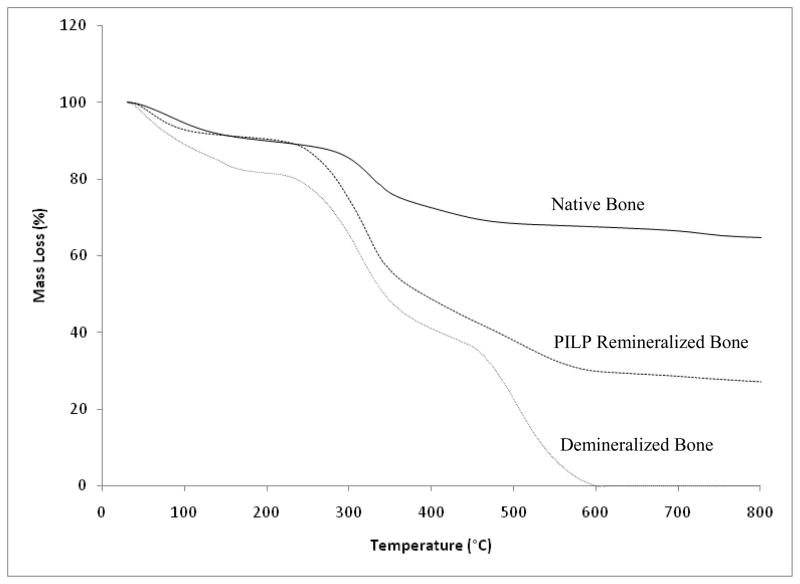
The mineral content of native, demineralized, and PILP-remineralized bone specimens was determined by thermogravimetric analysis (TGA). As shown in Figure 6, native bone had a mineral content of 68 wt.%, while the TGA result of demineralized bone showed 0 wt.% mineral content as expected. In the case of the PILP-remineralized specimen, the total mineral content reached 30 wt.% at 7 days of mineralization compared to 0 wt.% for the conventional mineralized sample (data not shown). The low mineral content of the remineralized sample correlates to the mineral distribution, where mineral only penetrated about 100 μm from the surfaces to the center of the specimen. Although the mineral content reached in the PILP-remineralized bone was less than 50% of the total mineral content of native bone, one has to keep in mind that only the outer regions of the samples were mineralized. Thus the results are still promising since the areas that were remineralized showed a bone-like architecture at the micro- and nano-structural levels.
Figure 6.
TGA curves of manatee bone. Native bone (solid line); PILP-remineralized bone treated with 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days (dashed line); and demineralized bone (dotted line).
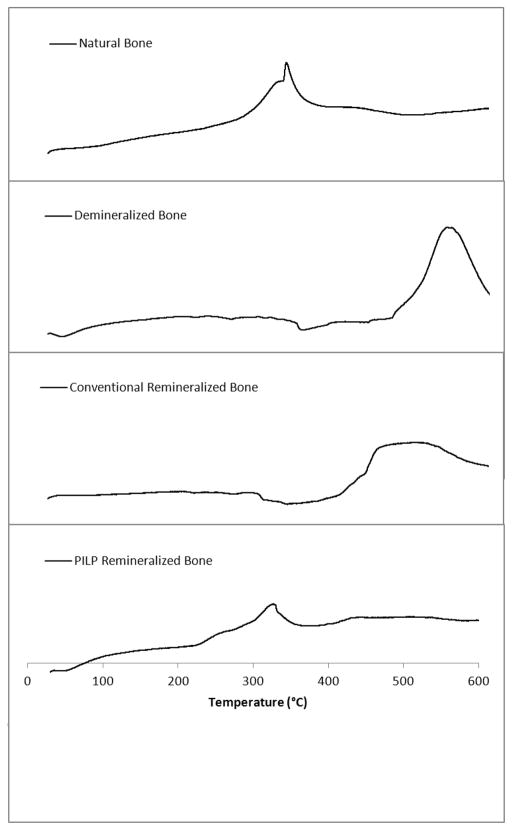
Thermal analysis of the bone specimens was done by thermogravimetric and differential thermal analysis (TG/DTA). Figure 7 displays the DTA curves for native, demineralized, conventional-mineralized (no polymer additive), and PILP-remineralized bone specimens. According to the literature, exothermic peaks in the range of 260 – 360°C correspond to collagen decomposition, while exothermic peaks in the range of 450 – 550°C correspond to collagen combustion [54]. DTA measurements showed a similar thermal behavior for native and PILP-remineralized bone samples. There is an exothermic peak with a maximum of about 337°C and 318°C for native bone and PILP-remineralized bone, respectively. In contrast, in the demineralized and conventional-mineralized (control) bone samples, the lower temperature peak is barely perceptible, and is replaced by a prominent peak at 548°C for the demineralized bone and at 500°C for the control. This strikingly different thermal behavior is not surprising though because the DTA curves for these two samples resemble the thermal behavior of pure collagen, which has been shown to have a high-temperature peak at 517°C (found in biological and reconstituted collagen) [54, 65]. These results demonstrate that the thermal stability of demineralized bone changed to a thermal behavior approaching that of native bone’s after PILP-remineralization, which was not the case for the conventional mineralized samples. This change in thermal stability could be attributed to the intimate structural relationship between hydroxyapatite and the collagen fibrils, as seen in native bone. Therefore, the thermal analysis of in-vitro remineralized specimens could be used as another tool to define intrafibrillar mineralization, where hydroxyapatite crystals embedded within the fibrils are intimately associated and alter its thermal stability.
Figure 7.
DTA curves of natural, demineralized, conventional remineralized (no polymer additive) and PILP remineralized manatee bone. PILP remineralized bone treated with 100 μg/ml of 10-KDa poly-L-aspartic acid for 7 days.
3.2. Effects of Specimen Dimension and Polymer-directing Agent on Mineral Penetration
Although the morphology and thermal stability results of our in-vitro remineralized bone compared to those of native bone, the degree of mineral penetration was still deficient. Since manatee bone is a very dense and compact material, smaller samples with 200-μm thickness were tested to improve the probability of mineral reaching across the entire sample cross-section. To further enhance mineral penetration, a higher molecular weight polyaspartate (27 KDa), which has been shown to improve collagen mineralization [49, 50], was used as the polymer-directing agent of the in-vitro remineralization process.
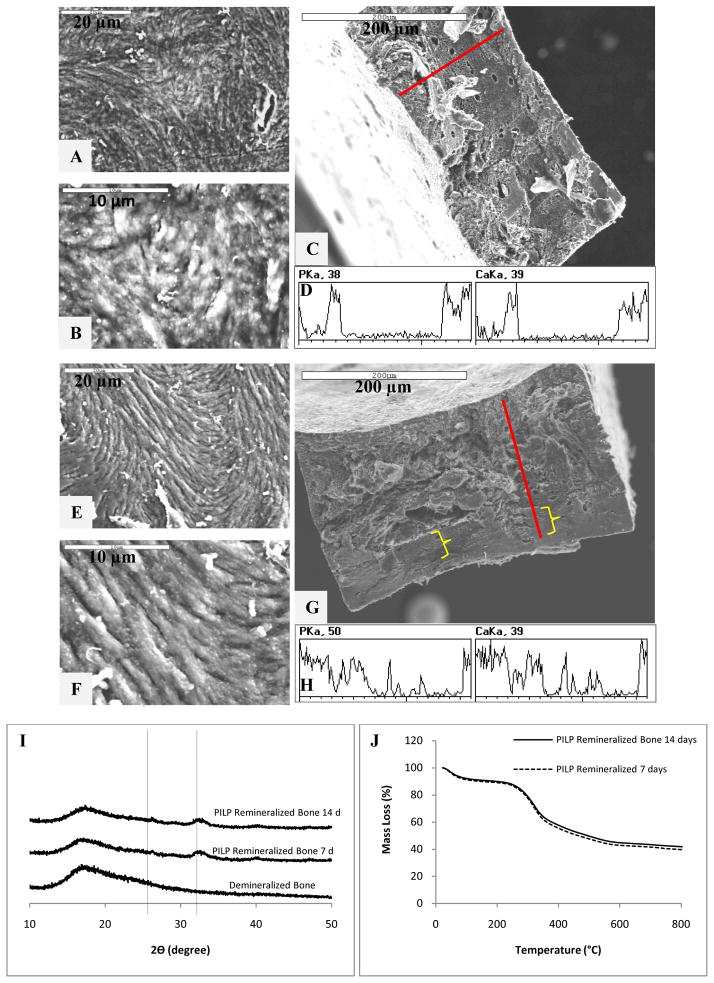
Interestingly, mineral penetration did not seem to improve by decreasing the specimen dimensions or using a more effective polymer-directing agent after 7 days of mineralization (Figs. 8A–C). However, when the remineralization process was carried out for 14 days, a deeper mineral penetration of about 100 μm (compared to the 25-μm mineral penetration at 7 days) was observed, which seemed to be initiated on only one side of the sample (Figs. 8D–G). Figures 8I and 8J show the XRD and TGA patterns for the remineralized specimens, respectively. The results for the crystallographic structure of the samples were comparable to hydroxyapatite in bone, as expected (Fig. 8I). In the case of the thermogravimetric analysis, the total mineral contents reached for the 7- and 14-day samples were 43 wt.% and 45 wt.%, respectively (not significantly different, although both being improved relative to the former data of 30 wt.%). It is puzzling that the TGA data showed no significant difference between the 7 and 14 day sample, while the depth profile seemed to show an improvement. As judging by the line scan, while the penetration seemed to reach a greater depth in the 14 day sample, the level of mineral was perhaps less and rather spotty, being predominantly on one side. This could be the result of the specimen resting on the bottom of the reaction vessel and one side being more exposed to the mineralization solution than the other. The discrepancy observed between the mineral content and penetration of the 7- and 14-day remineralized specimens is likely a consequence of structural variability between the examined samples. Even within the same sample, this was clearly seen in Fig. 4, which showed much enhanced mineral in the osteonal regions of the sample. It is important to consider the influence of specimen variability when using a natural collagen matrix. It is well-known that biological samples have certain degree of variability within the same species, which is more accentuated with age, sex and environmental conditions [62]. Since the bone tissue used was donated, we were not able to track down its origins and classify it to decrease variability within the sample group studied. This could explain some of the inconsistency in the mineral distribution and mineral content results for certain samples.
Figure 8.
Electron microscopy, wide-angle X-ray diffraction, and thermal analysis of PILP-remineralized manatee bone samples treated with 100 μg/ml of 27-KDa poly-L-aspartic acid. SEM of (A, B) surface and (C) cross-section of PILP-remineralized bone at 7 days. (D) Line scan spectroscopy of the 7-day remineralized bone cross-section. SEM of (E, F) surface and (G) cross-section of PILP remineralized bone at 14 days. (H) Line scan spectroscopy of the 14-day remineralized bone cross-section (brace symbols indicating areas of low mineral penetration). XRD (I) and TGA (J) curves of demineralized and PILP-remineralized manatee bone. PILP remineralized bone treated for 7 (dashed line) and 14 (solid line) days.
Certainly, mineral distribution and penetration across the bulk of the bone matrix seem to be a challenge for the PILP process. We believe the physical characteristics of the substrate used for in-vitro mineralization influence the kinetics of the process. Collagen in manatee bone is very dense, representing a material with a reduced surface area compared to porous materials. This compact substrate with limited surface area makes the penetration of the precursor nanodroplets more challenging. We speculate that while infiltration of the nanodroplets into the collagen may occur rapidly, the rate limiting step is for the nanodroplets to reach the collagen scaffold from the surrounding solution. Therefore, the infiltrated mineral, which solidifies with time, could potentially block further infiltration of the precursor that reaches it at a later time, thus limiting the ultimate depth of penetration.
In addition, one should note that the lamellae in bone’s osteons are only around 2 to 10 microns in thickness, which means that demands for depth of mineral penetration are not as high. Here, this PILP process has led to depths that far exceed the dimensions of the lamellae in natural bone formation. It is not known if the reason for the lamellar thickness in bone is related to limitations in the depth of mineral penetration, or if it is simply related to the dimensions and secretory ability of osteoblasts. In any case, these results suggest that it would be feasible for osteoblasts to secrete non-collagenous proteins (comparable to the polyaspartate used here) that could induce such a PILP-type process, which could then readily mineralize the thin lamellae as they are laid down in sequential fashion.
4. Conclusions
Using a biogenic collagen scaffold obtained from demineralized manatee bone, it was demonstrated that a bone-like nanostructure (intrafibrillar mineral) and microstructure (lamellar osteons) could be restored using a polymer-induced liquid-precursor (PILP) mineralization process. Mineral penetration depths of up to 100 μm were achieved using this PILP process, in stark contrast to the conventional crystallization process, which led to no penetration and only surface precipitates. The overall morphology of the PILP-remineralized specimen bore a strong resemblance to the native bone, showing an anisotropic fibrillar texture, with a smooth extrafibrillar mineral coating on the hypermineralized collagen fibrils. Under the most optimal conditions, using a thinner scaffold and a higher molecular weight polyaspartate (Mw 27 KDa), a degree of mineralization of 45wt% was attained. Regarding the substrate’s dimension, the content of mineral relative to the overall sample improved for the thinner substrate, although a full depth of penetration was not achieved. Comparison of morphology, crystallographic orientation of the intrafibrillar HA crystals, and thermal stability results of the PILP-remineralized bone, as well as the affinity of the mineral precursor for the osteonal structures, suggest that the function of polyaspartate in our in-vitro mineralization process emulates the role of the acidic non-collagenous proteins in bone formation. Although most of the analyses presented in this study were qualitative, the results are still promising. Hence the PILP process could be used as a biomimetic model system for the development of the next generation of synthetic bone-graft materials.
Acknowledgments
We are grateful to Dr. John Mecholsky (Materials Science and Engineering Department, University of Florida) for the donation of the manatee bone specimens, and Michael Kerschnitzki from Peter Fratzl’s group (Max Planck Institute of Colloids and Interfaces) for the microtome sample preparation of the ultrathin sections. We also would like to thank the Major Analytical Instrumentation Center, Department of Materials Science and Engineering, University of Florida for the assistance in sample preparation and analysis. This work was supported in part by a NSF Grants BES-0404000, DMR-0710606, and a NIH grant RO1 DE16849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiner S, Wagner HD. The material bone: Structure mechanical function relations. Annu Rev Mater Sci. 1998;28:271–298. [Google Scholar]
- 2.Baer E, Cassidy JJ, Hiltner A. Hierarchical structure of collagen composite systems: Lessons from biology. In: Sarikaya M, Aksay IA, editors. Biomimetics- Design and Processing of Materials. Woodbury, NY: AIP Press; 1995. pp. 13–34. [Google Scholar]
- 3.Currey JD. The design of mineralized hard tissues for their mechanical functions. Journal of Experimental Biology. 1999;202:3285–3294. doi: 10.1242/jeb.202.23.3285. [DOI] [PubMed] [Google Scholar]
- 4.Weiner S, Traub W, Wagner H. Lamellar Bone: structure-function relations. Journal of Structural Biology. 1999;126(3):241–256. doi: 10.1006/jsbi.1999.4107. [DOI] [PubMed] [Google Scholar]
- 5.Veis A, Dove PM, Yoreo JYD, Weiner S, editors. Biomineralization. Washington, D.C: Mineralogical Society of America; 2003. [Google Scholar]
- 6.Eastoe JE, Eastoe B. The organic constituents of mammalian compact bone. Biochemical Journal. 1954;57:453– 459. doi: 10.1042/bj0570453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currey JD. Role of collagen and other organics in the mechanical properties of bone. Osteoporosis International. 2003 Sep;14:S29–S36. doi: 10.1007/s00198-003-1470-8. [DOI] [PubMed] [Google Scholar]
- 8.Currey JD, Zioupos P, Davies P, Casinos A. Mechanical properties of nacre and highly mineralized bone. Proceedings of the Royal Society of London B. 2001;268:107– 111. doi: 10.1098/rspb.2000.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher LW, Termine JD. Non-collagenous proteins influencing the local mechanism of calcification. Clinical Orthopaedics. 1985;200:362. [PubMed] [Google Scholar]
- 10.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed. 2002;41:3130– 3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Helseth DL, Veis A. Collagen self-assembly - Role of telopeptides in in-vitro fibril formation. Federation Proceedings. 1979;38:819–819. [Google Scholar]
- 12.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, et al. Bone structure and formation: A new perspective. Materials Science & Engineering R-Reports. 2007 July 20;58(3–5):77–116. [Google Scholar]
- 13.Helseth DL, Lechner LH, Veis A. Role of the amino-terminal extrahelical region of type I collagen in directing the 4D overlap in fibrillogenesis. Biopolymers. 1979;18:3005–3014. [Google Scholar]
- 14.Weiner S, Traub W. Organization of Crystals in Bone. In: Suga S, Nakahara H, editors. Mechanisms and Phylogeny of Mineralization in Biological Systems. N.Y: Springer-Verlag; 1991. pp. 247–253. [Google Scholar]
- 15.Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF. Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction. Journal of Structural Biology. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- 16.Wagermaier W, Gupta HS, Gourrier A, Burghammer M, Roschger P, Fratzl P. Spiral twisting of fiber orientation inside bone lamellae. Biointerphases. 2006;1(1):1–5. doi: 10.1116/1.2178386. [DOI] [PubMed] [Google Scholar]
- 17.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nature Materials. 2005 Aug;4(8):612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 18.Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci U S A. 2006;103:17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair H. How the osteoclast degrades bone. BioEssays. 1998;20:10:837–846. doi: 10.1002/(SICI)1521-1878(199810)20:10<837::AID-BIES9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Heymann D, Bouler J, Daculsi G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/beta-tricalcium phosphate ratios. Biomaterials. 1997;18(15):1037–1041. doi: 10.1016/s0142-9612(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 21.Cui FZ, Li Y, Ge J. Self-assembly of mineralized collagen composites. Materials Science & Engineering R-Reports. 2007 Aug;57(1–6):1–27. [Google Scholar]
- 22.Lickorish D, Ramshaw JAM, Werkmeister JA, Glattauer V, Howlett CR. Collagen-hydroxyapatite composite prepared by biomimetic process. Journal of Biomedical Materials Research Part A. 2004 Jan 1;68A(1):19–27. doi: 10.1002/jbm.a.20031. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Cui FZ, Zhai Y, Wang XM, Kong XD, Fan DD. Investigations of the initial stage of recombinant human-like collagen mineralization. Mater Sci Eng C-Biomimetic Supramol Syst. 2006 May;26(4):635–638. [Google Scholar]
- 24.Liao S, Watari F, Uo M, Ohkawa S, Tamura K, Wang W, et al. The preparation and characteristics of a carbonated hydroxyapatite/collagen composite at room temperature. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2005 Aug;74B(2):817–821. doi: 10.1002/jbm.b.30315. [DOI] [PubMed] [Google Scholar]
- 25.Rhee SH, Lee JD, Tanaka J. Nucleation of hydroxyapatite crystal through chemical interaction with collagen. Journal of the American Ceramic Society. (83) 2000 Nov;(11):2890–2892. [Google Scholar]
- 26.Girija EK, Yokogawa Y, Nagata F. Apatite formation on collagen fibrils in the presence of polyacrylic acid. Journal of Materials Science-Materials in Medicine. 2004 May;15(5):593–599. doi: 10.1023/b:jmsm.0000026101.53272.86. [DOI] [PubMed] [Google Scholar]
- 27.Lin XY, Li XD, Fan HS, Wen XT, Lu J, Zhang XD. In situ synthesis of bone-like apatite/collagen nano-composite at low temperature. Materials Letters. 2004;58:3569–3572. [Google Scholar]
- 28.Yokoyama A, Gelinsky M, Kawasaki T, Kohgo T, Konig U, Pompe W, et al. Biomimetic porous scaffolds with high elasticity made from mineralized collagen - An animal study. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2005;75B:464–472. doi: 10.1002/jbm.b.30331. [DOI] [PubMed] [Google Scholar]
- 29.Goes JC, Figueiro SD, Oliveira AM, Silva CC, Ricardo NMPS, Sombra ASB. Apatite coating on anionic and native collagen films by an alternate soaking process. Acta Biomaterialia. 2007;3:773–778. doi: 10.1016/j.actbio.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Burger C, Krishnan CV, Chu B, Hsiao BS, Glimcher MJ. In vitro mineralization of collagen in demineralized fish bone. Macromolecular Chemistry and Physics. 2005;206:43– 51. [Google Scholar]
- 31.Deshpande AS, Beniash E. Bioinspired Synthesis of Mineralized Collagen Fibrils. Cryst Growth Des. 2008;8(8):3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Kim YK, Dai L, Li N, Khan SO, Pashley DH, Tay FR. Hierarchical and non-hierarchical mineralisation of collagen. Biomaterials. 2011;32:1291–1300. doi: 10.1016/j.biomaterials.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk NAJM. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 2010;9(12):1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gower LB, Odom DJ. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J Crystal Growth. 2000;210:4:719–734. [Google Scholar]
- 35.Dai L, Douglas EP, Gower LB. Compositional analysis of a polymer-induced liquid-precursor (PILP) amorphous CaCO3 phase. Journal of Non-Crystalline Solids. 2008;354:1845–1854. [Google Scholar]
- 36.Gower LB. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chemical Reviews. 2008;108:4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olszta M, Gajjeraman S, Kaufman M, Gower L. Nano-fibrous calcite synthesized via a solution-precursor-solid (SPS) mechanism. Chem Mat. 2004;16:12:2355–2362. [Google Scholar]
- 38.Cheng X, Gower LB. Molding Mineral within Microporous Hydrogels by a Polymer-Induced Liquid-Precursor (PILP) Process. Biotechnology Progress. 2006;22(1):141–149. doi: 10.1021/bp050166+. [DOI] [PubMed] [Google Scholar]
- 39.Amos FF, Dai L, · RK, Khan SR, Gower LB. Mechanism of formation of concentrically laminated spherules: implication to Randall’s plaque and stone formation. Urol Res. 2009;37:1:11–17. doi: 10.1007/s00240-008-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amos FF, Olszta MJ, Khan SR, Gower LB. Relevance of a Polymer-Induced Liquid-Precursor (PILP) Mineralization Process to Normal and Pathological Biomineralization. In: Königsberger E, Königsberger L, editors. Biomineralization- Medical Aspects of Solubility. West Sussex, England: John Wiley & Sons, Ltd; 2006. pp. 125–217. [Google Scholar]
- 41.Amos FF, Sharbaugh DM, Talham DR, Gower LB, Fricke M, Volkmer D. Formation of single-crystalline aragonite tablets/films via an amorphous precursor. Langmuir. 2006;23:1988–1994. doi: 10.1021/la061960n. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y-Y, Gower LB. Patterning Inorganic (CaCO3) Thin Films via a Polymer-Induced-Liquid-Precursor (PILP) Process. Langmuir. 2007;23:9:4862–4870. doi: 10.1021/la061975l. [DOI] [PubMed] [Google Scholar]
- 43.Olszta MJ, Douglas EP, Gower LB. Intrafibrillar mineralization of collagen using a liquid-phase mineral precursor. In: Thomas J, Kiick K, Gower L, editors. Mat Res Soc Symp Proc O- Materials Inspired by Biology. San Francisco: MRS; 2003. pp. 127–134. [Google Scholar]
- 44.Olszta MJ, Douglas EP, Gower LB. Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process. Calcif Tissue Int. 2003;72(5):583–591. doi: 10.1007/s00223-002-1032-7. [DOI] [PubMed] [Google Scholar]
- 45.Olszta MJ, Odom DJ, Douglas EP, Gower LB. A New Paradigm for Biomineral Formation: Mineralization via an Amorphous Liquid-Phase Precursor. Connective Tissue Research. 2003;44(Suppl 1):326–334. [PubMed] [Google Scholar]
- 46.Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proceedings of the National Academy of Sciences USA. 2008;105(35):12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li CH, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proceedings of the National Academy of Sciences USA. 2010;107(14):6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jee SS, Culver L, Li Y, Douglas EP, Gower LB. Biomimetic mineralization of collagen via an enzyme-aided PILP process. Journal of Crystal Growth. 2010;312:1249–1256. [Google Scholar]
- 49.Jee SS, Thula TT, Gower LB. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process: 1) Influence of polymer molecular weight. Acta Biomaterialia. 2010;6:3676–3686. doi: 10.1016/j.actbio.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 50.Thula TT, Svedlund F, Rodriguez DE, Podschun J, Pendi L, Gower LB. Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers. 2011;3:10–35. doi: 10.3390/polym3010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clifton KB, Reep RL, Mecholsky JJ., Jr Quantitative fractography for estimating whole bone properties of manatee rib bones. J Mater Sci. 2008;43:2026–2034. [Google Scholar]
- 52.Gu L, Kim YK, Liu Y, Ryou H, Wimmer CE, Dai L, Arola DD, Looney SW, Pashley DH, Tay FR. Biomimetic Analogs for Collagen Biomineralization. Journal of Dental Research. 2011;90(1):82–87. doi: 10.1177/0022034510385241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clifton KB, Yan J, Mecholsky JJ, Jr, Reep RL. Material properties of manatee rib bone. Journal of Zoology. 2008;274:150–159. [Google Scholar]
- 54.Bigi A, Ripamonti A, Cojazzi G, Pizzuto G, Roveri N, Koch MHJ. Structural analysis of turkey tendon collagen upon removal of the inorganic phase. Int J Biol Macromol. 1991 April;13:110–114. doi: 10.1016/0141-8130(91)90058-3. [DOI] [PubMed] [Google Scholar]
- 55.Jee SS, Kasinath RK, DiMasi E, Kim YY, Gower LB. Oriented hydroxyapatite in turkey tendon mineralized via the polymer-induced liquid-precursor (PILP) process. CrystEngComm. 2011 doi: 10.1039/c0ce00605j. Advance article. [DOI] [Google Scholar]
- 56.LeGeros RZ. Calcium Phosphate in Oral Biology and Medicine. N.Y: Karger; 1991. [PubMed] [Google Scholar]
- 57.Dorozhkin SV. Calcium Orthophosphates in Nature, Biology and Medicine. Materials. 2009;2:399–498. [Google Scholar]
- 58.Lowenstam HA, Weiner S. On Biomineralization. N.Y: Oxford University Press; 1989. [Google Scholar]
- 59.Glimcher MJ. The nature of the mineral phase in bone: Biological and clinical implications. In: Avioli LV, Krane SM, editors. Metabolic bone disease and clinically related disorders. San Diego, CA: Academic Press; 1998. pp. 23–50. [Google Scholar]
- 60.Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22(13):1705–1711. doi: 10.1016/s0142-9612(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 61.Murugan R, Ramakrishna S. Crystallographic study of hydroxyapatite bioceramics derived from various sources. Crystal Growth & Design. 2005;5:1:111–112. [Google Scholar]
- 62.Nyman JS, Roy A, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, Dean DD, Wang X. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone. 2006;39:1210–7. doi: 10.1016/j.bone.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zylberberg L, Traub W, De Buffrenil V, Allizard F, Arad T, Weiner S. Rostrum of a toothed whale: ultrastructural study of a very dense bone. Bone. 1998;23:241–247. doi: 10.1016/s8756-3282(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 64.Traub W, Arad T, Weiner S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci. 1989;86:9822–9826. doi: 10.1073/pnas.86.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lozano LF, Pena-Rico MA, Heredia A, N-Flores JO, Velazquez R, Belio IA, Bucio L. Thermal analysis study of human bone. Journal of Materials Science. 2003;38:4777–4782. [Google Scholar]