Abstract
Neurotrophin signaling at the neuromuscular junction modulates cholinergic transmission and enhances neuromuscular transmission via the tropomyosin-related kinase receptor subtype B (TrkB). A novel flavonoid, 7,8-dihydroxyflavone (7,8-DHF), selectively activates TrkB receptors. Using TrkBF616A mice that are susceptible to specific inhibition of TrkB activity by 1NMPP1, we show that neuromuscular transmission is enhanced by 7,8-DHF (~32%) via activation of TrkB in diaphragm muscle. The small molecule 7,8-DHF may constitute a novel therapy to improve neuromuscular function.
Keywords: Flavones, Neurotrophins, Neuromuscular Transmission Failure, Muscle fatigue, Synaptic efficacy
INTRODUCTION
Several studies demonstrate that neurotrophin signaling at the neuromuscular junction modulates cholinergic transmission.1–5 In particular, brain-derived neurotrophic factor (BDNF) potentiates neurotransmitter release in both developing neuromuscular synapses in culture,3,4,6 and at adult rat neuromuscular junctions.1,2,5 BDNF binds the tropomyosin-related kinase receptor subtype B (TrkB) with high affinity to exert its biological effects.7,8 A member of the flavonoid family of chemicals, 7,8-dihydroxyflavone (7,8-DHF), was recently reported to selectively activate TrkB,9,10 although some neuroprotective effects of 7,8-DHF may not be mediated via TrkB activation.11 The purpose of this study was to determine the effect of 7,8-DHF on neuromuscular transmission using TrkBF616A mice that are susceptible to specific inhibition of TrkB activity by derivatives of the kinase inhibitor PP1 (e.g., 1NMPP1).12 Administration of neurotrophins like BDNF has proven clinically unfeasible,13–15 but therapeutic limitations may be overcome by small molecule TrkB receptor agonists such as 7,8-DHF.
MATERIALS AND METHODS
Following institutional approval, adult male TrkBF616A mice (~24 g in body weight; gift from Dr. D.D. Ginty, John Hopkins University, Baltimore, MD) were sacrificed under deep ketamine and xylazine anesthesia.12 Diaphragm muscle preparations with the phrenic nerve attached (2 mm wide) were placed in a horizontal organ bath for isometric muscle force measurements as described previously.1,16,17 Three experimental groups (n=6 each) were used in this study: 1) vehicle control; 2) 7,8-DHF (10 µM, Tocris Bioscience, Ellisville, MO); and 3) 7,8-DHF and 1NMPP1 (Calbiochem, San Diego, CA). All preparations were treated for 30 min with the experimental drug(s) prior to and during force measurements.
The contribution of neuromuscular transmission failure to muscle fatigue with repetitive activation was determined as previously described.1,16–19 Briefly, neuromuscular transmission failure was defined as the difference in force elicited by phrenic nerve stimulation (supramaximal 0.2-ms pulses at 40 Hz, 330-ms trains repeated every 1 s for 2 min) and the force elicited by direct muscle stimulation (2-ms pulses at 40 Hz in 330-ms trains) superimposed every 15 s. This rate of stimulation results in muscle fatigue with no decrement in force within a train.18 In separate muscle segments, the contractile and fatigue properties of the diaphragm muscle were independently determined. All comparisons across groups were performed using repeated measures ANOVA (JMP 8.0, SAS Institute Inc., Cary, NC). Data are reported as mean ± SE. When appropriate, post hoc group differences were assessed using the Tukey-Kramer honestly significant difference test. A value of p<0.05 was considered statistically significant.
RESULTS
Diaphragm muscle force decreased with repetitive stimulation at 40 Hz, as previously reported in rats.1,16–19 Diaphragm muscle contractile and fatigue properties were not different across treatment groups. Specifically, twitch force generated by the diaphragm muscle was 7.9±0.6 N/cm2 for vehicle-treated, 7.4±0.3 N/cm2 for 7,8-DHF-treated and 7.5±0.2 N/cm2 for 1NMPP1- and 7,8-DHF-treated groups. Diaphragm muscle tetanic force was 21.4±0.4 N/cm2, 21.0±0.5 N/cm2 and 21.8±0.6 N/cm2 in the vehicle-, 7,8-DHF- and 1NMPP1-treated groups, respectively. Diaphragm muscle fatigability assessed as the decrement in force generated after 2 min of repetitive 40 Hz stimulation was also not different across groups (~35% of initial force). Thus, TrkB receptor signaling at the neuromuscular junction does not affect muscle fiber properties in mice, which is consistent with a previous report in rats.1
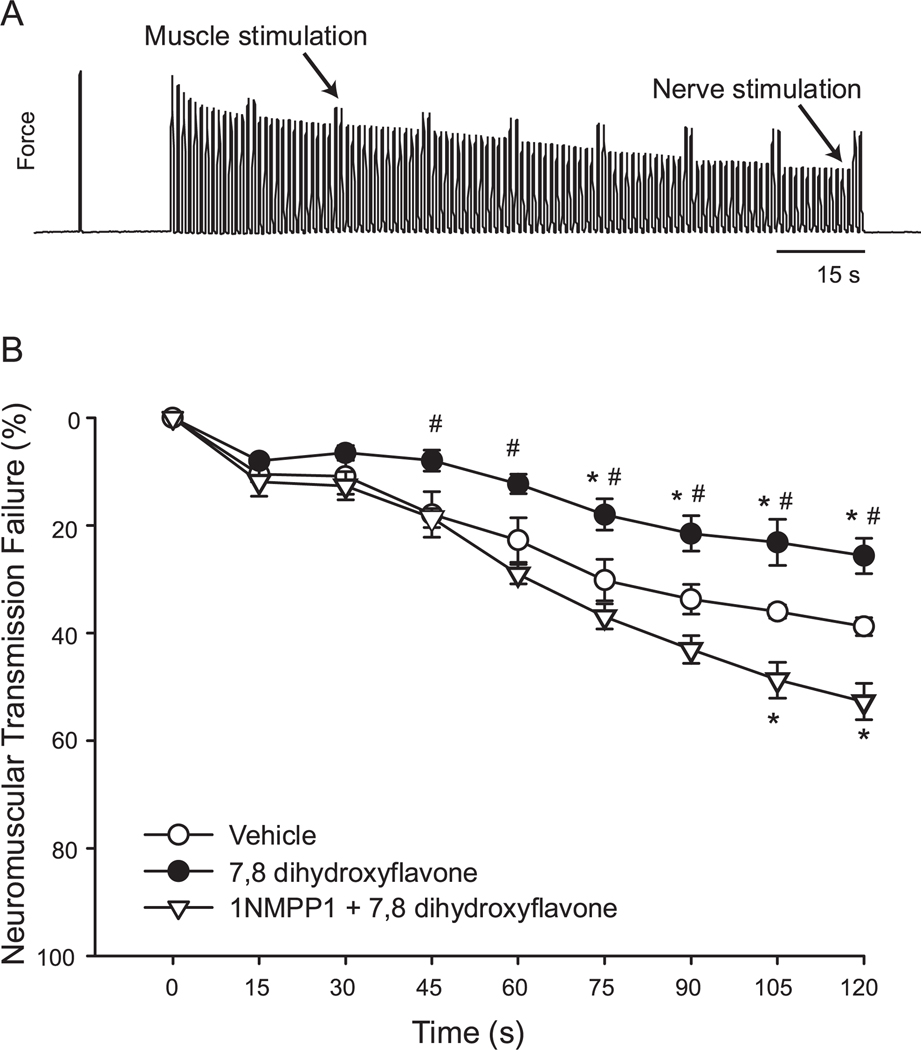
Neuromuscular transmission failure was estimated by comparing the forces generated by nerve and direct muscle stimulation (Fig. 1). A significant experimental group and time effect was observed (p<0.001). Compared to vehicle controls, 7,8-DHF treatment reduced the contribution of neuromuscular transmission failure to muscle fatigue after 45 s of repetitive 40-Hz stimulation (p<0.001), indicating an enhancement of neuromuscular transmission with 7,8-DHF treatment. Concomitant treatment with 1NMPP1 completely abolished the effect of 7,8-DHF, and after 105 s of repetitive stimulation, neuromuscular transmission was impaired compared to vehicle-treated controls (p<0.001). Following 2 min of repetitive nerve stimulation, the contribution of neuromuscular transmission failure to diaphragm muscle fatigue was 37.7±1.7% for vehicle-treated, 25.6±3.3% for 7,8-DHF-treated and 52.8±3.4% for 1NMPP1- and 7,8-DHF-treated groups (p<0.05 for both 7,8-DHF-treated groups compared to vehicle, and for the 1NMPP1-treated vs. 7,8-DHF alone groups). In summary, 7,8-DHF treatment enhanced neuromuscular transmission by ~32%, whereas concomitant treatment with 1NMPP1 impaired neuromuscular transmission by ~40%, indicating that 7,8-DHF rapidly potentiates neuromuscular transmission via activation of TrkB receptors.
Figure 1.
Effect of 7,8-dihydroxyflavone on neuromuscular transmission in diaphragm muscle of TrkBF616A knockin mice. A. Representative tracing of force evoked by repetitive stimulation of a diaphragm-phrenic nerve preparation. Maximal isometric twitch force was obtained by direct muscle stimulation (first peak). The phrenic nerve was then stimulated at 40-Hz. Direct muscle stimulation was superimposed every 15 s via plate electrodes. Repetitive stimulation results in muscle fatigue, which is evident as a decrease in force. The contribution of neuromuscular transmission failure to muscle fatigue can then be calculated from the difference in force elicited by nerve and muscle stimulation (see text for details). B. Time course of neuromuscular transmission failure for TrkBF616A diaphragm muscle. Vehicle treatment (open circles) shows increasing neuromuscular transmission failure over time, which is consistent with previous results in the rat.1,16–19 Treatment with 7,8-dihydroxyflavone (closed circles) reduced neuromuscular transmission failure, reflecting enhanced neuromuscular transmission. The effect of 7,8-dihydroxyflavone was completely blocked by 1NMPP1 treatment (open triangles), indicating a specific effect on TrkB receptor kinase activity, since TrkBF616A knockin mice harbor a mutation that renders TrkB susceptible to inhibition by 1NMPP1.12 *, different from vehicle-treated group at same time-point; #, different from 1NMPP1- and 7,8-dihydroxyflavone-treated group at same time-point (p<0.05).
DISCUSSION
A small molecule member of the flavonoid family of chemicals, 7,8-DHF, rapidly enhanced neuromuscular transmission in the diaphragm muscle of adult TrkBF616A mice. This improvement was produced by specific activation of the TrkB receptor, since inhibition of TrkB activity by 1NMPP1 abrogated this effect. TrkBF616A mice were genetically modified to stably harbor a knockin mutation in the exon encoding the Trk kinase ATP binding pocket that renders TrkB susceptible to inhibition by derivatives of the kinase inhibitor PP1.12
The compound 7,8-DHF likely enhances neuromuscular transmission pre-synaptically, since it does not directly affect isometric contractile and fatigue properties of the diaphragm muscle. The effects of 7,8-DHF are consistent with the role of neurotrophins on neuromuscular transmission.2–4,6 In rats, neuromuscular transmission is enhanced by treatment with the neurotrophins BDNF and NT-4 (~31%),1 both of which bind the TrkB receptor.7,8 Neither BDNF nor NT-4 treatment affected muscle contractile or fatigue properties in rats.
TrkB receptor activity may help sustain neuromuscular transmission during repetitive stimulation. Indeed, concomitant treatment with 1NMPP1 (and 7,8-DHF) worsened neuromuscular transmission compared to vehicle. In the rat diaphragm muscle, treatment with K252a, a TrkB receptor inhibitor, also impaired neuromuscular transmission.1 Furthermore, both K252a and the fusion protein TrkB-IgG, which binds endogenously released BDNF or NT-4, reduced cholinergic release in the mouse levator auris longus muscle.2 Of note, 7,8-DHF exerted neuroprotective effects in the hippocampus that were mediated by TrkB receptor signaling.9 However, 7,8-DHF prevented apoptosis of cultured hippocampal cells lacking TrkB receptor, and these effects were mediated by reduced generation of reactive oxygen species (ROS).11 In the frog neuromuscular junction, ROS inhibit transmitter release,20 but it is presently unknown if ROS exert a similar effect in rodents. Regardless, in this study, concomitant treatment with 1NMPP1 in TrkBF616A mice completely blocked the effect of 7,8-DHF.
Treatment with 7,8-DHF may yield the beneficial effects of neurotrophins,1–5 but without the side effect profile and bioavailability issues that have limited human application.13–15 Enhanced neuromuscular transmission by pharmacological agents such as 7,8-DHF may constitute a novel therapy for neuromuscular disorders, but the clinical impact of such therapy remains to be determined.
Acknowledgments
Supported by NIH grants HL096750 and AR051173 and the Mayo Clinic.
Abbreviations
- 7,8-DHF
7,8-dihydroxyflavone
- BDNF
brain-derived neurotrophic factor
- ROS
reactive oxygen species
- TrkB
tropomyosin-related kinase receptor subtype B
REFERENCES
- 1.Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004;29(3):381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- 2.Garcia N, Tomas M, Santafe MM, Besalduch N, Lanuza MA, Tomas J. The Interaction between Tropomyosin-Related Kinase B Receptors and Presynaptic Muscarinic Receptors Modulates Transmitter Release in Adult Rodent Motor Nerve Terminals. J Neurosci. 2010;30(49):16514–16522. doi: 10.1523/JNEUROSCI.2676-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XH, Poo MM. Potentiation of developing synapses by postsynaptic release of neurotrophin-4. Neuron. 1997;19(4):825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Berninger B, Poo M. Localized synaptic actions of neurotrophin-4. J Neurosci. 1998;18(13):4985–4992. doi: 10.1523/JNEUROSCI.18-13-04985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia N, Santafe MM, Tomas M, Lanuza MA, Besalduch N, Tomas J. Neurotrophin-4 couples to locally modulated ACh release at the end of neuromuscular synapse maturation. Neurosci Lett. 2010;468(1):72–74. doi: 10.1016/j.neulet.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168(2):163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Chua KW, Chua CC, Yu H, Pei A, Chua BH, Hamdy RC, Xu X, Liu CF. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011;499(3):181–185. doi: 10.1016/j.neulet.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46(1):13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(3):201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 14.Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, Naumann M. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(2):100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- 15.A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52(7):1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 16.Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22(3):307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low dose inhaled CO. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R784–R789. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett. 1991;125(1):34–36. doi: 10.1016/0304-3940(91)90124-c. [DOI] [PubMed] [Google Scholar]
- 19.Aldrich TK, Shander A, Chaudhry I, Nagashima H. Fatigue of isolated rat diaphragm: role of impaired neuromuscular transmission. J Appl Physiol. 1986;61(3):1077–1083. doi: 10.1152/jappl.1986.61.3.1077. [DOI] [PubMed] [Google Scholar]
- 20.Giniatullin AR, Grishin SN, Sharifullina ER, Petrov AM, Zefirov AL, Giniatullin RA. Reactive oxygen species contribute to the presynaptic action of extracellular ATP at the frog neuromuscular junction. J Physiol. 2005;565(Pt 1):229–242. doi: 10.1113/jphysiol.2005.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]