Abstract
Hepatitis C virus (HCV) infection is an important risk factor for insulin resistance (IR). The latter is the pathogenic foundation underlying metabolic syndrome, steatosis and cirrhosis, and possibly hepatocellular carcinoma (HCC). The interplay between genetic and environmental risk factors ultimately leads to the development of IR. Obesity is considered a major risk factor, with dysregulation of levels of secreted adipokines from distended adipose tissue playing a major role in IR. HCV-induced IR may be due to the HCV core protein inducing proteasomal degradation of insulin receptor substrates 1 and 2, blocking intracellular insulin signaling. The latter is mediated by increased levels of both tumour necrosis factor-α (TNF-α) and suppressor of cytokine signaling 3 (SOC-3). IR, through different mechanisms, plays a role in the development of steatosis and its progression to steatohepatitis, cirrhosis and even HCC. In addition, IR has a role in impairing TNF signaling cascade, which in turn blocks STAT-1 translocation and interferon stimulated genes production avoiding the antiviral effect of interferon.
Keywords: Hepatitis C virus-chronic hepatitis C, Insulin resistance, Sustained virological response, Homeostasis model assessment
INTRODUCTION
Infection with hepatitis C virus (HCV) is a common problem worldwide, affecting millions of people across all populations. Most acutely infected patients develop chronic hepatitis and become a potential source of virus transmission, and as many as 1 in 5 will develop cirrhosis and its complications[1]. Besides, HCV is an increasingly recognized important cause of extrahepatic manifestations, including insulin resistance (IR)[2].
IR is a complex pathophysiological condition where higher-than-normal concentrations of insulin are needed to maintain a normal glycemia and adequate glucose utilization in insulin target tissues[3]. IR is of global importance since is closely linked to the epidemic condition of obesity and it precedes and predicts the development of type 2 diabetes mellitus (T2DM) and increases the risk of life-threatening complications such as cardiovascular diseases, renal failure, and infections. However, these complications are not major causes of death in cirrhotic patients with IR[4]. In contrast, the development of intrahepatic complications, including HCC, is known to be associated with IR[5].
IR is extremely common in patients with chronic HCV infection and has been associated with increased disease severity, extrahepatic manifestations and decreased response to antiviral therapy[6]. Understanding the basis of such associations is of paramount importance to inform treatment strategies for patients with HCV. This review summarizes recent information on the different issues of HCV infection and IR.
CAUSES OF INSULIN RESISTANCE
It represents interplay between genetics (inherited) and environmental (acquired) factors. Genetic factor include abnormal insulin, abnormal insulin receptor and abnormal signaling proteins. The main acquired factors of IR include: abdominal obesity, aging, hyperglycemia, medications and recently HCV infection.
Abdominal obesity
Obesity is associated with IR, hepatic steatosis and over expression of tumor necrosis factor-α (TNF-α). All of these factors increase the risk of fibrosis and decreased antiviral efficacy. Also, obesity decreases interferon bioavailability and impairs immune stimulating properties of interferon. Hepatic steatosis, nonalcoholic steatohepatitis and fibrosis are associated with release of reactive oxygen species (ROS), which contribute to decreased HCV response to interferon (IFN)[7,8].
Moreover, obesity is associated with decreased number and downregulation of insulin receptors and impairment of postreceptor signaling. Overflow of free fatty acids (FFAs) from adipose tissue interferes with intrahepatic insulin signaling pathway via increased levels of pro-inflammatory cytokines such as TNF-α[9,10], and proteasomal degradation of the insulin receptor substrates (IRS) 1 and 2 ( Figure 1)[11].
Figure 1.
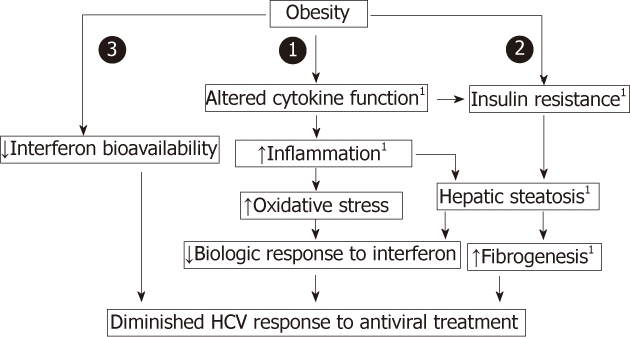
Obesity is associated with insulin resistance and decreased antiviral efficacy. 1Indicates potential for coexistent viral potentiation. HCV: Hepatitis C virus.
Aging
Aging is associated with increased levels of FFAs and triglycerides, in addition to glucose transporter-4 (GLUT4) decrease and mutation.
Hyperglycemia
Overflow of FFAs from adipose tissue to systemic circulation impairs insulin-mediated glucose uptake by the muscles resulting in hyperglycemia and peripheral IR.
Medications
Some medications can induce IR as glucocorticoids, cyclosporine, growth hormones, thyroid hormones, sex hormones, analogues, thiazides, β-blockers, protease inhibitors for HCV and nucleoside analogues for hepatitis B virus.
Hepatitis C virus infection
Recently has evolved as a significant risk factor for the development of IR.
ROLE OF ADIPOKINES IN INSULIN RESISTANCE
Adipokines
Adipokines are polypeptides secreted in the adipose tissue in a regulated manner and they include adiponectin, leptin, resistin, retinol-binding protein 4, visfatin, omentin, vaspin, chemerin, apelin, TNF-α, interleukin 6 (IL-6), and monocyte chemoattractant protein-1[12].Chronic HCV infection is associated with changes in the serum level of some adipokines[13].
Adiponectin
Decreased serum levels of adiponectin have been reported in chronic hepatitis C (CHC), especially in patients with steatosis. Deficiency of adiponectin is associated with obesity, IR, glucose intolerance, triglyceride (TG) accumulation and steatosis, and metabolic syndrome[13].
Leptin
Leptin insufficiency is associated with increased body weight, increased FA synthesis, decreased FA oxidation, decreased TG excretion, increased steatosis, and impaired insulin sensitivity and secretion[13]. In a recent controlled study of HCV-infected nondiabetic males compared to matched uninfected controls, circulating levels of the adipokines leptin and adiponectin were independently associated with IR, but not with the presence of HCV, and it was concluded that HCV-associated IR does not seem to be mediated by adipokines or proinflammatory cytokines[14].
Tumor necrosis factor-α
TNF-α is also considered one of the adipokines secreted in adipose tissue. HCV may also induce IR by triggering the production of proinflammatory cytokines, because in hepatitis C, circulating TNF-α levels are increased[15,16]. TNF-α can induce IR by several mechanisms, both direct and indirect. TNF-α interferes with the insulin signaling pathway, via induction of the suppressor of cytokines 3 (SOCS-3) that inactivates phosphatidylinositol-3-kinase (PI3K). The latter inhibits GLUT-4 translocation to cell membrane and intracellular glucose entry[13]. Increased TNF-α-mediated expression of SOCS-3 has been identified in obese, genotype 1 patients with chronic HCV infection[17].
In addition, TNF-α triggers lipolysis of FFAs from adipose tissue, leading to increased serum levels of free fatty acids and it has also a direct inhibitory effect on insulin action in the liver. These effects lead to reduced glucose uptake in muscle, and to increased hepatic glucose production[18,19]. TNF-α is also suggested to regulate expression of several adipocyte genes known to modulate insulin sensitivity/resistance[20], therefore, it is suggested that TNF-α acts as a possible link between HCV and diabetes[21].
Apeline
Apeline, an adipocytokine derived from adipose tissue, has been recently suggested to be associated with fibrosis progression and development of cirrhosis in HCV infection. In addition, apeline levels are more elevated in IR subjects than in non-IR. Both TNF-α and apeline may rather express complementary effect in fibrosis progression as well as impaired response to IFN therapy.
HEPATITIS C VIRUS AND INSULIN RESISTANCE
Hepatitis C virus induces insulin resistance
The causal relationship of HCV infection and IR development has been demonstrated by the increased prevalence of IR in chronic HCV infection. Whereas the overall prevalence of IR is 10%-25% of the population[22], the prevalence IR in HCV infection reaches figures ranging between 30% to 70%[23,24]. Moreover, IR with HCV infection is increased at early stages of liver disease without liver fibrosis, and is on average significantly higher than that found in patients with chronic hepatitis B, matched for age and body mass index[25].
The causal relationship of HCV infection and IR development has also been demonstrated by improvement of IR after successful therapy in CHC patients, whereas no improvement is observed in nonresponders[26-28].
Mechanism
HCV virus, through both direct and indirect pathways, affects the insulin signaling pathways, promoting IR at a cellular level. Insulin effects are elicited after binding of insulin to its receptor that is linked to a complex signaling pathway that involves sequential activation of IRS, PI3K, Akt, a protein kinase which is a downstream of PI3K activation, and protein kinase C. This cascade of events eventually results in stimulation of glucose uptake after translocation of the GLUT4 to the plasma membrane. IR results from defects at any level of the insulin receptor-related signaling pathway[29] (Figure 2).
Figure 2.
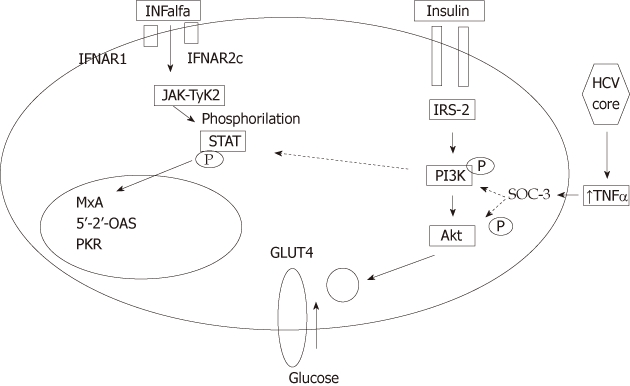
Interaction between hepatitis C virus core, insulin- and interferon-α signaling pathways continuous lines represent activation. Dotted lines represent inhibition. Hepatitis C virus core protein induces expression of tumour necrosis α (TNF-α), which in turn activates suppressor of cytokines-3 (SOCS-3). Activation of SOCs-3 leads to proteasomal degradation of insulin receptor substrate and inactivates phosphatidyl-inositol-3-kinase (PI3K), leading to inhibition of translocation of glucose transferase (GLUT-4) to cell membrane, blocking intracellular glucose entry, with subsequent hyperglycemia, hyperinsulinemia and peripheral insulin resistance. Activation of SOCS-3 also leads leading to inhibition of Tyr-phosphorylation of signal transducers and activators of transcription 1 leading to impaired TNF-α signaling. HCV: Hepatitis C virus; IFNAR2c: Interferon receptor chain 2; IRS2: Insulin receptor substrate 2; JAK: Janus kinase; TYK2: Tyrosine kinase 2; STAT: Signal transducer and activator of transcription.
Following inflammatory response in the liver to HCV infection, a profound impairment of insulin signaling occurs at the level of IRS tyrosine phosphorylation and PI3K activation[30]. HCV core protein induces expression of TNF-α, which activates SOCS-3, leading to subsequent proteasomal degradation of IRS1 and IRS2, resulting in the development of IR. Meanwhile, SOCS-3 inactivates PI3K, which in turn inhibits translocation of GLUT-4 to cell membrane, thus blocking intracellular glucose uptake[10,11,31] (Figure 3).
Figure 3.
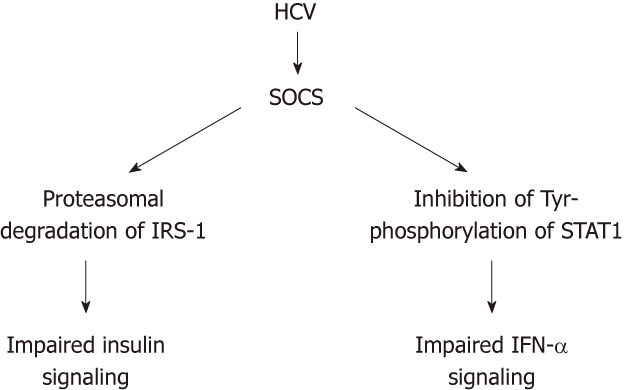
Proposed dual role of the upregulation of members of the suppressor of cytokines family by hepatitis C virus: simultaneous impairment of the insulin and IFN-a signaling systems. HCV: Hepatitis C virus; SOC: Suppressor of cytokines; IRS-1: Insulin receptor substrate 1; STAT1: Signal transducers and activators of transcription1; IFN-α: Interferon-α.
It has been also suggested that increased levels of pro-inflammatory cytokines such as interleukin 1, TNF-α, IL-6 and leptin, and reduced levels of adiponectin may directly contribute to the occurrence of HCV-related IR[23].
Viral load and insulin resistance
The relationship between the severity of IR and HCV replicative levels has been very difficult to prove. However, recent work seems to suggest so[32]. In addition, it has been shown that IR is modified by treatment and that the incidence of T2DM in patients achieving sustained virological response (SVR), defined as undetectable HCV RNA 24 wk after completing treatment, is significantly lower than that seen in nonresponders[26-28]. However, it is still not clear whether HCV replication directly increases IR, or whether hyperinsulinemia stimulates viral replication, as suggested by in vitro data[33]. It is to be noted that the global level of IR is likely to depend on the contribution from the adipose tissue and the muscle, two extrahepatic compartments that are not infected by HCV.
Viral genotype and insulin resistance
Although the interference with the insulin effects shows some HCV genotype-specificity, IR has been reported to occur in all HCV genotypes, but to a different extent[34]. HCV genotype 3a, in addition, may alter the intrahepatic insulin signaling through a downregulation of peroxisome proliferator-activated receptor[35]. In HCV genotype 1b infections, substitutions of amino acids 70 and/or 91 in HCV-1b core were found to be significant determinants of severe IR, in patients without cirrhosis and diabetes mellitus, which suggests a real connection between HCV-1b infection and IR at early stages of liver disease[36].
Hepatitis C virus, iron overload, oxidative stress and insulin resistance
Hepatic iron overload has been repeatedly reported in patients with chronic HCV infection[37-39]. Although the mechanisms of hepatic iron overload remains unclear, recent studies showed a decreased hepatic expression of hepcidin, a negative regulator of duodenal iron absorption, in patients with HCV infection[40-42], in addition to increased hepatic expression of transferring receptor 2, a mediator of iron uptake, which is responsible for the hepatic iron overload[43].
The mechanisms through which iron causes IR are not clear. Hepatic iron overload produces oxidative stress and is a factor responsible for the development of HCV-associated IR[44-47]. Oxidative stress, itself, is an independent factor in the development of IR in patients with signaling[47], and has been associated with altered IFN-α signaling via decreased phosphorylation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway[48]. In addition, a cross-talk between iron metabolism and insulin-glucose metabolism has recently been documented[49]. Iron has been found to reduce hepatic extraction/metabolism of insulin and to interfere with insulin action on the liver, leading to peripheral hyperinsulinemia[50,51]. In contrast, hyperinsulinemia may cause rapid stimulation of iron uptake into the liver, because insulin is known to redistribute transferrin receptors from an intracellular membrane compartment to the cell surface[52].
EFFECTS OF HEPATITIS C VIRUS-INDUCED INSULIN RESISTANCE
Increased incidence of type 2 diabetes mellitus
An important clinical implication of IR in chronic HCV infection is the strong relationship of IR and T2DM development[53]. One recent meta-analysis has confirmed that chronic HCV is associated with an increased risk of developing T2DM compared with both non-infected controls and patients with chronic HBV infection, which is independent of the presence of cirrhosis[54]. Nearly 30%-70% of patients with CHC display some form of IR[23]. accordingly worldwide, 47 million patients may have HCV-associated DM[21].
On the other hand, IFN therapy is often implicated in the literature as having a role in the development of diabetes in HCV patients. However, this association is rare, and the few cases of DM developing during IFN therapy had T1DM, in line with other autoimmune manifestations induced by IFN[55,56].
Insulin resistance-induced hepatic steatosis, fibrosis and hepatocellular carcinoma
HCV, IR and steatosis: The overall prevalence of steatosis in patients with HCV infection is approximately 55% ranging from 35% to 81% in various studies, which is approximately 2-3 folds higher than the prevalence of steatosis in other liver disease[57]. One-to two-thirds of liver biopsies from CHC patients have histological evidence of steatosis, which has been associated with being overweight, hepatic fibrosis and increased TG levels[58,59].
The relationship between IR and HCV infection is complex and bidirectional; HCV induces steatosis[57], and the latter could also cause IR[60]. In addition to inflammation, HCV proteins also play a role in the development of IR and oxidative stress, the two key pathways in the pathogenesis of non alcoholic fatty liver disease (NAFLD)[61]. On the other hand, insulin is an anabolic hormone and promotes hepatic lipogenesis[62], and inhibits lipolysis[63]. Therefore, the initial step in HCV-related metabolic disorders remains unclear.
The current evidence suggests that HCV-associated hepatic steatosis is mainly virus-induced in genotype-3a infected patients[64]. which seem to be mediated by an impaired very low-density lipoprotein (VLDL) secretion, most likely via an impaired activity of the liver microsomal triglyceride transfer protein (MTP)[65]. On the contrary, the host-factors (mainly IR) play a major role in steatosis in non-3 genotypes[64]. In addition to inflammation, HCV proteins also play a role in the development of IR and oxidative stress, the two key pathways in the pathogenesis of NAFLD[65]. It is well known that IR causes impaired metabolic clearance of glucose and hyperglycemia. In addition, peripheral IR also increases adipose tissue lipolysis, leading to increased plasma and hepatic uptake of FFAs. Increased hepatic uptake of FFAs impairs β-oxidation in mitochondria, together with decreased excretion of VLDL, resulting in TG retention with subsequent development of hepatic steatosis[66,67].
A direct relationship between HCV replication and steatosis has been extensively documented by both clinical and experimental data[68,69]. HCV core protein also regulates secretion of VLDL, TG, and apoliprotein B through regulation of MTP peroxisome proliferator-activated receptor γ (PPAR-γ), and sterol regulatory element binding protein-1c (SREBP-1c)[70-72]. The latter is a protein central to insulin signaling, also involved in upregulation of de novo lipogenesis and inhibition of fatty acid β-oxidation; two events that can favour intracellular accumulation of triglycerides.
Recently, there has been considerable interest in the role of micro-RNAs (miRNA) in the genesis of both fatty liver and HCV replication, in particular, mir122, the most abundant liver miRNA, has been shown to affect the development of steatosis by increasing lipogenesis and by enhancing HCV virus replication[73].
HCV, IR and fibrosis: Both IR and hepatic steatosis have been closely associated with progression of hepatic fibrosis in patients with HCV infection[57]. A recent meta-analysis using 3068 patients recruited at 10 centers in 5 countries suggests that steatosis and diabetes are both independent factors of fibrogenesis in patients with genotype 1 infection[74]. Significant fibrosis has also been associated with IR independent of steatosis in CHC genotype 1 and 4 infections.
Recently, several reports have suggested that IR may contribute to the progression of fibrosis[75-77]. IR may directly stimulate the proliferation of hepatic stellate cells (HSCs) promoting collagen I synthesis[78] or IR-induced hepatic lipid accumulation and generation of ROS can activate HSCs, initiating progression of fibrosis to cirrhosis[79].
HCV, IR and HCC: Subjects with HCV and diabetes have a higher risk of developing HCC[80]. In a very large, general population-based, cohort study comprising a total of 23 820 residents in Taiwan and followed up for 14 years[81], diabetes was associated with HCC especially among individuals with HCV infection, with lower risk values among carriers of the hepatitis B virus or uninfected individuals. Recent evidence strongly suggests that steatosis and diabetes may also significantly enhance the risk of HCC[82,83].
HCV core protein was reported to induce HCC in transgenic mice, providing a direct experimental evidence for the contribution of HCV core protein in the development of HCC in human HCV infection[84]. More recent reports support the oncogenic potential of HCV and linked clinically the substitutions of amino acid 70 and/or 91 in HCV-1b core region to HCC[85,86]. This may suggest the presence of IR-dependent pathway as a mechanism of HCV-1b core region-associated hepatocarcinogenesis, and the importance of eradicating such mutant HCV in reducing the development of HCC[36].
IR is recognized as an independent risk factor for the development of HCC worldwide[87-89], and the development of diabetes-related HCC suggests that IR has direct effects on hepatocarcinogenesis, although precise mechanisms for this effect remain unclear. IR causes lipid accumulation, which results in changes in serum adipocytokine levels, including reduction of adiponectin, which has suppressive effects for hepatocarcinogenesis[90] Hepatic lipid accumulation also increases oxidative stress, which may be responsible for the development of HCC[88,89]. Hyperinsulinaemia and increased levels of insulin growth factors have been shown to promote cell proliferation[90,91], and IL-6. Another possibility is that insulin has a mitogenic effect, through activation of a mitogen-activated protein kinase pathway, suggesting that insulin may be directly linked to hepatocarcinogenesis[92]. However, the specific cytokines and mechanisms that promote neoplasia during IR have not yet been fully defined.
Insulin resistance-induced interferon resistance
IR is associated with a poor response to anti-viral treatment in patients with HCV infections, both for initial virological response[93,94] and SVR[76,95]. This negative association has been reported to occur both in patients infected with the genotype 1[76,96] and in those with genotypes 2 and 3[97].
Although the reason for such association is largely unknown, several possibilities have been suggested. In one study, obese HCV patients have approximately an 80% lower chance of achieving SVR compared with non-obese patients[98]. Obese HCV patients with steatosis are thought to have increased lipid droplets in hepatocytes, which can act as a functional barrier for the interaction between antiviral drugs and hepatocytes[8,99]. Alternatively, lipids are important for HCV replication[100],and accumulation of hepatic lipid droplets may increase HCV replication and results in poor responses to anti-viral treatment[100]. Obese people are known to have a poor lymphatic circulation[101], this could result in suboptimal serum levels of pegylated interferons (PEG-IFNs) and a reduced response to antivirals, as certain PEG-IFNs may be preferentially absorbed through blood capillaries or the lymphatic circulation. Obesity may also affect the antiviral response by modulating the IFN signaling pathway, as a recent study showed that obese HCV genotype 1 patients had increased mRNA expression of SOCS-3 compared with normal controls[102] (Figure 2).
However, in a recent large-scale study, IR but not steatosis or fibrosis was the most important predictor for response to PEG-IFN and ribavirin therapy[103]. Moreover, the molecular link between insulin signaling and reduced response to IFN-α has been demonstrated in several studies[104-106]. HCV core protein stimulates the SOCS-3, which is a negative regulator of IFN-α signaling. SOCS-3 upregulation inhibits expression of interferon stimulated genes as 2’,5’-oligoadenylate synthetase and protein kinase receptor (PKR), through inactivation of the JAK-STAT pathway[105].
Downregulation of PPAR-γ and an upregulation of SOCS-7 were also observed upon expression of the HCV genotype 3 core protein[107], and it was suggested that the activation of SOCS family members may be a mechanism common to all major HCV genotypes[108]. However, activation of SOCS family members is not the only mechanism suggested to account for HCV-induced IR, as it has been shown that HCV core protein also suppresses insulin signaling through a proteasomal activator 28 γ-dependent pathway[109]. This is worthy of note, because this activator plays a role also in the development of steatosis and HCC[110].
Insulin resistance-associated extrahepatic manifestations
In patients with extrahepatic manifestations of HCV, fasting insulin levels and homeostasis model assessment (HOMA) for IR are significantly higher than for patients without extrahepatic manifestations[111]. Among various extrahepatic manifestations, IR is associated with oral lichen planus[112], oral squamous cell carcinoma and multiple primary cancers including gastric cancer[113]. Although reasons for this association remain unclear, a high prevalence of precancerous lesions and cancers are seen in patients with T2 DM[114,115], suggesting that IR or hyperinsulinemia may enhance carcinogenic activities.
THERAPEUTIC IMPLICATIONS
The clinical implications of the presence of IR in patients with CHC have become evident in many studies that consistently showed that these cofactors are related to both disease progression and a poorer response to antiviral therapy.
Effect of insulin resistance on sustained virologicresponse
The negative impact of IR on response to antiviral therapy has been demonstrated in several studies[93-97]. Romero-Gómez et al[76], showed marked differences in the rates of SVR in HCV infected patients with and without IR, assessed by HOMA-IR. In this study, 23 of 70 (32.8%) patients with genotype 1 CHC and IR (HOMA-IR > 2) achieved a SVR vs 26 of 43 (60.5%) genotype 1 CHC patients without IR. These findings were independently confirmed[65], and extended to genotypes 2 and 3[103].
In a recent large-scale study in CHC patients, pretreatment HOMA-IR was associated with SVR to combination therapy with (PEG-IFN)/ribavirin, in particular among ‘‘difficult-to-treat” patients (genotype 1b and high baseline viral loads). These findings suggest that pretreatment measurement of HOMA-IR, in combination with tests of HCV genotypes and viral load, may be used as the determinants for selecting regimens in CHC patients (Figure 4)[103].
Figure 4.
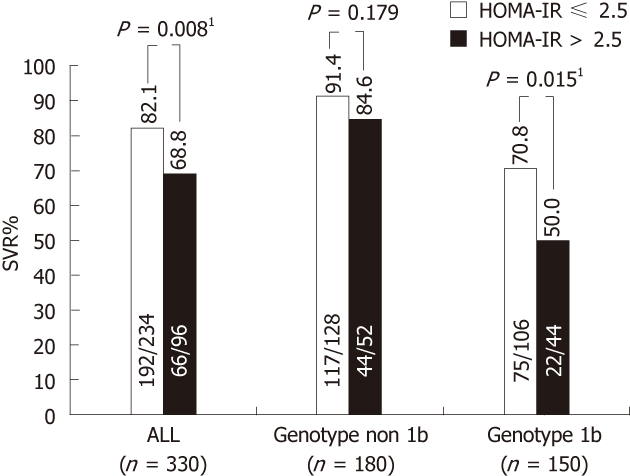
Insulin resistance predicts response to peginterferon-α/ribavirin combination therapy in chronic hepatitis C patients[106]. 1Statistical significance. SVR: Sustained virological response; HOMA-IR: Homeostasis model assessment-insulin resistance.
Effect of improving insulin resistance on sustained virologicresponse
Since IR is considered a factor that can be modified and improved by various interventions, it would be valuable to evaluate by prospective studies whether the improvement of IR before initiation of the combination therapy for CHC can significantly increase the SVR rate. It has been shown that weight reduction may have an impact on both liver histology and biochemistry in patients with CHC[116,117]. Moreover, a strict low calorie diet for three months, aiming to achieve a 10% reduction in body mass index before starting treatment has determined higher rates of response to Peg-IFN plus ribavirin therapy[118].
Amelioration of IR may improve the response to anti-viral treatment. However, the impact of insulin-sensitizing agents, biguanides and thiazolidinediones, on SVR rates has not yet been established.
Recently, metformin, a biguanide agent that decreases the production of glucose in hepatocytes and increases the utilization of glucose within skeletal muscle, has been reported to ameliorate HCV-associated IR, and increase the SVR rate in HCV genotype 1- infected patients with normalization of HOMA-IR at week 24 of therapy[119].
Thiazolidinediones improve insulin sensitivity through activation of the peroxisome proliferator-activated receptor-γ (PPAR-γ) in adipocytes and skeletal muscle[120]. Pioglitazone, a thiazolidinedione agent, has been found to decrease SOCS-3 expression in diabetic patients[121], and has been also reported to ameliorate IR and increase SVR rates in Egyptian patients with HCV genotype 4 infections[122]. In a recent randomized, double-blind, placebo-controlled study, adding pioglitazone 30 mg once daily simultaneously to the standard of care clearly increased the on-treatment virological response, but failed to increase the sustained virological response after the end of treatment[123]. A recent prospective, multicenter study aimed to investigate the efficacy and safety of pioglitazone, 15 mg once daily, added to the Peg-IFN-α2a, 180 μg once weekly/ribavirin and 1200 mg once daily combination therapy in chronic hepatitis C patients who were previously nonresponders to a Peg-IFN-α/ribavirin combination without the insulin sensitizer. All patients had a baseline HOMA > 2 as additional inclusion criterion and diabetic patients were however excluded. Unfortunately, none of the first five patients enrolled into the trial had a satisfactory virological response after 12 wk of retreatment, despite the fact that in at least three of them the IR score improved, and thus the study was prematurely terminated[124].
Because both metformin and thiazolidinedione agents have severe adverse effects, neither is recommended for patients with liver cirrhosis. Biguanides predispose cirrhotic patients to lactic acidosis[125], while thiazolidinediones may cause significant hepatotoxicity[126]. Therefore further validation for safety is required.
It is still unclear whether one should start the antiviral retreatment together with the insulin sensitizer or only once the HOMA-IR score has decreased to a level predicting a sufficient SVR rate[76]. Also, insulin sensitizing therapy might need to be tailored according to HCV genotype, and PPARs agonists should probably be considered only in insulin-resistant patients with HCV genotype 3a[127]. This approach, however, should also take into consideration the known effects of PPAR agonists on serum lipid profile and their potential consequences on the HCV life cycle. HCV circulates bound to lipoproteins in complexes known as lipoviroparticles[128]. As a result, HCV entry into hepatocytes appears to be mediated and facilitated, among others, by the low density lipoprotein (LDL) receptor[129]. In keeping with this, at least two recent studies have suggested that baseline LDL-associated cholesterol levels may affect response to antiviral therapy[130,131]. In fact, higher levels of cholesterol-and ApoB-rich lipoproteins could facilitate viral clearance by impeding HCV interaction with cell surface receptors. Thus, drugs like thiazolidinediones that modify the circulating lipoprotein profile may have unexpected and potentially unwanted effects on the HCV life cycle. Although highly speculative, these hypotheses deserve being appropriately evaluated in clinical trials.
Finally, dipeptidyl peptidase (DPPIV) inhibitor is a new therapeutic agent[132] that has shown its clinical efficacy in T2 DM[133]. It may be suited for ameliorating HCV-associated IR, as activation of DPPIV was considered a factor responsible for HCV-associated IR[134].
Effect of sustained virologicresponse on insulin resistance
Evidence that effective antiviral treatment will result in improved glucose homeostasis in patients with T2DM is at best preliminary. It has been shown that IR is modified by treatment and that the incidence of T2DM in patients achieving SVR is significantly lower than that seen in non-responders[25,26]. Kawaguchi et al[28], reported a significant decrease in HOMA-IR values in patients who attained a SVR, while no changes occurred in HOMA-IR non-responders and relapsers.
Effect of sustained virologicresponse on steatosis
In HCV genotype 3 infections, the severity of steatosis is directly related to the HCV RNA viral load, and steatosis often resolves with the loss of viremia after antiviral treatment[8,135,136], and reappears after the end of therapy in relapsers[7].
On the contrary, in genotype 1 infection, the severity of steatosis is independent of the HCV viral load and antiviral therapy alone does not improve steatosis in these patients[7,137]. Similar data have been obtained for genotype 4 infections, while little data are available for genotype 2[138].
Therapeutic effects of phlebotomy
In order to reduce hepatic iron deposition, both dietary iron restriction and phlebotomy are effective. It has been shown that dietary iron restriction (less than 7 mg/d) decreases serum alanine aminotransferase levels in patients with HCV infection[138], and phlebotomy reduces oxidative stress as well as IR in patients with HCV infection[47,138-140].
A number of studies have shown that phlebotomy to induce iron depletion may lead to regression of fibrosis. In the three trials in which paired liver biopsies before and after treatment with IFN or phlebotomy plus IFN were evaluated, all reported histological improvements in the phlebotomy group[141-144]. Di Bisceglie et al[145] compared treatment with phlebotomy alone to phlebotomy plus IFN, and found that, after 1 year, the phlebotomy-only group demonstrated mild histological improvement that did not reach statistical significance.A recent meta-analysis of six prospective randomized controlled trials found that phlebotomy also improves therapeutic response to interferon in patients with CHC[146].
Additionally, it has been reported that hepatic iron concentration is correlated with the risk of developing HCC[147]. Moreover, a long-term combination treatment with phlebotomy and dietary iron restriction has been found to reduce the risk of development of HCC in patients with HCV infection[143]. If these observations are correct, it is possible that therapeutic phlebotomy in patients with CHC may provide a benefit to patients even in the absence of an SVR to current therapy[147].
Effect of anti-diabetic agents
Anti-diabetic agents such as exogenous insulin and sulfonylurea agents, are effective for decreasing plasma glucose leading to prevention of diabetes mellitus-associated complications including cardiovascular diseases[148-150]. However, the use of exogenous insulin or sulfonylurea agents may worsen hyperinsulinemia. Recently, an association has been reported between exogenous sulphonylurea or insulin treatment and the development of HCC in patients with HCV infection[151,152]. The use of exogenous insulin has also been reported to be associated with the development of colon cancer[153], and other malignancies[154,155]. Although a causal relationship between exogenous insulin and the development of HCC remains controversial[122], the reduction of serum insulin levels is a first line therapeutic strategy for IR[156].
CONCLUSION
IR is one of the pathological features in patients with HCV infection. IR plays a crucial role in the development of various complications and events associated with HCV infection. Mounting evidence indicates that HCV-associated IR may cause hepatic steatosis, hepatic fibrosis, resistance to anti-viral treatment, hepatocarcinogenesis and proliferation of hepatocellular carcinoma; and extrahepatic manifestations.
Thus, HCV-associated IR is a therapeutic target at any stage of HCV infection. However, therapeutic guidelines for preventing the distinctive complications of HCV-associated insulin resistance have not yet been established. Insulin-sensitizing agents are reported to improve sustained virologic response rates, but further validation for safety is required. Little is known regarding the effect of anti-diabetic agents on HCV infection, and a possible association between use of exogenous insulin or a sulfonylurea agent and the development of HCC has recently been reported.
Footnotes
Peer reviewers: Takumi Kawaguchi, MD, PhD, Department of Digestive Disease Information and Research, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan; Vance Matthews, PhD, BS, Cellular and Molecular Metabolism Laboratory, Baker University of Texas Medical Branch, IDI, PO Box 6492, St Kilda Road Central, VIC 8008 Melbourne, Australia
S- Editor Tian L L- Editor Kerr C E- Editor Xiong Lv
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29:74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic Manifestations of Chronic HCV Infection. J Gastrointestin Liver Dis March. 2007;16:65–73. [PubMed] [Google Scholar]
- 3.Bloomgarden ZT. Insulin resistance concepts. Diabetes Care. 2007;30:1320–1326. doi: 10.2337/dc07-zb05. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi G, Marchesini G. Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119–125. doi: 10.1016/0270-9139(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 5.Machado MV, Cortez-Pinto H. Insulin resistance and steatosis in chronic hepatitis C. A. nn Hepatol. 2009;8:S67–S75. [PubMed] [Google Scholar]
- 6.Alberti A, Vario A, Ferrari A, Pistis R. Review article: chronic hepatitis C--natural history and cofactors. Aliment Pharmacol Ther. 2005;22(Suppl 2):74–78. doi: 10.1111/j.1365-2036.2005.02602.x. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 8.Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, Heaton S, Conrad A, Pockros PJ, McHutchison JG. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Knobler H, Schattner A. TNF-{alpha}, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98:1–6. doi: 10.1093/qjmed/hci001. [DOI] [PubMed] [Google Scholar]
- 10.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 13.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66–73. doi: 10.1002/hep.21703. [DOI] [PubMed] [Google Scholar]
- 15.Zylberberg H, Rimaniol AC, Pol S, Masson A, De Groote D, Berthelot P, Bach JF, Bréchot C, Zavala F. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185–191. doi: 10.1016/s0168-8278(99)80060-9. [DOI] [PubMed] [Google Scholar]
- 16.Crespo J, Rivero M, Fábrega E, Cayón A, Amado JA, García-Unzeta MT, Pons-Romero F. Plasma leptin and TNF-alpha levels in chronic hepatitis C patients and their relationship to hepatic fibrosis. Dig Dis Sci. 2002;47:1604–1610. doi: 10.1023/a:1015835606718. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 19.Cheung AT, Wang J, Ree D, Kolls JK, Bryer-Ash M. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes. 2000;49:810–819. doi: 10.2337/diabetes.49.5.810. [DOI] [PubMed] [Google Scholar]
- 20.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 21.Knobler H, Zhornicky T, Sandler A, Haran N, Ashur Y, Schattner A. Tumor necrosis factor––induced insulin resistance may mediate the hepatitis C virus–diabetes association. Am J Gastroenterol. 2003;98:2751–2756. doi: 10.1111/j.1572-0241.2003.08728.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Järvinen H. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR) Hypertension. 1997;30:1144–1149. doi: 10.1161/01.hyp.30.5.1144. [DOI] [PubMed] [Google Scholar]
- 23.Harrison SA. Insulin resistance among patients with chronic hepatitis C: etiology and impact on treatment. Clin Gastroenterol Hepatol. 2008;6:864–876. doi: 10.1016/j.cgh.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075–7080. doi: 10.3748/wjg.v12.i44.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–2466. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
- 27.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 29.Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356–4364. doi: 10.3748/wjg.15.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.el-Zayadi AR, Selim OE, Hamdy H, Dabbous H, Ahdy A, Moniem SA. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141–144. [PubMed] [Google Scholar]
- 32.Harrison SA. Correlation between insulin resistance and hepatitis C viral load. Hepatology. 2006;43:1168; author reply 1168–1169. doi: 10.1002/hep.21125. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Chand N, Comar K, Mirshahi F. Hyperinsulinemia blocks the inhibition of hepatitis C virus (HCV) replication by interferon:a potential mechanism for failure of interferon therapy in subjects with HCV and nonalcoholic liver disease. Hepatology. 2004;40:179A. [Google Scholar]
- 34.Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ, et al. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41–46. doi: 10.1016/j.jhep.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 35.de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107–114. doi: 10.1111/j.1365-2036.2006.02729.x. [DOI] [PubMed] [Google Scholar]
- 36.Akuta1 N, Suzuki1 F, Hirakawa1 M, Kawamura1 Y, Yatsuji1 H, Sezaki1 H, Suzuki1 Y, Hosaka1 T, Kobayashi1 M, Kobayashi M, et al. Amino acid substitutions in the hepatitis C virus core region of genotype 1b are the important predictor of severe insulin resistance in patients without cirrhosis and diabetes mellitus. J Med Virol. 2009;81:1032–1039. doi: 10.1002/jmv.21473. [DOI] [PubMed] [Google Scholar]
- 37.Piperno A, D'Alba R, Fargion S, Roffi L, Sampietro M, Parma S, Arosio V, Faré M, Fiorelli G. Liver iron concentration in chronic viral hepatitis: a study of 98 patients. Eur J Gastroenterol Hepatol. 1995;7:1203–1208. doi: 10.1097/00042737-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Riggio O, Montagnese F, Fiore P, Folino S, Giambartolomei S, Gandin C, Merli M, Quinti I, Violante N, Caroli S, et al. Iron overload in patients with chronic viral hepatitis: how common is it? Am J Gastroenterol. 1997;92:1298–1301. [PubMed] [Google Scholar]
- 39.Bonkovsky HL, Banner BF, Rothman AL. Iron and chronic viral hepatitis. Hepatology. 1997;25:759–768. doi: 10.1002/hep.510250345. [DOI] [PubMed] [Google Scholar]
- 40.Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, et al. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36:288–293. doi: 10.1016/j.hepres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol. 2009;15:538–551. doi: 10.3748/wjg.15.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeo M, Kobayashi Y, Fujita N, Urawa N, Iwasa M, Horiike S, Tanaka H, Kaito M, Adachi Y. Upregulation of transferrin receptor 2 and ferroportin 1 mRNA in the liver of patients with chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:562–569. doi: 10.1111/j.1440-1746.2005.03770.x. [DOI] [PubMed] [Google Scholar]
- 44.Furutani M, Nakashima T, Sumida Y, Hirohama A, Yoh T, Kakisaka Y, Mitsuyoshi H, Senmaru H, Okanoue T. Insulin resistance/beta-cell function and serum ferritin level in non-diabetic patients with hepatitis C virus infection. Liver Int. 2003;23:294–299. doi: 10.1034/j.1600-0676.2003.00841.x. [DOI] [PubMed] [Google Scholar]
- 45.Garrido Serrano A, Guerrero Igea FJ, Lepe Jiménez JA, Palomo Gil S, Grilo Reina A. Hepatitis C virus infection, increased serum ferritin and hyperinsulinemia. Rev Esp Enferm Dig. 2001;93:639–648. [PubMed] [Google Scholar]
- 46.Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403–410. doi: 10.1002/dmrr.972. [DOI] [PubMed] [Google Scholar]
- 47.Mitsuyoshi H, Itoh Y, Sumida Y, Minami M, Yasui K, Nakashima T, Okanoue T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2008;38:348–353. doi: 10.1111/j.1872-034X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 48.Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, Craxì A. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol. 2006;45:271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 49.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 50.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, Siegel E, Creutzfeldt W. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984;26:441–444. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 51.Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharamacol Ther. 2005;22(Suppl 2):61–63. doi: 10.1111/j.1365-2036.2005.02599.x. [DOI] [PubMed] [Google Scholar]
- 52.Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986;261:8708–8711. [PubMed] [Google Scholar]
- 53.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–1547. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mofredj A, Howaizi M, Grasset D, Licht H, Loison S, Devergie B, Demontis R, Cadranel JF. Diabetes mellitus during interferon therapy for chronic viral hepatitis. Dig Dis Sci. 2002;47:1649–1654. doi: 10.1023/a:1015852110353. [DOI] [PubMed] [Google Scholar]
- 56.Schattner A. Lymphokines in autoimmunity--a critical review. Clin Immunol Immunopathol. 1994;70:177–189. doi: 10.1006/clin.1994.1027. [DOI] [PubMed] [Google Scholar]
- 57.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 58.Conjeevaram HS, Kleiner DE, Everhart JE, Hoofnagle JH, Zacks S, Afdhal NH, Wahed AS. Race, insulin resistance and hepatic steatosis in chronic hepatitis C. Hepatology. 2007;45:80–87. doi: 10.1002/hep.21455. [DOI] [PubMed] [Google Scholar]
- 59.Hsieh MH, Lee LP, Hsieh MY, Tsai KB, Huang JF, Hou NJ, Chen SC, Lin ZY, Hsieh MY, Wang LY, et al. Hepatic steatosis and fibrosis in chronic hepatitis C in Taiwan. Jpn J Infect Dis. 2007;60:377–381. [PubMed] [Google Scholar]
- 60.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 61.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–2133. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- 62.Geelen MJ, Harris RA, Beynen AC, McCune SA. Short-term hormonal control of hepatic lipogenesis. Diabetes. 1980;29:1006–1022. doi: 10.2337/diab.29.12.1006. [DOI] [PubMed] [Google Scholar]
- 63.Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009;16:4843–4857. doi: 10.2174/092986709789909620. [DOI] [PubMed] [Google Scholar]
- 64.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 65.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260–269. doi: 10.1136/gut.46.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 67.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 68.Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106–115. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 69.Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–130. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 71.Negro F. Peroxisome proliferator-activated receptors and hepatitis C virus-induced insulin resistance. PPAR Res. 2009;2009:483485. doi: 10.1155/2009/483485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McPherson S, Jonsson JR, Barrie HD, O’Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;49:1046–1054. doi: 10.1016/j.jhep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 73.Henke JI, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, Jünemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 75.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 77.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 79.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 80.Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465–473. [PubMed] [Google Scholar]
- 81.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 82.Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710–715. doi: 10.1023/a:1014715327729. [DOI] [PubMed] [Google Scholar]
- 83.Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 84.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 85.Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, et al. Amino acid substitutions in the hepatitis C virus core region are the important predictor of hepatocarcinogenesis. Hepatology. 2007;46:1357–1364. doi: 10.1002/hep.21836. [DOI] [PubMed] [Google Scholar]
- 86.Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, et al. Substitution of amino acid 70 in the hepatitis C virus core region of genotype 1b is an important predictor of elevated alpha-fetoprotein in patients without hepatocellular carcinoma. J Med Virol. 2008;80:1354–1362. doi: 10.1002/jmv.21202. [DOI] [PubMed] [Google Scholar]
- 87.Donadon V, Balbi M, Perciaccante A, Casarin P. Insulin Resistance and Hyperinsulinemia in patients with chronic Liver Disease and Hepatocellular carcinoma. Clinical Medicine: Endocrinology and Diabetes. 2009;2:25–33. [Google Scholar]
- 88.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 Suppl 2:S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 89.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 90.Yang SQ, Lin HZ, Mandal AK, Huang J, Diehl AM. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 2001;34:694–706. doi: 10.1053/jhep.2001.28054. [DOI] [PubMed] [Google Scholar]
- 91.Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, Liedtke C, Wasmuth HE, Trautwein C, Streetz KL. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology. 2010;51:463–473. doi: 10.1002/hep.23322. [DOI] [PubMed] [Google Scholar]
- 92.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–486. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 93.Huang JF, Yu ML, Dai CY. Pretreatment insulin sensitivity contributes to the treatment response to peginterferon plus ribavirin combination therapy for patients with chronic hepatitis C. Hepatology. 2007;46:349A. [Google Scholar]
- 94.Bortoletto G, Realdon S, Dal Pero F. Insulin resistance (IR) defined by the homeostasis model of assessment insulin resistance (HOMA-IR) index has a direct effect on early viral kinetics during pegylated-interferon therapy for chronic hepatitis C. Hepatology. 2007;46:361A. [Google Scholar]
- 95.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 96.Chu CJ, Lee SD, Hung TH, Lin HC, Hwang SJ, Lee FY, Lu RH, Yu MI, Chang CY, Yang PL, et al. Insulin resistance is a major determinant of sustained virological response in genotype 1 chronic hepatitis C patients receiving peginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther. 2009;29:46–54. doi: 10.1111/j.1365-2036.2008.03823.x. [DOI] [PubMed] [Google Scholar]
- 97.Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 98.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 99.Giannini E, Ceppa P, Testa R. Steatosis in chronic hepatitis C: can weight reduction improve therapeutic efficacy? J Hepatol. 2001;35:432–433. doi: 10.1016/s0168-8278(01)00107-6. [DOI] [PubMed] [Google Scholar]
- 100.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 101.Banerjee D, Williams EV, Ilott J, Monypenny IJ, Webster DJ. Obesity predisposes to increased drainage following axillary node clearance: a prospective audit. Ann R Coll Surg Engl. 2001;83:268–271. [PMC free article] [PubMed] [Google Scholar]
- 102.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 104.Vlotides G, Sörensen AS, Kopp F, Zitzmann K, Cengic N, Brand S, Zachoval R, Auernhammer CJ. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007–1014. doi: 10.1016/j.bbrc.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 105.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 106.Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014–5019. doi: 10.3748/wjg.15.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010;91:1678–1686. doi: 10.1099/vir.0.020644-0. [DOI] [PubMed] [Google Scholar]
- 108.Pascarella S, Clément S, Guilloux K, Conzelmann S, Penin F, Negro F. Effects of hepatitis C virus on suppressor of cytokine signaling mRNA levels: comparison between different genotypes and core protein sequence analysis. J Med Virol. 2011;83:1005–1015. doi: 10.1002/jmv.22072. [DOI] [PubMed] [Google Scholar]
- 109.Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727–1735. doi: 10.1128/JVI.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Miyamura T, Suzuki T, et al. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci USA. 2007;104:1661–1666. doi: 10.1073/pnas.0607312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagao Y, Kawaguchi T, Tanaka K, Kumashiro R, Sata M. Extrahepatic manifestations and insulin resistance in an HCV hyperendemic area. Int J Mol Med. 2005;16:291–296. [PubMed] [Google Scholar]
- 112.Nagao Y, Kawasaki K, Sata M. Insulin resistance and lichen planus in patients with HCV-infectious liver diseases. J Gastroenterol Hepatol. 2008;23:580–585. doi: 10.1111/j.1440-1746.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 113.Nagao Y, Sata M. High incidence of multiple primary carcinomas in HCV-infected patients with oral squamous cell carcinoma. Med Sci Monit. 2009;15:CR453–CR459. [PubMed] [Google Scholar]
- 114.Husseini A, Abu-Rmeileh NM, Mikki N, Ramahi TM, Ghosh HA, Barghuthi N, Khalili M, Bjertness E, Holmboe-Ottesen G, Jervell J. Cardiovascular diseases, diabetes mellitus, and cancer in the occupied Palestinian territory. Lancet. 2009;373:1041–1049. doi: 10.1016/S0140-6736(09)60109-4. [DOI] [PubMed] [Google Scholar]
- 115.Ship JA. Diabetes and oral health: an overview. J Am Dent Assoc. 2003;134 Spec No:4S–10S. doi: 10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 116.Hickman IJ, Clouston AD, Macdonald GA, Purdie DM, Prins JB, Ash S, Jonsson JR, Powell EE. Effect of weight reduction on liver histology and biochemistry in patients with chronic hepatitis C. Gut. 2002;51:89–94. doi: 10.1136/gut.51.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tarantino G, Conca P, Ariello M, Mastrolia M. Does a lower insulin resistance affect antiviral therapy response in patients suffering from HCV related chronic hepatitis? Gut. 2006;55:585. [PMC free article] [PubMed] [Google Scholar]
- 119.Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M, et al. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702–1708. doi: 10.1002/hep.23206. [DOI] [PubMed] [Google Scholar]
- 120.Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 121.Kanatani Y, Usui I, Ishizuka K, Bukhari1 A, Fujisaka1 S, Urakaze1 M, Haruta1 T, Kishimoto2 T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression. Potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes. 2007;56:795–803. doi: 10.2337/db06-1039. [DOI] [PubMed] [Google Scholar]
- 122.Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30:447–454. doi: 10.1111/j.1478-3231.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 123.Conjeevaram H, Burant CF, McKenna B. A randomized, double-blind, placebo-controlled study of PPAR-gamma agonist pioglitazone given in combination with peginterferon and ribavirin in patients with genotype-1 chronic hepatitis C. Hepatology. 2008:48: 384A. [Google Scholar]
- 124.Overbeck K, Genné D, Golay A, Negro F. Pioglitazone in chronic hepatitis C not responding to pegylated interferon-alpha and ribavirin. J Hepatol. 2008;49:295–298. doi: 10.1016/j.jhep.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 125.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 126.Shishido S, Koga H, Harada M, Kumemura H, Hanada S, Taniguchi E, Kumashiro R, Ohira H, Sato Y, Namba M, et al. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003;37:136–147. doi: 10.1053/jhep.2003.50014. [DOI] [PubMed] [Google Scholar]
- 127.Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 128.Thomssen R, Bonk S, Propfe C, Heermann KH, Köchel HG, Uy A. Association of hepatitis C virus in human sera with beta-lipoprotein. Med Microbiol Immunol. 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 129.André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Economou M, Milionis H, Filis S, Baltayiannis G, Christou L, Elisaf M, Tsianos E. Baseline cholesterol is associated with the response to antiviral therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2008;23:586–591. doi: 10.1111/j.1440-1746.2007.04911.x. [DOI] [PubMed] [Google Scholar]
- 131.del Valle J, Mira JA, de los Santos I, López-Cortés LF, Merino D, Rivero A, Girón JA, Ríos-Villegas MJ, González-Serrano M, Collado A, et al. Baseline serum low-density lipoprotein cholesterol levels predict response to hepatitis C virus therapy in HIV/hepatitis C virus coinfected patients. AIDS. 2008;22:923–930. doi: 10.1097/QAD.0b013e3282ff8ad3. [DOI] [PubMed] [Google Scholar]
- 132.Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibitors: a promising new therapeutic approach for the management of type 2 diabetes. Int J Biochem Cell Biol. 2006;38:831–844. doi: 10.1016/j.biocel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 133.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–2317. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 135.Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420–424. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hézode C, Roudot-Thoraval F, Zafrani ES, Dhumeaux D, Pawlotsky JM. Different mechanisms of steatosis in hepatitis C virus genotypes 1 and 3 infections. J Viral Hepat. 2004;11:455–458. doi: 10.1111/j.1365-2893.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 137.Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266–1272. doi: 10.1053/jhep.2002.36370. [DOI] [PubMed] [Google Scholar]
- 138.Iwasa M, Iwata K, Kaito M, Ikoma J, Yamamoto M, Takeo M, Kuroda M, Fujita N, Kobayashi Y, Adachi Y. Efficacy of long-term dietary restriction of total calories, fat, iron, and protein in patients with chronic hepatitis C virus. Nutrition. 2004;20:368–371. doi: 10.1016/j.nut.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 139.Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986–988. [PubMed] [Google Scholar]
- 140.Kaito M, Iwasa M, Kobayashi Y, Fujita N, Tanaka H, Gabazza EC, Adachi Y, Kojima Y, Nakagawa N, Watanabe S. Iron reduction therapy by phlebotomy reduces lipid peroxidation and oxidative stress in patients with chronic hepatitis C. J Gastroenterol. 2006;41:921–922. doi: 10.1007/s00535-006-1871-5. [DOI] [PubMed] [Google Scholar]
- 141.Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K. Hepatic iron accumulation may be associated with insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2007;37:932–940. doi: 10.1111/j.1872-034X.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- 142.Van Thiel DH, Friedlander L, Molloy PJ, Kania RJ, Fagiuoli S, Wright HI, Gasbarrini A, Caraceni P. Retreatment of hepatitis C interferon non-responders with larger doses of interferon with and without phlebotomy. Hepatogastroenterology. 1996;43:1557–1561. [PubMed] [Google Scholar]
- 143.Fontana RJ, Israel J, LeClair P, Banner BF, Tortorelli K, Grace N, Levine RA, Fiarman G, Thiim M, Tavill AS, et al. Iron reduction before and during interferon therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31:730–736. doi: 10.1002/hep.510310325. [DOI] [PubMed] [Google Scholar]
- 144.Fargion S, Fracanzani AL, Rossini A, Borzio M, Riggio O, Belloni G, Bissoli F, Ceriani R, Ballarè M, Massari M, et al. Iron reduction and sustained response to interferon-alpha therapy in patients with chronic hepatitis C: results of an Italian multicenter randomized study. Am J Gastroenterol. 2002;97:1204–1210. doi: 10.1111/j.1572-0241.2002.05705.x. [DOI] [PubMed] [Google Scholar]
- 145.Di Bisceglie AM, Bonkovsky HL, Chopra S, Flamm S, Reddy RK, Grace N, Killenberg P, Hunt C, Tamburro C, Tavill AS, et al. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: a multicenter, prospective, randomized, controlled trial. Hepatology. 2000;32:135–138. doi: 10.1053/jhep.2000.8700. [DOI] [PubMed] [Google Scholar]
- 146.Desai TK, Jamil LH, Balasubramaniam M, Koff R, Bonkovsky HL. Phlebotomy improves therapeutic response to interferon in patients with chronic hepatitis C: a meta-analysis of six prospective randomized controlled trials. Dig Dis Sci. 2008;53:815–822. doi: 10.1007/s10620-007-9945-7. [DOI] [PubMed] [Google Scholar]
- 147.Chapoutot C, Esslimani M, Joomaye Z, Ramos J, Perney P, Laurent C, Fabbro-Peray P, Larrey D, Domergue J, Blanc F. Liver iron excess in patients with hepatocellular carcinoma developed on viral C cirrhosis. Gut. 2000;46:711–714. doi: 10.1136/gut.46.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830–836. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 149.Ajjan RA, Grant PJ. Cardiovascular disease prevention in patients with type 2 diabetes: The role of oral anti-diabetic agents. Diab Vasc Dis Res. 2006;3:147–158. doi: 10.3132/dvdr.2006.023. [DOI] [PubMed] [Google Scholar]
- 150.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 151.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–486. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 153.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 154.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kath R, Schiel R, Müller UA, Höffken K. Malignancies in patients with insulin-treated diabetes mellitus. J Cancer Res Clin Oncol. 2000;126:412–417. doi: 10.1007/pl00021277. [DOI] [PubMed] [Google Scholar]
- 156.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14 Suppl 2:E1–40. doi: 10.1097/01.hjr.0000277984.31558.c4. [DOI] [PubMed] [Google Scholar]


