Abstract
Posttranscriptional gene silencing is mediated by RNA-induced silencing complexes (RISCs) that contain AGO proteins and single-stranded small RNAs. The assembly of plant AGO1-containing RISCs depends on the molecular chaperone HSP90. Here, we demonstrate that cyclophilin 40 (CYP40), protein phosphatase 5 (PP5), and several other proteins with the tetratricopeptide repeat (TPR) domain associates with AGO1 in an HSP90-dependent manner in extracts of evacuolated tobacco protoplasts (BYL). Intriguingly, CYP40, but not the other TPR proteins, could form a complex with small RNA duplex-bound AGO1. Moreover, CYP40 that was synthesized by in-vitro translation using BYL uniquely facilitated binding of small RNA duplexes to AGO1, and as a result, increased the amount of mature RISCs that could cleave target RNAs. CYP40 was not contained in mature RISCs, indicating that the association is transient. Addition of PP5 or cyclophilin-binding drug cyclosporine A prevented the association of endogenous CYP40 with HSP90–AGO1 complex and inhibited RISC assembly. These results suggest that a complex of AGO1, HSP90, CYP40, and a small RNA duplex is a key intermediate of RISC assembly in plants.
Keywords: ARGONAUTE, cyclophilin 40 (CYP40), HSP90, posttranscriptional gene silencing (PTGS), RNA-induced silencing complex (RISC)
Introduction
Posttranscriptional gene silencing (PTGS) is a conserved eukaryotic gene regulatory mechanism that is important for a variety of biological processes such as development, stress responses, and defence. The effector complex of PTGS, called RNA-induced silencing complex (RISC), contains a single-stranded (ss) small RNA such as small interfering RNA (siRNA) and microRNA (miRNA) that is bound to an ARGONAUTE family protein (AGO). RISCs prevent the production of proteins from mRNAs that contain sequences complementary to the ss small RNAs, through cleavage or translational repression (Czech and Hannon, 2010).
siRNAs and miRNAs are produced by Dicer endonucleases as duplexes with 3′ overhangs of 2 nucleotides (nt) (Ghildiyal and Zamore, 2009; Voinnet, 2009). In plants, the 3′-terminal nucleotides of these small RNAs are 2′-O-methylated (Li et al, 2005; Yu et al, 2005; Yang et al, 2006), and this modification prevents uridylation and subsequent degradation of the small RNAs. The small RNA duplexes bind to AGO proteins, and later the unnecessary strands, called siRNA passenger strands and miRNA* strands, are removed through cleavage and unwinding, respectively (Matranga et al, 2005; Miyoshi et al, 2005; Rand et al, 2005; Iki et al, 2010). The other strands of small RNAs, siRNA guide strands and miRNA strands, remain associated with AGO1 to form mature RISCs.
The model plant Arabidopsis thaliana has 10 AGO family proteins, among which AGO1 plays major roles in PTGS and is loaded with both miRNAs and siRNAs (Vaucheret, 2008; Mallory and Vaucheret, 2010). Previously, we have developed a plant cell-free RISC assembly system using extracts of evacuolated tobacco BY-2 protoplasts (BYL) (Iki et al, 2010). In this system, synthetic siRNA and miRNA duplexes bind to AGO1 proteins that have been newly synthesized in BYL by in-vitro translation, then the passenger siRNA strands and the miRNA* strands are removed and RISCs that show target cleavage activity are formed. Using this system, we have demonstrated that the molecular chaperone HSP90 facilitates RISC assembly through a chaperone cycle that involves ATP binding and hydrolysis (Iki et al, 2010). It has also been reported that the HSP70/HSP90 chaperone machinery mediates ATP-dependent RISC loading in animals (Iwasaki et al, 2010; Miyoshi et al, 2010).
In this study, to unravel the detailed mechanisms underlying the HSP90-mediated plant AGO1 RISC assembly, we searched for factors that physically and functionally associate with HSP90-bound AGO1, and found that cyclophilin 40 (CYP40) plays a unique and important role in plant RISC assembly.
Results
Identification of proteins associated with HSP90–AGO1 complexes
RISC assembly is assisted by the molecular chaperones HSP70 and HSP90 (Iki et al, 2010; Iwasaki et al, 2010; Miyoshi et al, 2010). In a cell-free system using BYL, AGO1 binds to ATP-bound HSP90 and becomes competent for accepting small RNA duplexes (Iki et al, 2010). To better understand the mechanisms underlying the HSP90-mediated RISC assembly, we searched for factors that associate with HSP90-bound AGO1. Our previous study showed that the binding of HSP90 to AGO1 is transient and is greatly stabilized by the addition of ATPγS, an ATP analogue that binds to the ATP-binding pocket of HSP90 but is very slowly hydrolyzed (Hessling et al, 2009; Iki et al, 2010). It was also shown that the binding of HSP90 to AGO1 is inhibited by geldanamycin (GA), which competitively binds to the ATP-binding pocket of HSP90 (Roe et al, 1999; Iki et al, 2010).
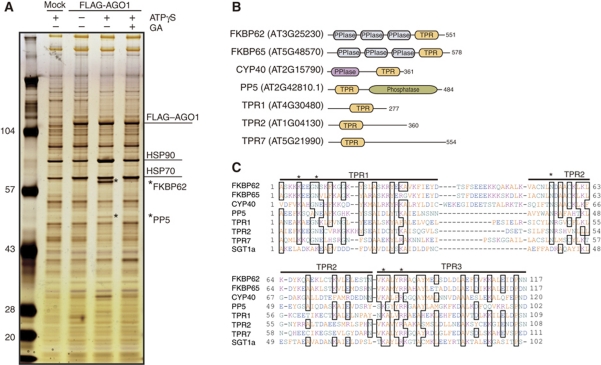
Based on the above facts, we synthesized N-terminally FLAG-tagged AGO1 (FLAG–AGO1) by in-vitro translation in BYL, incubated the reaction mixture in either [−ATPγS −GA], [+ATPγS −GA], or [+ATPγS +GA] conditions, immunopurified FLAG–AGO1 with an anti-FLAG antibody, and searched for proteins that were specifically copurified with FLAG–AGO1 in the [+ATPγS −GA] condition but not in the other conditions. In addition to HSP90, several such proteins were detected (Figure 1A, asterisks), among which mass spectrometry analysis identified a 60–65 kDa protein and a 50–55 kDa protein as FK506-binding protein 62 (FKBP62), and protein phosphatase 5 (PP5), respectively.
Figure 1.
Identification of factors that associate with AGO1. (A) Analysis of factors that are copurified with AGO1. FLAG–AGO1 mRNA-translated BYL was incubated at 25°C for 30 min in the presence of additional 2 mM ATP, 3 mM MgCl2, and 2% DMSO (−ATPγS –GA); 2 mM ATPγS, 3 mM MgCl2, and 2% DMSO (+ATPγS –GA); or 2 mM ATPγS, 3 mM MgCl2, 2% DMSO, and 20 μM GA (+ATPγS +GA). In parallel, mock-translated BYL was incubated in the +ATPγS –GA condition. FLAG–AGO1 was immunopurified with anti-FLAG antibodies, and copurified proteins were analysed by SDS–PAGE followed by silver staining. LC–MS/MS analysis identified two FLAG–AGO1-associated proteins corresponding to bands that were found only in the +ATPγS –GA condition (indicated with asterisks) to be FKBP62 and PP5. (B) Schematic representation of the domain structures of the TPR proteins. (C) Alignment of the amino-acid sequences of the TPR domains from A. thaliana proteins. The amino-acid sequences of the TPR domains of Arabidopsis FKBP62, FKBP65, CYP40, PP5, TPR1, TPR2, TPR7, and SGT1a were aligned using Clustal W (DNA Data Bank of Japan). Asterisks indicate the conserved residues required for interaction with the EEVD motif of HSP90 or HSP70.
FKBP62, FKBP65, CYP40, PP5, and TPR2 associate with AGO1 in an HSP90-dependent manner
FKBP62 is a plant orthologue of animal FKBP51 and FKBP52, which are targets of the immunosuppressive drug FK506 (He et al, 2004), and consists of three N-terminal peptidyl prolyl cis–trans isomerase (PPIase) domains and a C-terminal tetratricopeptide repeat (TPR) domain (Figure 1B). PP5 is a member of the serine/threonine-specific phosphoprotein phosphatases (Farkas et al, 2007), and consists of an N-terminal TPR domain and a C-terminal phosphatase domain (Figure 1B). In animals, FKBP51, FKBP52, and PP5 are known as components of steroid receptor–HSP90 complexes. The TPR domains bind to the C-terminal EEVD motif of HSP90 (Pratt and Toft, 1997, 2003; Scheufler et al, 2000). These facts are consistent with the result that FKBP62 and PP5 were copurified with AGO1 along with HSP90.
The genome of the model plant A. thaliana encodes many other TPR domain-containing proteins (TPR proteins) such as FKBP65, Cyclophilin 40/SQUINT (CYP40/SQN, hereafter CYP40), TPR1, TPR2, and TPR7 (Prasad et al, 2010) (Figure 1B and C). FKBP65 is a homologue of FKBP62. FKBP62 and FKBP65 each interacts with HSP90 and is involved in plant thermotolerance (Blecher et al, 1996; Aviezer-Hagai et al, 2007; Meiri and Breiman, 2009; Meiri et al, 2010). CYP40 is a plant orthologue of animal Cyp40, one of the targets of the immunosuppressive drug cyclosporin A (CsA) (Romano et al, 2004). CYP40 contains a C-terminal TPR domain and an N-terminal PPIase domain. The PPIase domain of CYP40 is structurally distinct from that of FKBPs. Like FKBP51 and FKBP52, animal Cyp40 binds to HSP90 in steroid receptor complexes (Pratt and Toft, 1997, 2003). Notably, A. thaliana cyp40 (sqn) mutants show abnormality in miRNA-related functions (Berardini et al, 2001; Smith et al, 2009). Recently, it was reported that, like animal Cyp40, plant CYP40 interacts with HSP90, and this interaction is essential for the function of CYP40 in planta (Earley and Poethig, 2011). We also focused on TPR1 and TPR2, each of which interacts with HSP90 (Prasad et al, 2010), and TPR7 that has a TPR domain that is homologous to that of FKBP62.
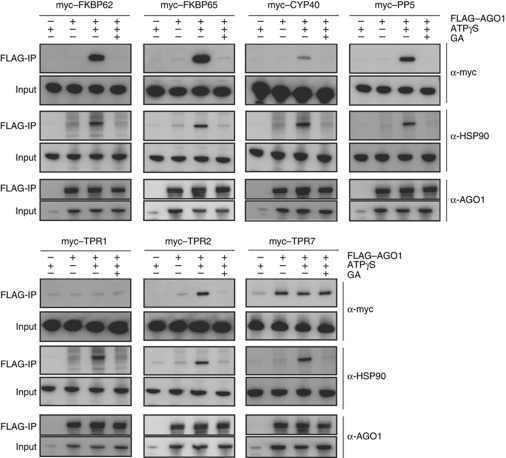
For these TPR proteins, we first examined the association with AGO1 and HSP90. Complementary DNAs for the coding regions of these TPR proteins were cloned from A. thaliana. N-terminally myc-tagged forms of the proteins were synthesized by translating in vitro-synthesized mRNAs using the BYL system (Supplementary Figure S1), mixed with FLAG–AGO1 mRNA-translated BYL. The mixtures were further incubated in [−ATPγS −GA], [+ATPγS −GA], or [+ATPγS +GA] conditions, and FLAG–AGO1 was immunopurified using the anti-FLAG antibody. The myc-tagged FKBP62, FKBP65, CYP40, PP5, and TPR2 proteins were copurified with FLAG–AGO1 in the [+ATPγS −GA] condition and less efficiently in the [−ATPγS −GA] and [+ATPγS +GA] conditions (Figure 2). The results are consistent with those presented in Figure 1A for FKBP62 and PP5, and suggest that FKBP65, CYP40, and TPR2 behave similarly to FKBP62 and PP5. The myc–TPR1 protein was not copurified with FLAG–AGO1 in any conditions, and myc–TPR7 was copurified with FLAG–AGO1 in an HSP90-independent manner (Figure 2).
Figure 2.
Association of TPR proteins and HSP90 with AGO1. FLAG–AGO1 and N-terminally myc-tagged TPR proteins were synthesized in BYL, mixed, and incubated at 25°C for 30 min in the presence of additional 1 mM MgCl2 and 2% DMSO (−ATPγS −GA); 0.75 mM ATPγS, 1 mM MgCl2 and 2% DMSO (+ATPγS −GA); or 0.75 mM ATPγS, 1 mM MgCl2, 2% DMSO, and 20 μM GA (+ATPγS +GA). The myc–TPR proteins synthesized in BYL were also mixed with mock-translated BYL and incubated in the [+ATPγS −GA] condition. The samples before (input) and after (FLAG-IP) immunopurification with anti-FLAG antibodies were analysed by immunoblotting using anti-myc, anti-HSP90, and anti-AGO1 antibodies.
CYP40 facilitates RISC assembly
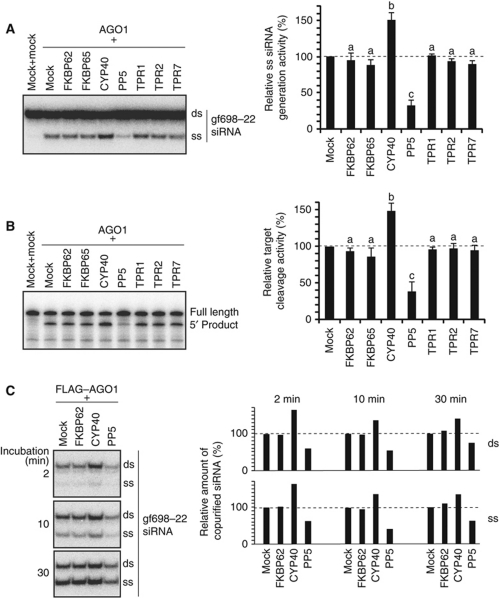
The above results suggest that some of the TPR proteins function as cochaperons of HSP90 and control the efficiency of RISC assembly. To test for this possibility, the effect of TPR protein addition on RISC assembly was examined. Non-tagged TPR and AGO1 proteins were separately synthesized in BYL (Supplementary Figure S2A), mixed, and incubated with a 22-nt siRNA (gf698-22) duplex. RISC assembly was assessed by generation of the ss siRNA guide strand (removal of the passenger strand) and by target cleavage activity. The generation of the ss siRNA guide strand in the CYP40-added mixture was ∼150% of that in the control sample (AGO1 mRNA-translated BYL mixed with mock-translated BYL), and in the PP5-added mixture was ∼30% of that in the control sample (Figure 3A). The effect of the other TPR proteins was not significant (Figure 3A). Similar results were obtained for a 21-nt siRNA (gf698-21) and miR168, although enhancement of the generation of ss gf698-21 siRNA guide strand by the addition of CYP40 was not so drastic (Supplementary Figure S2B). Target cleavage activity measured using the GF-s target RNA (433 nt), which is cleaved by a RISC programmed with the gf698-22 guide strand into a 335-nt 5′ product and a 98-nt 3′ product (Iki et al, 2010), strongly correlated with generation of the ss gf698-22 siRNA guide strand (Figure 3B). These results indicate that RISC assembly is facilitated by the addition of CYP40, while inhibited by the addition of PP5.
Figure 3.
Effects of the addition of TPR proteins on RISC assembly. (A) Effect of the addition of TPR proteins on the generation of ss siRNA. AGO1 and non-tagged TPR proteins were synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex containing the 32P-labelled guide strand at 25°C for 30 min. RNA was extracted from the reaction mixtures and analysed by 15% native PAGE (left panel). Negative control reactions using mock-translated BYL were performed in parallel. The ss siRNA generation activity was calculated by dividing the intensity of ss siRNA band by the sum of intensities of ss and duplex siRNA bands. The graph shows the averages and s.d. of the relative ss siRNA generation values (‘AGO1+mock’=100%) obtained in three independent experiments (right panel). Different letters indicate statistically significant differences (Student’s t-test, P<0.01). (B) Effect of the addition of TPR proteins on target cleavage activity. AGO1 and TPR proteins were synthesized in BYL, mixed, incubated with gf698-22 siRNA duplex at 25°C for 30 min, and further incubated with 32P-labelled GF-s target RNA. RNA was extracted and analysed by denaturing 5% PAGE (left panel). To calculate relative target cleavage activity, the intensity of the full-length GF-s target RNA and the 5′ cleavage product bands was quantified. Because full-length GF-s and the 5′ product contain 104 and 86 cytosine residues, respectively, the intensity of the 5′ product bands was multiplied by 104/86 (∼1.21), and divided by the sum of intensity of full-length GF-s and that of the 5′ product multiplied by 1.21. The graph shows the averages and s.d. of the relative target cleavage activity values (‘AGO1+mock’=100%) obtained in three independent experiments (right panel). Different letters indicate statistically significant differences (Student’s t-test, P<0.01). (C) Effect of the addition of TPR proteins on the amount of ss and ds small RNAs copurified with AGO1. FLAG–AGO1 and non-tagged TPR proteins were synthesized in BYL, mixed, and incubated at 25°C for 2, 10, and 30 min with gf698-22 siRNA duplex containing the 32P-labelled guide strand. FLAG–AGO1 was immunopurified with anti-FLAG antibodies and copurified RNA was analysed by 15% native PAGE. The intensity of copurified ds and ss siRNA bands was measured, and the relative amount (‘AGO1+mock’=100%) was shown in the left graph. Experiments were repeated three times, and the representative data were shown.
To confirm that the Nicotiana tabacum TPR proteins function in similar ways, cDNAs for FKBP62/65, CYP40, and PP5 orthologues were cloned from BY-2 cells. The amino-acid sequence identities/similarities were for FKBP62 (FKBP65) from A. thaliana and N. tabacum (Nt) FKBP62/65, 82% (76%)/96% (94%); for CYP40 from A. thaliana and NtCYP40, 76%/94%; and for PP5 from A. thaliana and NtPP5, 89%/98%. The NtFKBP62/65, NtCYP40, and NtPP5 proteins were synthesized by in-vitro translation using BYL (Supplementary Figure S2C), and the effect of the addition of these proteins on RISC assembly was examined as above. Consistent with the results for the A. thaliana orthologues, the addition of NtFKBP62/65 did not affect RISC assembly. NtCYP40 and NtPP5 facilitated and inhibited RISC assembly to levels comparable to those with A. thaliana CYP40 and PP5, respectively (Supplementary Figure S2D and E).
Our next question was whether the TPR proteins affect binding of small RNA duplexes onto AGO1 or later steps such as removal of passenger strands. To answer this question, TPR proteins and FLAG–AGO1 were separately synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex containing the 32P-labelled guide strand for 2, 10, and 30 min. FLAG–AGO1-associated factors were then immunopurified with the anti-FLAG antibody, and copurified RNA was extracted and analysed. The addition of CYP40 and PP5 increased and decreased, respectively, not only the amount of ss guide strand siRNA but also that of siRNA duplex (Figure 3C). This suggests that the addition of CYP40 and PP5 primarily affects the amount of small RNA duplex-bound AGO1.
CYP40 forms a complex with HSP90, AGO1, and a small RNA duplex
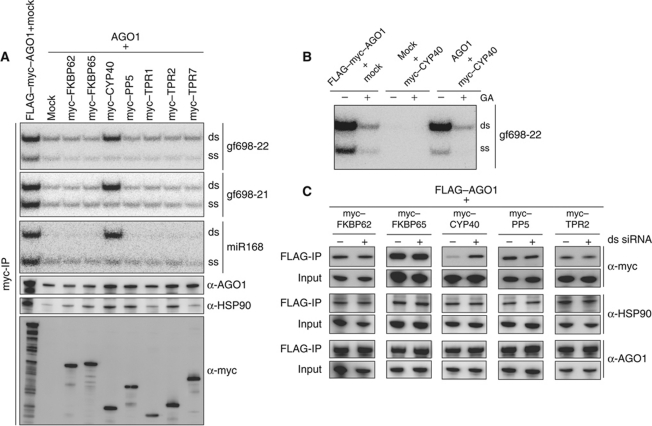
To further investigate the roles of TPR proteins in RISC assembly, we examined whether small RNAs are copurified with the TPR proteins. The myc–TPR proteins and AGO1 were separately synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex containing the 32P-labelled guide strand in the +ATPγS condition. Then, myc–TPR protein-associated factors were purified with an anti-myc antibody, and copurified RNA was extracted and analysed. A significant amount of gf698-22 siRNA duplex as well as HSP90 and AGO1 were copurified with myc–CYP40 (Figure 4A). Similar results were obtained for a 21-nt siRNA (gf698-21) and miR168 (Figure 4A). Copurification of the gf698-22 siRNA duplex with CYP40 was not observed in the absence of newly synthesized AGO1, and it was sensitive to GA (Figure 4B). These results suggest that CYP40 forms a complex with AGO1, HSP90, and small RNA duplexes during RISC assembly. When myc-tagged AGO1 was immunopurified with anti-myc antibody (FLAG–myc–AGO1+mock condition), not only duplexes but also ss siRNA guide strands or miRNA strands were copurified. In contrast, ss small RNAs were not copurified with myc–CYP40 (Figure 4A). This result suggests that CYP40 is not associated with mature RISCs that contain ss small RNAs.
Figure 4.
Association of the TPR proteins with small RNA. (A) Copurification of small RNA duplexes with CYP40. FLAG–AGO1 and N-terminally myc-tagged TPR proteins were synthesized in BYL, mixed, and incubated at 25°C for 30 min with small RNA duplexes (gf698-22 siRNA, gf698-21 siRNA, or miR168/168*) containing 32P-labelled guide siRNA strand or miRNA strand in the presence of additional 0.75 mM ATPγS and 1 mM MgCl2. As controls, FLAG–myc–AGO1 and AGO1 synthesized in BYL were mixed with mock-translated BYL and processed in the same way. Immunopurification was performed using anti-myc antibodies and copurified RNA was analysed by 15% native PAGE. Similar experiment using unlabelled ds gf698-22 siRNAs was also performed, and copurified proteins were analysed by immunoblotting using anti-myc, anti-HSP90, and anti-AGO1 antibodies. (B) Effect of the omission of AGO1 and addition of GA on the association of small RNA with CYP40. FLAG–myc–AGO1 mRNA-translated BYL, mock-translated BYL, and AGO1 mRNA-translated BYL were mixed with mock-translated BYL, myc–CYP40 mRNA-translated BYL, and myc–CYP40 mRNA-translated BYL, respectively, and incubated with gf698-22 siRNA duplexes containing 32P-labelled guide strand, at 25°C for 30 min in the presence of additional 0.75 mM ATPγS, 1 mM MgCl2, and 2% DMSO with (+) or without (−) 20 μM GA. RNAs were immunopurified with anti-myc antibodies and analysed by 15% native PAGE. (C) Effect of the addition of the siRNA duplex on the association of the TPR proteins with AGO1. FLAG–AGO1 and myc-tagged TPR proteins were synthesized in BYL, mixed, and incubated at 25°C for 30 min in the presence of additional 0.75 mM ATPγS, and 1 mM MgCl2, with (+) or without (−) gf698-22 siRNA duplex (50 nM). The reaction mixtures (input) and the proteins immunopurified with anti-FLAG antibodies (FLAG-IP) were analysed by immunoblotting using anti-myc, anti-HSP90, and anti-AGO1 antibodies.
The amount of gf698-22 siRNA duplex and ss guide strand that were copurified with the other myc–TPR proteins was similar to that of the respective RNA in the control sample in which a myc-tagged protein was not added (Figure 4A, compare the ‘mock’ lane to the other lanes). Similar results were obtained for gf698-21 siRNA and miR168 (Figure 4A). AGO1 and HSP90 were copurified with myc–PP5 and myc–TPR2; AGO1, but not HSP90, was copurified with myc–TPR7; and neither AGO1 nor HSP90 was copurified with myc–TPR1 (Figure 4A, compare the signals to those in the respective control samples without myc-tagged protein [‘mock’]). These results are consistent with those shown in Figure 2. However, although the result shown in Figure 2 suggests that FKBP62 and FKBP65 bind to AGO1 in an HSP90-dependent manner, neither AGO1 nor HSP90 was efficiently copurified with myc–FKBP62 or myc–FKBP65, as shown in Figure 4A (similar levels to the negative control). Considering that this result might be due to a problem with the myc-tag fused at the N-termini of FKBP62 and FKBP65, we performed similar experiments using C-terminally myc-tagged FKBP62 and FKBP65 along with CYP40 and PP5. With the C-terminally myc-tagged FKBP62, FKBP65, and PP5, AGO1 and HSP90 were efficiently copurified (Supplementary Figure S3), confirming the result shown in Figure 2. Copurification of small RNAs with these proteins was not observed (similar level to the control sample without myc-tagged protein; Supplementary Figure S3). With regard to CYP40–myc, although the copurification of AGO1 and HSP90 was inefficient, that of the gf698-22 siRNA duplex was still observed (Supplementary Figure S3). These results suggest that each of the FKBP62, FKBP65, PP5 and TPR2 proteins forms a complex with HSP90 and AGO1, but unlike CYP40, not with small RNAs.
We next examined whether addition of siRNA duplexes affects the association of the TPR proteins with AGO1. FLAG–AGO1 and myc-tagged TPR proteins were synthesized in BYL, mixed, and incubated in the +ATPγS condition with or without gf698-22 siRNA duplex. FLAG–AGO1-associated factors were then purified using the anti-FLAG antibody and analysed by immunoblotting. Remarkably, the addition of the siRNA duplex increased the amount of myc–CYP40 copurified with FLAG–AGO1, while it did not increase the amount of the other TPR proteins copurified with FLAG–AGO1 (Figure 4C). The amount of HSP90 was not affected by the addition of the siRNA duplex (Figure 4C). These results suggest that CYP40 associates more strongly with the HSP90–AGO1–small RNA duplex complex than with the HSP90–AGO1 complex free of small RNA duplex.
CsA inhibits RISC assembly
The above results indicate that CYP40 facilitates RISC assembly. In these experiments, addition of 10-fold or larger excess of CYP40 over endogenous CYP40 (unpublished observation) resulted in formation of only 150% of RISC, and it remained unclear whether CYP40 (and equivalent factors) is essential for RISC assembly or whether it simply enhances RISC assembly. If the activity of endogenous CYP40 could be suppressed by some means, useful information on this issue might be obtained.
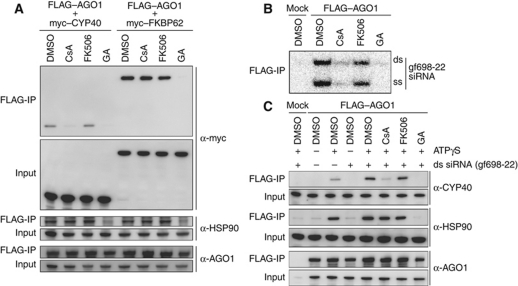
The immunosuppressive drugs CsA and FK506 bind to the PPIase domains of animal cyclophilins and FKBPs, respectively (Pflugl et al, 1993; Theriault et al, 1993; Sinars et al, 2003; Wu et al, 2004), and inhibit their activity (Takahashi et al, 1989). We hypothesized that CsA and FK506 may inhibit the binding of plant CYP40 and FKBP62 to HSP90–AGO1, respectively. To test this possibility, FLAG–AGO1 and myc–CYP40 or myc–FKBP62 were synthesized in BYL, mixed, and incubated in the [+ATPγS], [+ATPγS +CsA], [+ATPγS +FK506], or [+ATPγS +GA] conditions. AGO1-associated factors were then immunopurified using the anti-FLAG antibody. As expected, copurification of HSP90, myc–CYP40, and myc–FKBP62 was significantly inhibited in the [+ATPγS +GA] condition (Figure 5A). In the [+ATPγS +CsA] condition, copurification of myc–CYP40 was significantly inhibited but that of HSP90 and myc–FKBP62 was not affected (Figure 5A). FK506 slightly inhibited the copurification of myc–FKBP62 but had no significant effect on copurification of myc–CYP40 or HSP90 (Figure 5A).
Figure 5.
Involvement of endogenous CYP40 in RISC assembly. (A) Effect of the addition of CsA, FK506, and GA on the association of CYP40 and FKBP62 with AGO1. FLAG–AGO1, myc–CYP40, and myc–FKBP62 were synthesized in BYL. FLAG–AGO1 was mixed with either the myc-tagged TPR proteins, incubated at 25°C for 30 min in the presence of additional 0.75 mM ATPγS, and 1 mM MgCl2, with 2% DMSO alone, 2% DMSO and 20 μM CsA, 2% DMSO and 20 μM FK506, or 2% DMSO and 20 μM GA. FLAG–AGO1 was immunopurified using the anti-FLAG antibody. The samples before (input) and after (FLAG-IP) purification were analysed by immunoblotting using anti-myc, anti-HSP90, and anti-AGO1 antibodies. (B) Effect of the addition of CsA, FK506, and GA on the association of small RNA with AGO1. FLAG–AGO1 mRNA-translated BYL was incubated at 25°C for 30 min with gf698-22 siRNA duplex containing 32P-labelled guide strand in the presence of 2% DMSO alone or 2% DMSO plus 20 μM CsA, 20 μM FK506, or 20 μM GA. FLAG–AGO1 was immunopurified with anti-FLAG antibody and copurified RNA was analysed by 15% native PAGE. (C) Effect of the addition of CsA on the association of endogenous CYP40 with AGO1. FLAG–AGO1 mRNA-translated BYL was incubated at 25°C for 30 min in the presence of additional 0.75 mM ATPγS, 1 mM MgCl2, and other chemicals and gf698-22 siRNA duplex (50 nM) indicated above the panel. FLAG–AGO1 was immunopurified with anti-FLAG antibodies. Samples before (input) and after (FLAG-IP) immunopurification were analysed by immunoblotting using anti-CYP40, anti-HSP90, and anti-AGO1 antibodies.
We then examined whether CsA and FK506 inhibit RISC assembly. AGO1 mRNA-translated BYL was incubated with gf698-22 siRNA duplex containing the 32P-labelled guide strand in the absence or presence of CsA, FK506, or GA. Analysis of RNA extracted from the reaction mixtures showed that generation of the ss siRNA guide strand in the +CsA condition and +FK506 condition was 20 and 70%, respectively, of that in the control (no drug) condition (Supplementary Figure S4A). Consistent results were obtained in the target cleavage assay (Supplementary Figure S4B). In the +GA condition, generation of the ss siRNA guide strand was hardly detectable as expected. Furthermore, the efficiency of copurification of gf698-22 siRNA duplex as well as the ss siRNA guide strand with FLAG–AGO1 was drastically decreased by CsA and GA (Figure 5B). As observed for newly synthesized myc–CYP40 (Figures 4C and 5A), the copurification of endogenous CYP40 with FLAG–AGO1 was facilitated by adding small RNA duplexes in the +ATPγS condition and inhibited by further adding CsA (Figure 5C). These results indicate that RISC assembly, more specifically, small RNA duplex–AGO1 association, in plants depends on CYP40 (and possibly other CsA-sensitive factors) and HSP90.
PP5 interferes with the association of CYP40 with AGO1
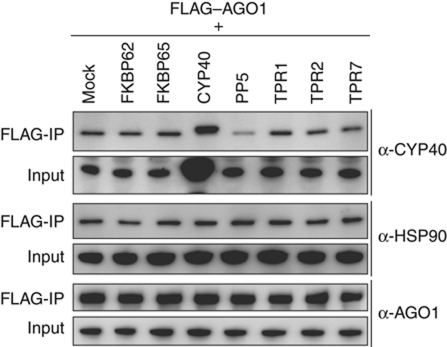
RISC assembly was inhibited by the addition of PP5 (Figure 3). To explore how PP5 inhibits RISC assembly, the effect of its addition on the association of endogenous CYP40 with AGO1 was examined. FLAG–AGO1 was synthesized in BYL, and mixed with each of PP5 and other TPR proteins synthesized in the same way. Then, the mixtures were incubated with small RNA duplexes in the +ATPγS condition, and immunopurified with the anti-FLAG antibody. As expected, the addition of CYP40 increased the amount of CYP40 copurified with FLAG–AGO1 (Figure 6, compare the signal to that for the control without the addition of the TPR proteins). The addition of PP5 significantly decreased the amount of endogenous CYP40 copurified with FLAG–AGO1, whereas the addition of the other TPR proteins did not affect the copurification (Figure 6). This result suggests that the addition of PP5 inhibits RISC assembly by interfering with a step at or before the formation of a complex that contains AGO1, HSP90, and CYP40.
Figure 6.
Effect of the addition of TPR proteins on the association of endogenous CYP40 with AGO1. FLAG–AGO1 and TPR proteins were synthesized in BYL, mixed, and incubated with 50 nM gf698-22 siRNA duplex at 25°C for 30 min, in the presence of additional 0.75 mM ATPγS and 1 mM MgCl2. FLAG–AGO1 was immunopurified with the anti-FLAG antibody. Protein samples before (input) and after (FLAG-IP) immunopurification were analysed by immunoblotting using the anti-CYP40, anti-HSP90, and anti-AGO1 antibodies.
Both PPIase and TPR domains of CYP40 are required for RISC assembly
CYP40 consists of the N-terminal PPIase domain and the C-terminal TPR domain (Figures 1B and 7A). The former exhibits the PPIase activity and is the site of CsA binding, whereas the latter directly binds to the EEVD motif of HSP90 (Hoffmann and Handschumacher, 1995; Owens-Grillo et al, 1995; Ratajczak and Carrello, 1996; Ward et al, 2002). We examined the requirement of these domains in RISC assembly using several mutant forms of A. thaliana CYP40.
Figure 7.
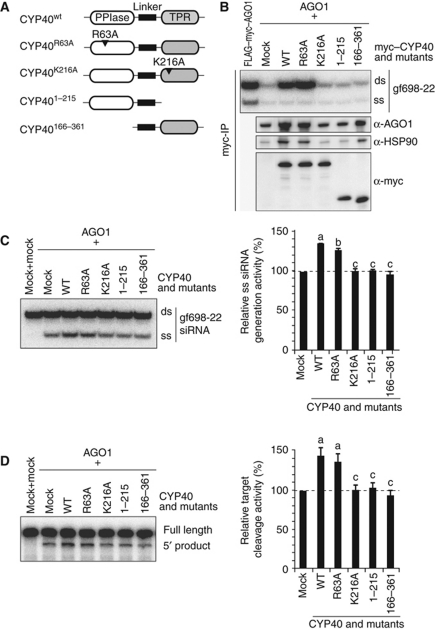
Effect of deletion and point mutations in CYP40 on RISC assembly. (A) CYP40 mutant proteins used in this study. (B) Copurification of HSP90, AGO1, and small RNA with CYP40 mutant proteins. AGO1 and myc–CYP40 mutant proteins were synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex containing 32P-labelled guide strand at 25°C for 30 min in the presence of additional 0.75 mM ATPγS and 1 mM MgCl2. The myc–CYP40 proteins were immunopurified with anti-myc antibodies and copurified RNA was analysed by 15% native PAGE. A similar experiment using unlabelled gf698-22 siRNA duplex was performed in parallel, and copurified proteins were analysed by immunoblotting using anti-myc, anti-HSP90, and anti-AGO1 antibodies. (C) Effect of the CYP40 mutant proteins on generation of ss small RNA. AGO1 and non-tagged CYP40 mutant proteins were synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex containing 32P-labelled guide strand at 25°C for 30 min. After the reaction, RNA was extracted and analysed by 15% native PAGE (left panel). The ss siRNA generation activity was calculated as described in the legend to Figure 3A. The graph shows the averages and s.d. of the relative ss siRNA generation activity values obtained in three independent experiments (right panel). Different letters indicate statistically significant differences (Student’s t-test, P<0.01). (D) Effect of the addition of the CYP40 mutant proteins on target cleavage activity. AGO1 and non-tagged CYP40 mutant proteins were synthesized in BYL, mixed, incubated with gf698-22 siRNA duplex at 25°C for 30 min, and further incubated with internally 32P-labelled GF-s target RNA at 25°C for 10 min. RNA was extracted from the mixtures and analysed by 5% denaturing PAGE (left panel). Relative target cleavage activity was calculated as described in the legend to Figure 3B. The graph shows the averages and s.d. of the relative target cleavage activity values obtained in four independent experiments (right panel). Different letters indicate statistically significant differences (Student’s t-test, P<0.01).
The 55th arginine residue of human CypA is involved in the binding to substrate peptides (Pflugl et al, 1993; Theriault et al, 1993), and the CypAR55A mutant is defective in PPIase activity (Zydowsky et al, 1992). We thus constructed CYP40R63A, in which the arginine residue that corresponds to the 55th residue of human CypA was changed to an alanine residue. We also constructed CYP40K216A, in which the lysine residue in the TPR domain that is important for the binding to HSP90 was replaced by an alanine residue (Figure 1C) (Ward et al, 2002), and two deletion mutants: CYP401−215, which lacks the TPR domain, and CYP40166−361, which lacks the PPIase domain (Figure 7A).
These four mutant proteins were first tested for the ability to associate with HSP90, AGO1, and small RNA duplexes. The N-terminally myc-tagged CYP40 mutant and AGO1 proteins were synthesized in BYL, mixed, and incubated with gf698-22 siRNA duplex. Then, myc–CYP40 derivative-associated factors were immunopurified with the anti-myc antibody. As expected based on the importance of the TPR domain for binding to HSP90, neither myc–CYP401−215 nor myc–CYP40K216A enriched HSP90, AGO1, or siRNAs. In contrast, myc–CYP40166−361 enriched HSP90 and AGO1, but not siRNAs. Like myc–CYP40WT, myc–CYP40R63A enriched HSP90, AGO1, and siRNA duplexes (Figure 7B).
Next, we examined whether these CYP40 mutants could facilitate RISC assembly. Non-tagged CYP40 and the mutant proteins were synthesized in BYL (Supplementary Figure S5A). Generation of the ss siRNA guide strand increased to 140% by the addition of CYP40WT (Figure 7C). The increase in the amount of ss siRNA guide strand was not detectable when CYP401−215, CYP40166−361, or CYP40K216A were added (Figure 7C), while an attenuated increase was detected when CYP40R63A was added (130% of the negative control without CYP40: the ‘AGO1+mock’ lane in Figure 7C). Consistent results were obtained in the target cleavage assay (Figure 7D). Similar results were obtained with the N-terminally myc-tagged CYP40 mutant proteins (Supplementary Figure S5B and C). These results suggest that both the PPIase and TPR domains of CYP40 are essential for facilitation of RISC assembly, but PPIase activity may not be as important in this regard.
Discussion
A previous study established a cell-free plant AGO1 RISC assembly system, and showed that HSP90 and HSP70 play important roles in the reaction (Iki et al, 2010). Here, using the same in-vitro system, we demonstrated that several HSP90 cochaperones associate with HSP90–AGO1 complexes, and that CYP40 facilitates RISC assembly by promoting or stabilizing the binding of small RNA duplexes to AGO1. Taking advantage of the property of ATPγS that stabilizes HSP90–AGO1 association by inhibiting ATP hydrolysis by HSP90, we identified a CYP40–HSP90–AGO1–small RNA duplex-containing complex as an important intermediate that is transiently formed during RISC assembly.
HSP90 chaperone machinery mediates folding of a wide but specific range of proteins called ‘clients’. HSP90 binds to the client proteins with ordered assistance of HSP40, HSP70, and the HSP-organizing protein (Hop) (Pratt and Toft, 2003; Grad and Picard, 2007). Hop consists of two TPR domains that bind to the EEVD motifs of HSP70 and HSP90, respectively (Scheufler et al, 2000). Also, TPR domain-containing cochaperones (TPR cochaperones), such as FKBPs, CYP40, and PP5, bind to the EEVD motif of HSP90 (Carrello et al, 1999), and can replace Hop on HSP90–client protein complexes (Pratt and Toft, 2003; Grad and Picard, 2007).
Mammalian steroid receptors, well-studied clients of HSP90, are incorporated into complexes called ‘heterocomplexes’ that contain an HSP90 dimer and typically one of the TPR cochaperones (Pratt and Toft, 2003). In the heterocomplexes, the cochaperones differently influence the hormone-binding affinity of steroid receptors and their ability to activate downstream signalling (Smith and Toft, 2008). For example, FKBP52 potentiates glucocorticoid signalling by increasing hormone-binding affinity, while FKBP51 antagonizes the FKBP52 function (Riggs et al, 2003). On the other hand, PP5 modulates glucocorticoid receptor phosphorylation and hormone-dependent transcriptional activity (Wang et al, 2007). Cyp40 is also incorporated into steroid receptor heterocomplexes, but its functional importance is not known (Smith and Toft, 2008).
The role of CYP40 in RISC assembly
How does CYP40 promote RISC assembly? The addition of CYP40 increased not only the amount of ss siRNA copurified with FLAG–AGO1, but also that of siRNA duplex in the presence of ATP (Figure 3C). On the other hand, the addition of PP5 and CsA interfered with the association of CYP40 with AGO1 in the presence of ATPγS (Figures 5C and 6) and inhibited the copurification of siRNA duplex as well as ss siRNA guide strand with FLAG–AGO1 in the presence of ATP (Figures 3C and 5B). These results together suggest that CYP40 increases the number of AGO1 molecules that bind small RNA duplexes. While it is possible that CYP40 also has some effects on steps following small RNA duplex binding to AGO1, such as passenger strand removal, we consider that these are not major factors influencing RISC assembly because the effect of CYP40 on the amount of AGO1-bound small RNA duplex was comparable to that on the amount of formed RISCs (Figure 3C).
Here, we showed that, in the presence of ATPγS, myc–CYP40 is associated with FLAG–AGO1 (Figure 2) and small RNA duplexes (Figure 4A) in a GA-sensitive (HSP90-dependent) manner, and that the CYP40–small RNA duplex association depends also on newly synthesized AGO1 (Figure 4B). Therefore, it is likely that a stable CYP40–HSP90–AGO1–small RNA duplex-containing complex is formed in this condition. Furthermore, addition of CYP40 increased the efficiency of small RNA duplex copurification with FLAG–AGO1 (Figure 3C), while addition of small RNA duplexes also increased the efficiency of CYP40 copurification with FLAG–AGO1 (Figures 4C and 5C). The latter observation is reminiscent of the hormone-induced enhancement of FKBP52 copurification with glucocorticoid and mineralocorticoid receptors in animals (Davies et al, 2002; Galigniana et al, 2010). These results suggest that, in the presence of ATPγS, CYP40, and small RNA duplexes cooperatively bind to HSP90-bound AGO1, to form the stable CYP40–HSP90–AGO1–small RNA duplex-containing complex. If, to form this complex, CYP40 and the small RNA duplex join the AGO1–HSP90-containing complex in a specific order, putative intermediate complexes that contain AGO1, HSP90, and either CYP40 or small RNA duplex are likely unstable and show low abundance. We presume that in the presence of ATP (in the absence of ATPγS), the same scenario applies except that the additional process, that is, dissociation of HSP90 and cochaperones from AGO1 triggered by ATP hydrolysis by HSP90, proceeds much faster.
The CYP40 derivative that lacks the PPIase domain copurified HSP90 and AGO1 but did not copurify small RNA duplexes (Figure 7B), and did not facilitate RISC assembly (Figure 7C and D). In addition, CsA that binds to the PPIase domain of CYP40 prevented the copurification of CYP40 with HSP90–AGO1 (Figure 5A and C), and inhibited RISC assembly (Figure 5B). Although the involvement of the PPIase domain in the association of CYP40 with HSP90–AGO1 complex remains controversial, these results suggest that the PPIase domain of CYP40, in addition to the TPR domain, is essential for formation of the CYP40–HSP90–AGO1–small RNA duplex-containing complex. This finding contrasts with the fact that CsA does not affect the formation or function of steroid receptor–HSP90–CYP40 complexes in mammals (Owens-Grillo et al, 1995; Renoir et al, 1995), and that a derivative of the yeast CYP40 orthologue Cpr7 that lacks the PPIase domain complements defects in the cell growth and activation of steroid receptor activity caused by cpr7 deletion (Duina et al, 1996, 1998).
All of our results taken together, we propose a model of plant AGO1 RISC assembly (Figure 8). Aided by HSP70, HSP40, and HOP, small RNA-free AGO1 forms complexes with HSP90ATP(γS) and one of the TPR cochaperones. The complex is in a dynamic equilibrium in which TPR cochaperones interchange and small RNA duplexes dissociate and re-associate. We presume that the AGO1–HSP90ATP(γS)–CYP40–small RNA duplex-containing complex is more stable than, and therefore dominates, the complexes that contain AGO1 and small RNA duplexes with other TPR cochaperones. As a result, virtually only CYP40 allows AGO1 and small RNA duplexes to associate. Finally, triggered by ATP hydrolysis by HSP90, HSP90, CYP40, and passenger strand leave the complex, and a mature RISC that contains AGO1 and the ss siRNA guide strand is formed.
Figure 8.
A possible mechanism of plant RISC assembly. By analogy with steroid receptor activation and other systems, we hypothesize that AGO1 binds to HSP90 with the ordered assistance of HSP40, HSP70, and HOP. On HSP90–AGO1, HOP is displaced by one of the TPR proteins. While CYP40, FKBP62, FKBP65, PP5, and TPR2 each binds to the HSP90–AGO1 complex, only CYP40 can form a complex with AGO1, HSP90, and a small RNA duplex that has a high competence to form mature RISCs. ATP hydrolysis induces dissociation of HSP90 and CYP40 from the complex, followed by removal of the siRNA passenger strand or miRNA*. Finally, a mature RISC that contains AGO1 and siRNA guide strand or miRNA is formed.
Previously, Poethig and colleagues isolated a mutant of A. thaliana, squint (sqn), that has abnormalities in the vegetative phase change, and mapped the responsible mutation in the CYP40 gene (Berardini et al, 2001). They further revealed that the sqn mutant shows reduced miRNA activity and the phenotype is attributable to an increase in the expression of miRNA (miR156)-regulated genes (Smith et al, 2009). In keeping with the genetic findings, the above model predicts that loss of CYP40 functions attenuates HSP90-mediated miRISC assembly, which results in over-accumulation of the target transcripts of the miRISCs.
Inhibition of RISC assembly by PP5
In the presence of ATPγS, not only CYP40 but also other TPR domain-containing proteins (FKBP62, FKBP65, PP5, and TPR2) associated with the HSP90–AGO1 complex (Figure 2). Silver staining of proteins that copurified with FLAG–AGO1 in the presence of ATPγS suggested that FKBP62 is a major cochaperone component of the AGO1–HSP90 complex in BYL (Figure 1A). Because TPR cochaperones compete for binding to the EEVD motif of HSP90 (Barent et al, 1998; Carrello et al, 1999), the population of AGO1–HSP90–cochaperone complexes should exist in a dynamic mixture reflecting the relative abundance and affinity of each TPR cochaperone for AGO1–HSP90. When myc–PP5 and myc–CYP40 were tested for copurification with FLAG–AGO1, myc–PP5 was more efficiently copurified compared with myc–CYP40 regardless of small RNA addition (Figures 2 and 4C, compare the signals in ‘FLAG-IP’ panels with those in ‘input’ panels), suggesting that PP5 has higher affinity for AGO1–HSP90 than CYP40. Therefore, inhibition of the association of CYP40 with AGO1–HSP90 by addition of the PP5 protein (Figure 6) could be ascribed to simple competition between CYP40 and PP5 for binding at the same acceptor site on an HSP90 dimer. Further analysis is required to determine whether PP5 is involved in PTGS in planta.
Materials and methods
Preparation of BYL and in-vitro translation
An evacuolated protoplast extract, BYL, was prepared from cells of the suspension-cultured tobacco cell line BY-2 (Iki et al, 2010). In-vitro translation was performed using BYL as described previously (Ishibashi et al, 2006). AGO1 and TPR proteins were synthesized in BYL by adding mRNAs to concentrations of 0.05 and 0.025 μg/μl, respectively. Preparation of mRNAs is shown in the Supplementary data and Supplementary Table SI. The translation reaction was performed at 25°C for 90 min, and terminated by adding puromycin to a concentration of 2 μM.
Small RNA analysis
AGO1 and TPR proteins were synthesized in BYL, mixed (1:1 v/v) and incubated for 30 min at 25°C in the presence of additional 0.75 mM ATP, 1 mM MgCl2, 20 mM creatine phosphate (CP), and 0.4 mg/ml creatine kinase (CK). Then, the mixture was further incubated with 5 nM small RNA duplexes containing 32P-labelled siRNA guide or miRNA strands. To analyse RNAs, the reaction mixtures were diluted 10-fold with 10 mM Tris–1 mM EDTA (TE, pH 8.0) and extracted with equal volumes of TE-saturated phenol. The resulting aqueous phase that contained RNA was recovered, mixed with an equal volume of native dye solution (1 × TBE (100 mM Tris, 90 mM boric acid, 2 mM EDTA-2Na), 0.2 mg/ml bromophenol blue, 0.2 mg/ml xylene cyanol, 10% (v/v) glycerol), and analysed with native 15% PAGE using 0.5 × TBE as running buffer (200 V, 40 min). The 32P signals were detected and quantified using an image analyser (BAS2500, Fujifilm, Japan).
Target cleavage assay
Target RNA (GF-s) was prepared as described previously, using [α-32P]CTP (0.37 TBq/mmol). AGO1 and TPR proteins were synthesized in BYL, mixed (1:1 v/v) and incubated with 5 nM gf698-22 siRNA duplexes at 25°C for 30 min in the presence of additional 0.75 mM ATP, 1 mM MgCl2, 20 mM CP, and 0.4 mg/ml CK. Then, 5 nM target RNA was added to the mixture and further incubated at 25°C for 10 min, followed by 10-fold dilution with TE and extraction with TE-saturated phenol. The resulting aqueous phase that contained RNA was mixed with 1.6 volume of urea dye mix (12.5 M urea, 0.2 mg/ml bromophenol blue, and 0.2 mg/ml xylene cyanol) and analysed with 7 M urea–5% PAGE using 0.5 × TBE as running buffer (200 V, 35 min).
Immunopurification with anti-FLAG antibodies
Samples to be analysed (50 μl) were mixed with 10 μl (packed volume) of EZview Red anti-FLAG M2 Affinity Gel (Sigma-Aldrich Co.) that had been equilibrated in 100 μl TR buffer (30 mM HEPES (pH 7.6), 80 mM KOAc, 1.8 mM MgCl2, 2 mM DTT, and 1 tablet/50 ml Complete protease inhibitor (Roche, Switzerland)), and incubated on ice for 90 min with occasional swirling. Then, the gel was washed with 100 μl TR buffer three times, mixed with 25 μl TR buffer containing 0.17 mg/ml 3 × FLAG peptide (Sigma-Aldrich Co.), and incubated for 30 min on ice with occasional swirling to elute FLAG-tagged and associated proteins. The copurified proteins were analysed by SDS–PAGE and silver staining or immunoblotting. To analyse small RNA, the purified samples were extracted with equal volumes of TE-saturated phenol without dilution, and the resulting aqueous phase was analysed as described above.
Immunopurification with anti-myc antibodies
Samples to be analysed (20 μl) were incubated on ice for 40 min with 0.3 μl anti-myc antibodies (1 μg/μl, clone 4A6; Millipore) and 4.7 μl TR buffer (total 25 μl), and further incubated on ice for 20 min with 3 μl (packed volume) protein G-conjugated Dynabeads (Invitrogen, USA) that was pre-equilibrated in 15 μl TR buffer. The magnet beads were washed twice with 100 μl TR buffer. To extract RNAs from the beads, 20 μl TE buffer and 20 μl TE-saturated phenol were added. After vortexing and centrifugation (16 000 g, 3 min), the aqueous phase that contained RNA was recovered and analysed as described above.
Immunoblot analysis
Proteins were separated by NuPAGE 4–12% Bis–Tris gel (Invitrogen), and immunoblot analysis was performed using a standard protocol. The anti-N. tabacum HSP90 antibody and the anti-N. tabacum AGO1 antibody were described previously (Iki et al, 2010). Anti-CYP40 antibody was raised in rabbits using synthetic peptides (NH2-CGIGPNTGVPLHYKGNCFHR-COOH, underlined residues correspond to 45th to 63rd amino acids of tomato CYP40 (SGN-U571739; http://solgenomics.net/)) as an antigen (Kojin Bio, Japan), and used at 1:1000 dilution. The anti-HSP90, anti-AGO1, and anti-CYP40 antibodies detected respective proteins as major bands in total BYL proteins on immunoblots. The mouse monoclonal anti-myc antibody (Millipore, USA) was used at 1:2000 dilution. To detect myc-tagged proteins immunopurified with the monoclonal anti-myc antibody (Millipore), we used rabbit polyclonal anti-myc antibody (ABM, Canada) at 1:2000 dilution. The horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG secondary antibodies (GE Healthcare, UK) were used at 1:2000 dilution. The signals were detected with an ECL kit (GE Healthcare) using an image analyser (LAS-3000, Fujifilm).
Supplementary Material
Acknowledgments
We thank our laboratory members for helpful discussion. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan to TM and MI, and by a grant from PRESTO of JST to MY.
Author contributions: MI and TM supervised, and TI performed all the experiments, with contribution of MY. TI and MI wrote the paper. All the authors understood their responsibilities connected to authorship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aviezer-Hagai K, Skovorodnikova J, Galigniana M, Farchi-Pisanty O, Maayan E, Bocovza S, Efrat Y, von Koskull-Doring P, Ohad N, Breiman A (2007) Arabidopsis immunophilins ROF1 (AtFKBP62) and ROF2 (AtFKBP65) exhibit tissue specificity, are heat-stress induced, and bind HSP90. Plant Mol Biol 63: 237–255 [DOI] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF (1998) Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol 12: 342–354 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Bollman K, Sun H, Poethig RS (2001) Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291: 2405–2407 [DOI] [PubMed] [Google Scholar]
- Blecher O, Erel N, Callebaut I, Aviezer K, Breiman A (1996) A novel plant peptidyl-prolyl-cis-trans-isomerase (PPIase): cDNA cloning, structural analysis, enzymatic activity and expression. Plant Mol Biol 32: 493–504 [DOI] [PubMed] [Google Scholar]
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T (1999) The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and hop is located in the dimerization domain of Hsp90. J Biol Chem 274: 2682–2689 [DOI] [PubMed] [Google Scholar]
- Czech B, Hannon GJ (2010) Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet 12: 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER (2002) A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem 277: 4597–4600 [DOI] [PubMed] [Google Scholar]
- Duina AA, Chang HC, Marsh JA, Lindquist S, Gaber RF (1996) A cyclophilin function in Hsp90-dependent signal transduction. Science 274: 1713–1715 [DOI] [PubMed] [Google Scholar]
- Duina AA, Marsh JA, Kurtz RB, Chang HC, Lindquist S, Gaber RF (1998) The peptidyl-prolyl isomerase domain of the CyP-40 cyclophilin homolog Cpr7 is not required to support growth or glucocorticoid receptor activity in Saccharomyces cerevisiae. J Biol Chem 273: 10819–10822 [DOI] [PubMed] [Google Scholar]
- Earley KW, Poethig RS (2011) Binding of SQUINT to Hsp90 is required for the function of this Cyclophilin 40-related protein in Arabidopsis. J Biol Chem in press (doi: 10.1074/jbc.M111.290130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C (2007) Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci 12: 169–176 [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G (2010) The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol 30: 1285–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad I, Picard D (2007) The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 275: 2–12 [DOI] [PubMed] [Google Scholar]
- He Z, Li L, Luan S (2004) Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol 134: 1248–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessling M, Richter K, Buchner J (2009) Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol 16: 287–293 [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Handschumacher RE (1995) Cyclophilin-40: evidence for a dimeric complex with hsp90. Biochem J 307(Part 1): 5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iki T, Yoshikawa M, Nishikiori M, Jaudal MC, Matsumoto-Yokoyama E, Mitsuhara I, Meshi T, Ishikawa M (2010) In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol Cell 39: 282–291 [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Komoda K, Ishikawa M (2006) In vitro translation and replication of tobamovirus RNA in a cell-free extract of evacuolated tobacco BY-2 protoplasts. In Biotechnology in Agriculture and Forestry, Nagata T, Matsuoka K, Inze D (eds) Vol. 58, pp 183–194. Berlin, Germany: Springer [Google Scholar]
- Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, Tomari Y (2010) Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell 39: 292–299 [DOI] [PubMed] [Google Scholar]
- Li J, Yang Z, Yu B, Liu J, Chen X (2005) Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol 15: 1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory A, Vaucheret H (2010) Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22: 3879–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD (2005) Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 123: 607–620 [DOI] [PubMed] [Google Scholar]
- Meiri D, Breiman A (2009) Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J 59: 387–399 [DOI] [PubMed] [Google Scholar]
- Meiri D, Tazat K, Cohen-Peer R, Farchi-Pisanty O, Aviezer-Hagai K, Avni A, Breiman A (2010) Involvement of Arabidopsis ROF2 (FKBP65) in thermotolerance. Plant Mol Biol 72: 191–203 [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC (2005) Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev 19: 2837–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Takeuchi A, Siomi H, Siomi MC (2010) A direct role for Hsp90 in pre-RISC formation in Drosophila. Nat Struct Mol Biol 17: 1024–1026 [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Hoffmann K, Hutchison KA, Yem AW, Deibel MR Jr, Handschumacher RE, Pratt WB (1995) The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J Biol Chem 270: 20479–20484 [DOI] [PubMed] [Google Scholar]
- Pflugl G, Kallen J, Schirmer T, Jansonius JN, Zurini MG, Walkinshaw MD (1993) X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature 361: 91–94 [DOI] [PubMed] [Google Scholar]
- Prasad BD, Goel S, Krishna P (2010) In silico identification of carboxylate clamp type tetratricopeptide repeat proteins in Arabidopsis and rice as putative co-chaperones of Hsp90/Hsp70. PLoS One 5: e12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Toft DO (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18: 306–360 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228: 111–133 [DOI] [PubMed] [Google Scholar]
- Rand TA, Petersen S, Du F, Wang X (2005) Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell 123: 621–629 [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A (1996) Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J Biol Chem 271: 2961–2965 [DOI] [PubMed] [Google Scholar]
- Renoir JM, Mercier-Bodard C, Hoffmann K, Le Bihan S, Ning YM, Sanchez ER, Handschumacher RE, Baulieu EE (1995) Cyclosporin A potentiates the dexamethasone-induced mouse mammary tumor virus-chloramphenicol acetyltransferase activity in LMCAT cells: a possible role for different heat shock protein-binding immunophilins in glucocorticosteroid receptor-mediated gene expression. Proc Natl Acad Sci USA 92: 4977–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF (2003) The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J 22: 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42: 260–266 [DOI] [PubMed] [Google Scholar]
- Romano PG, Horton P, Gray JE (2004) The Arabidopsis cyclophilin gene family. Plant Physiol 134: 1268–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101: 199–210 [DOI] [PubMed] [Google Scholar]
- Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J (2003) Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci USA 100: 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Toft DO (2008) Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol 22: 2229–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Willmann MR, Wu G, Berardini TZ, Moller B, Weijers D, Poethig RS (2009) Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc Natl Acad Sci USA 106: 5424–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Hayano T, Suzuki M (1989) Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337: 473–475 [DOI] [PubMed] [Google Scholar]
- Theriault Y, Logan TM, Meadows R, Yu L, Olejniczak ET, Holzman TF, Simmer RL, Fesik SW (1993) Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature 361: 88–91 [DOI] [PubMed] [Google Scholar]
- Vaucheret H (2008) Plant ARGONAUTES. Trends Plant Sci 13: 350–358 [DOI] [PubMed] [Google Scholar]
- Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687 [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen W, Kono E, Dang T, Garabedian MJ (2007) Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol 21: 625–634 [DOI] [PubMed] [Google Scholar]
- Ward BK, Allan RK, Mok D, Temple SE, Taylor P, Dornan J, Mark PJ, Shaw DJ, Kumar P, Walkinshaw MD, Ratajczak T (2002) A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem 277: 40799–40809 [DOI] [PubMed] [Google Scholar]
- Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, Ye S, Bartlam M, Shen B, Rao Z (2004) 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci USA 101: 8348–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ebright YW, Yu B, Chen X (2006) HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res 34: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307: 932–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zydowsky LD, Etzkorn FA, Chang HY, Ferguson SB, Stolz LA, Ho SI, Walsh CT (1992) Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci 1: 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.