Abstract
In addition to acting as a transcriptional cofactor for p53, ASPP1 has been shown to function in the cytoplasm to regulate the nuclear localization and activity of YAP/TAZ. We show here that the ability of ASPP1 to activate YAP results in the decreased expression of LATS2, which lowers the ability of p53 to induce p21, cell-cycle arrest and senescence. ASPP1 expression peaks in S-phase, and down-regulation of ASPP1 leads to a reduction in DNA synthesis and enhanced senescence in response to drugs that impede DNA replication. These activities of cytoplasmic ASPP1 in opposing p53-mediated p21 expression are in contrast to the role of nuclear ASPP1 in cooperating with p53 to induce the expression of apoptotic target genes, and may help to dampen p53 activity in normal cells.
Keywords: ASPP1, LATS2, p21, p53, YAP
Introduction
The transcription factor p53 plays an important role in preventing tumourigenesis and is activated by most types of oncogenic stress (Brady and Attardi, 2010). p53 is mutated in >50% of human tumours, while the p53 pathway is attenuated in cancer cells that retain wild-type p53 (Vogelstein et al, 2000). p53 can contribute to a number of mechanisms that help to restrain tumour progression, including apoptosis and senescence, and tumour-associated defects in p53 prevent the full activation of these responses (Vousden and Prives, 2009). Interestingly, however, a number of more recently described p53 activities, such as the activation of autophagy or metabolic adaptation, may be beneficial to cancer cell survival (Maddocks and Vousden, 2011).
The ASPP proteins (ASPP1, ASPP2 and iASPP) are important regulators of p53 activity and specifically control the apoptotic function of p53 (Samuels-Lev et al, 2001; Bergamaschi et al, 2003, 2004). iASPP functions to inhibit p53 binding to the regulatory regions of apoptotic target genes via its interaction with p53's proline-rich and DNA-binding domains (Bergamaschi et al, 2006; Ahn et al, 2009). Conversely, nuclear ASPP1 and ASPP2 promote p53 binding to these same apoptotic target genes by competing with iASPP for p53 binding. These data suggest that while iASPP could act as an oncogene by opposing p53's apoptotic activity, ASPP1 and ASPP2 may function as tumour suppressor genes that cooperate with p53. Consistently, increased iASPP expression (Chen et al, 2010) and decreased ASPP2 expression (by promoter methylation) or mutation within ASPP2 has been described in several different tumour types (Sullivan and Lu, 2007; Park et al, 2010; Zhao et al, 2010b). Furthermore, mouse models have also confirmed ASPP2 as a tumour suppressor (Vives et al, 2006; Kampa et al, 2009). However, p53-independent activities of ASPP2 that may contribute to tumour suppression have also been described. Cytoplasmic ASPP2 has been shown to play a role in the maintenance of cell polarity and formation of tight junctions (Cong et al, 2010; Sottocornola et al, 2010). ASPP2 also contributes to the induction of senescence by preventing the nuclear accumulation of Cyclin D1 in response to Ras activation (Wang et al, 2011). Similarly, ASPP1 also shows p53-independent activities. For example, ASPP1-deficient mice show a defect in lymphatic vessel assembly, but this phenotype is not dependent on p53 (Hirashima et al, 2008). In general, the tumour suppressor activity of ASPP1 is less clear than for ASPP2 in both mouse models and human cancer.
Our previous work showed that cytoplasmic ASPP1 activates the closely related transcriptional cofactors YAP and TAZ by inhibiting their phosphorylation, thereby promoting nuclear accumulation (Vigneron et al, 2010). Further studies have shown that ASPP2 can regulate TAZ in a similar way (Liu et al, 2011). YAP and TAZ are important regulators of development; they induce progenitor cells expansion and restrain cell differentiation in tissues such as the liver, skin, intestine and lung (Lian et al, 2010; Zhao et al, 2010a). Their activity is regulated by the Hippo pathway, an important tumour suppressor pathway that controls organ size in mammals (Zhang et al, 2008). YAP and TAZ have also been shown to promote the epithelial–mesenchymal transition in different cellular and orthotopic xenograft models, helping cells to survive stress and increasing migration and invasion (Chan et al, 2008; Lei et al, 2008; Zhao et al, 2008).
The observation that YAP and TAZ can be activated by ASPP1 and ASPP2 suggests that in addition to some tumour suppressor function, ASPP1 and ASPP2 expression may also help to support tumour development under some conditions. While down-regulation of ASPP1/YAP was shown to oppose some of the transformed characteristics of a tumour cell line—such as resistance to stress or ability to invade (Vigneron et al, 2010)—inhibition of ASPP1 function would also be expected to impede p53 functions, which might contribute to these activities. We therefore sought to explore in more detail the relationship between ASPP1 in the regulation of YAP and the functions of p53.
Results
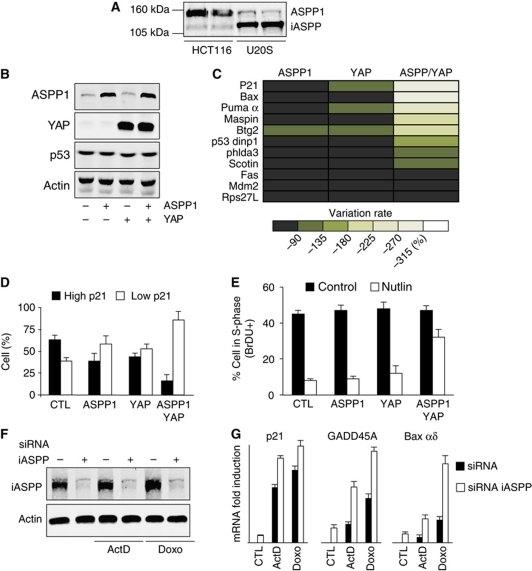
While nuclear ASPP1 can directly modulate p53's transcriptional activity, our recent studies showed that many cells contain cytoplasmic ASPP1, which can function by binding to LATS1 to promote YAP/TAZ nuclear accumulation and transcriptional activity. YAP can act as a transcriptional cofactor with several transcription factors, including the p53-related protein p73 (Matallanas et al, 2007). We therefore sought to examine whether cytoplasmic ASPP1 can influence p53 transcriptional activity indirectly. To investigate this possibility, we examined the effect of ASPP1 and YAP on the regulation of p53-target gene expression. We focussed our studies on the analysis of two cell lines; U2OS cells that express wild-type p53, low levels of endogenous ASPP1 and high levels of the inhibitor protein iASPP (in which we examined the effect of overexpression of ASPP1 and YAP) and HCT116 cells, also wild type for p53 but expressing higher levels of endogenous ASPP1and low levels of iASPP (in which we studied the consequences of ASPP1 and YAP depletion) (Figure 1A).
Figure 1.
ASPP1/YAP overexpression inhibits p53 transcriptional activity. (A) ASPP1 and iASPP expression detected by western blot with specific antibodies in HCT116 and U2OS cells. Duplicate samples from each cell line are shown. (B) U2OS cells were infected with retroviral constructs coding for ASPP1 and YAP as shown and expression of p53, ASPP1 and YAP detected by western blot 2 days later. Actin expression was used as a loading control. (C) U2OS cells were infected as in (B) and mRNA expression of different p53-target genes tested by RT–qPCR using specific primers. The results were normalized against two different standard genes, and the graphs represent the mean of three independent experiments. (D) U2OS cells were transfected with vectors coding for ASPP1 and YAP as indicated and treated for 24 h with 10 μM Nutlin. ASPP1 and p21 expression was detected by immunofluorescence with specific antibodies. In the absence of treatment, p21 staining was extremely low in all cells—following treatment cells staining brightly for p21 were scored as showing high expression while cells staining at background levels were scored as showing low expression. (E) Cells infected as in (B) and treated with Nutlin as in (D) were analysed for BrdU incorporation and DNA content analysis by flow cytometry. (F) U2OS cells were transfected with control siRNA or specific siRNAs directed against iASPP. iASPP expression was detected by western blot 2 days later. Actin was used as a loading control. (G) U2OS cells were transfected as in (F) and mRNA expression of indicated p53-target genes was tested by RT–qPCR using specific primers after treatment with 5 nM of Actinomycin D (ActD) or 200 ng/ml of Doxorubicin (Doxo) for 24 h. The results were normalized against two different standard genes, and the graphs represent the mean of three independent experiments.
Ectopic expression of ASPP1 and/or YAP in U2OS cells did not affect the overall levels of endogenous p53 (Figure 1B). We have shown previously that ASPP1 is predominantly cytoplasmic in both of these cell lines (Vigneron et al, 2010), and consistently the ectopically expressed ASPP1 was also found predominantly in the cytoplasm (Supplementary Figure S1). While overexpression of either ASPP1 or YAP alone did not have a profound effect on p53 activity as measured by activation of several established p53-target genes (Figure 1C), overexpression of both ASPP1 and YAP simultaneously strongly repressed a number of p53-target genes, including p21, Bax and Puma, while other p53 responsive genes (such as Mdm2) were not clearly affected (Figure 1C). The requirement for both ASPP1 and YAP to modulate p53 activity indicates that these two proteins work together, and that expression of either one alone (these cells have very low endogenous levels of ASPP1 and YAP) is not sufficient. To confirm the down-regulation of p21 expression, we examined the effect of ASPP1 and YAP expression on p21 protein levels in cells treated with Nutlin to activate the p53 pathway. While expression of ASPP1 or YAP independently very modestly reduced the number of cells expressing high levels of p21, coexpression of ASPP1 and YAP strongly repressed p21 levels (Figure 1D). These effects on p21 expression were reflected in the cell-cycle progression of these cells, where coexpression of ASPP1 and YAP clearly relieved the cell-cycle arrest normally seen following p53 activation (Figure 1E).
At first glance, these results were in contrast to several previous studies that have shown a role for ASPP1 (and the related protein ASPP2) in enhancing the transcriptional activity of p53, by interfering with the binding of p53 to the inhibitory family member iASPP (Samuels-Lev et al, 2001; Bergamaschi et al, 2003, 2004, 2006). To determine whether this well-established function for the ASPP family is still functional in our cells, we examined the consequences of depletion of iASPP. Following efficient knockdown of iASPP (Figure 1F), we clearly detected the expected enhanced up-regulation of p53-target genes such as GADD45A and Bax, an effect that was most evident after activation of a p53 response by treatment of the cells with Actinomycin D or Doxorubicin (Figure 1G; Supplementary Figure S2). As previously described, the effect of iASPP depletion on p21 and MDM2 expression was more modest. These results show that the established functions of nuclear ASPP proteins are conserved in these cells. We therefore propose that the effects of ASPP1 and YAP in repressing p53-target gene expression reflect functions of cytoplasmic ASPP1, and so an indirect regulation of p53 transcriptional activity.
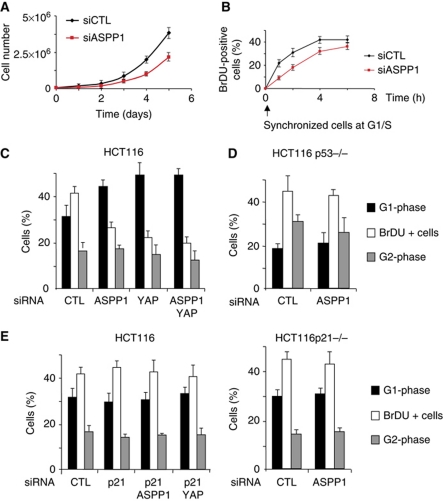
To confirm that endogenous ASPP1 and YAP play a similar role in the regulation of the p53 response, we turned to HCT116 cells, which have also been shown to express predominantly cytoplasmic ASPP1 and relatively high levels of YAP (Vigneron et al, 2010). siRNA-mediated depletion of ASPP1 resulted in a slower growth rate of these cells (Figure 2A) and a delayed ability to reenter cell cycle as measured by BrdU incorporation after release from a G1/S block (Figure 2B). Cell-cycle analysis of cells depleted for either ASPP1 or YAP showed that depletion of each resulted in an accumulation of cells in G1 with a clear loss of cells actively undergoing DNA synthesis (as measured by BrdU incorporation) (Figure 2C). Importantly, depletion of both ASPP1 and YAP simultaneously did not further strengthen this effect, suggesting that ASPP1 and YAP function in the same pathway to mediate the cell-cycle arrest and that both are required for the response. These results are consistent with those seen in U2OS cells (Figure 1), where expression of both ASPP1 and YAP is necessary to affect p21 levels. Similar analyses in HCT116 cells that are deficient for p53 showed that the block in cell-cycle progression in response to ASPP1 or YAP depletion was p53 dependent (Figure 2D), and use of cells depleted of p21 (by either gene deletion or siRNA) demonstrated that this response is also p21 dependent (Figure 2E). Importantly, although ASPP1 and YAP modulated p53-dependent expression of apoptotic genes like PUMA and Bax in U2OS cells (Figure 1C), HCT116 cells express very low levels of PUMA and Bax in the absence of a p53-inducing signal. Furthermore, PUMA and Bax expression was not affected by siRNA-mediated depletion of basal p53 expression—unlike p21 and MDM2 expression, which was significantly lower following knockdown of p53 (Supplementary Figure S3). This lack of modulation of genes like PUMA and Bax in HCT116 cells would explain the strong contribution of p21 to the cell-cycle arrest seen in response to ASPP1 depletion. The results are therefore consistent with a role for ASPP1 and YAP in modulating p53's ability to activate the expression of a subset of target genes, including p21.
Figure 2.
Inhibition of HCT116 cell proliferation after ASPP1/YAP down-regulation is p53 and p21 dependent. (A) Cell proliferation of HCT116 cells transfected with control or ASPP1 siRNA was quantified by Trypan blue exclusion. (B) Cells transfected as in (A) were synchronized at the G1/S-phase transition by double thymidine block, and analysed for BrdU incorporation at the indicated times following release. (C) Cell cycle of HCT116 transfected by the indicated siRNA was analysed by BrdU and PI incorporation, measured by flow cytometry. Results represent the mean of three independent experiments. (D) p53−/− HCT116 cells were transfected and analysed as in (C). Results represent the mean of three independent experiments. (E) HCT116 cells transfected with siRNA targeting p21 (LHS), or p21−/− cells (RHS) were treated and analysed as in (C). Results represent the mean of three independent experiments. Note the control siRNA data in (E) are the same as those shown in (C).
Previous studies have shown that YAP can enhance the activity of p73, the p53 family member (Strano et al, 2005). To determine whether the ASPP1/YAP pathway under study here might also function to control p73-dependent expression of p21, we examined p21 expression in p53 null cells (Supplementary Figure S4). However, in the absence of p53, the expression of p21 was very low and not affected by ASPP1 or YAP depletion. The effects of YAP and ASPP1 therefore appear to be mediated primarily through p53 in these cells.
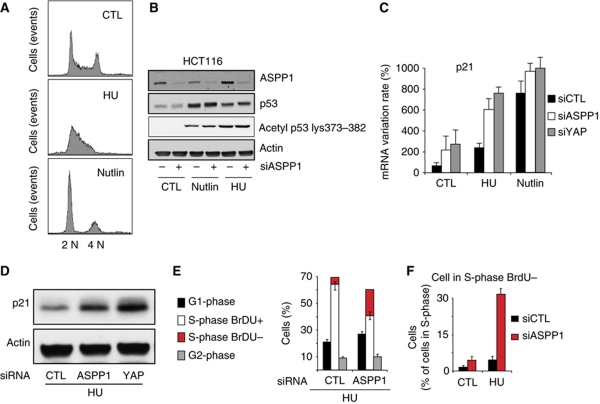
To determine the effect of endogenous ASSP1 on stress-induced p53 activation, we used siRNA to deplete ASPP1 levels in HCT116 cells where p53 was stabilized by using either Nutlin (which stabilizes p53 directly and promotes a p53-dependent G1 arrest) or low levels of hydroxyurea (HU) (which reduces the available intracellular dNTP leading to p53-independent stalled replication and S-phase delay) (Figure 3A). Depletion of ASPP1 had no effect on the stabilization or acetylation of p53 in response to either Nutlin or HU (Figure 3B), suggesting that ASPP1 is not necessary for the activation of p53. Interestingly, the levels of ASPP1 protein appear to be cell-cycle regulated, with increased ASPP1 protein in HU-treated (S-phase arrested) cells, and lower levels of ASPP1 in Nutlin (G1-phase arrested) cells (Figure 3B). Analysis of ASPP1 mRNA levels also revealed an increase in ASPP1 expression in cells treated with HU or Doxorubicin (Supplementary Figure S5), an effect that was much less apparent following Nutlin or Actinomycin D treatment. This effect was observed in both p53 expressing and p53-depleted cells (data not shown), and is consistent with previous work showing that ASPP1 is an E2F1 responsive gene (Fogal et al, 2005; Hershko et al, 2005), since both HU and Doxorubicin treatment lead to elevated E2F1 activity.
Figure 3.
Increase of the p53 response to DNA replication inhibition following ASPP1 and YAP down-regulation. (A) Cell cycle of HCT116 treated for 24 h with 400 μM of HU or 10 μM of Nutlin, analysed by BrdU and PI incorporation and measured by flow cytometry. Result shows a typical histogram for the different conditions. (B) HCT116 cells transfected by control siRNA or siRNA against ASPP1 and treated as in (A) were analysed by western blot with specific antibodies against ASPP1, p53 and p53 acetylated on residues K373/K382. (C) HCT116 cells were treated as in (B) and mRNA expression of p21 was tested by RT–qPCR using specific primers. The results were normalized against two different standard genes, and the graphs represent the mean of three independent experiments. (D) p21 protein expression was measured by western blot. Actin was used as a loading control. (E) Cell cycle of HCT116 transfected with control or ASPP1-directed siRNA and treated 24 h with HU, analysed by BrdU and PI incorporation measured by flow cytometry. Results represent the mean of three independent experiments. (F) Cells were treated as in (E), then cells with an S-phase DNA content were gated and analysed for BrdU incorporation by flow cytometry.
In the light of the increase of ASPP1 levels in S-phase arrested cells, we examined the effect of ASPP1 and YAP modulation in HU cells more closely. Consistent with the results seen following overexpression of ASPP1 and YAP1, depletion of either ASPP1 or YAP in HU-treated cells resulted in a significantly enhanced activation of several p53-target genes, including p21 (Figure 3C; Supplementary Figure S6). Previous work has shown that transcriptional activation by p53 is impaired during S-phase arrest induced by HU treatment, with lower p21 accumulation due to reduced transcriptional elongation (Mattia et al, 2007). We also detected much lower levels of activation of p21 expression in cells treated with HU compared with Nutlin (Figure 3C). However, following depletion of ASPP1 or YAP, a significant increase in p21mRNA and p21 protein expression was detected in the HU-treated cells (Figure 3C and D). By contrast, the high levels of p21 induced by Nutlin treatment were not further enhanced by ASPP1 or YAP knockdown. BrdU incorporation studies showed that depletion of ASPP1 resulted in a further reduction of DNA synthesis in HU-treated HCT116 cells. A concomitant increase of cells with an S-phase DNA content but negative for BrdU suggests that p21 induction in ASPP1-depleted cells can reinforce the block in S-phase progression and DNA synthesis in these HU-treated cells (Figure 3E and F), as previously shown (Rohaly et al, 2005).
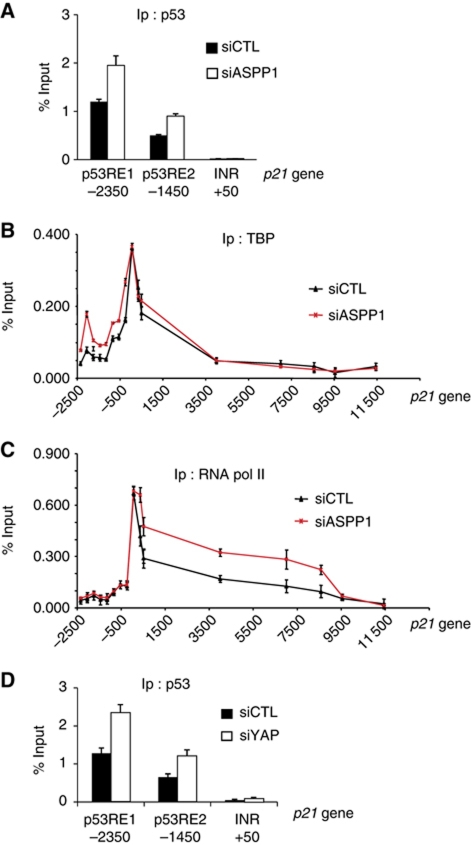
Our observations suggest that depletion of ASPP1 can enhance the ability of p53 to promote p21 expression. ChIP analysis showed an increase of p53 at the p53-binding site of the p21 promoter following ASPP1 knockdown in HU-treated cells (Figure 4A). Interestingly, modulation of ASPP1 levels did not obviously affect TBP recruitment to the p21 promoter in HU-treated cells (Figure 4B). Previous studies have shown that p53-driven expression of p21 can also be regulated by control of mRNA elongation (Mattia et al, 2007). We therefore examined the RNA polymerase II occupancy of sites downstream of the promoter and found an enhancement following ASPP1 knockdown in HU-treated cells (Figure 4C). Interestingly, in the light of the contribution of YAP to the ASPP1 response, we also found an increase in p53 at the p21 promoter in YAP-depleted cells (Figure 4D). Taken together, these results show that ASPP1 and YAP1 depletion results in an elevation of p21 expression that reflects both increased p53 recruitment to the promoter of p21, and enhanced transcriptional elongation—probably reflecting a complex remodelling of the p21 promoter.
Figure 4.
ASPP1/YAP down-regulation in HCT116 cells increases p53 binding and RNA polymerase II elongation on p21. (A) HCT116 cells transfected with siRNA control or directed against ASPP1 and treated 24 h with 400 μM of HU, were fixed and chromatin immunoprecipitated with an antibody against p53. p53-bound DNA was then analysed by qPCR with specific primers amplifying the indicated region of the p21 gene. The results are expressed as a percentage of input and the mean of three experiments. (B, C) Cells were treated as in (A), then TBP or RNA polymerase II catalytic subunit bound DNA was analysed by qPCR with specific primers amplifying the indicated region of the p21 gene. The results are expressed as a percentage of input and the mean of three experiments. (D) Cells transfected with control or YAP-directed siRNA were treated as in (A), then p53-bound DNA analysed by qPCR with specific primers amplifying the indicated region of the p21 gene. The results are expressed as a percentage of input and the mean of three experiments.
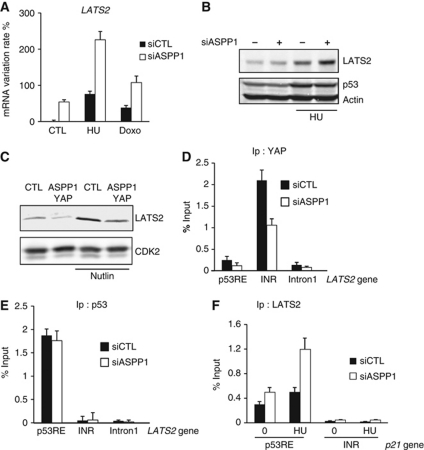
Since ASPP1 is predominantly cytoplasmic in these cells, the ability of ASPP1 to regulate p53 transcriptional activity is likely to be indirect. We showed previously that cytoplasmic ASPP1 can influence transcriptional activity through the regulation of YAP (Vigneron et al, 2010), consistent with the role for YAP in the regulation of p21 described in this study. We therefore sought to determine whether there is a link between the regulation of YAP by ASPP1, and the p53-dependent effects seen here. YAP has not been reported to directly regulate p21 transcription, and we were unable to detect any interaction of YAP with the p21 promoter (data not shown). An alternative candidate mediator of ASPP1 function in this context is LATS2, which has recently been shown to be recruited to the p21 promoter by p53, and help to promote the expression of p21 (Aylon et al, 2010). Most simply, we considered that the previously described interaction of ASPP1 with LATS2 might result in the cytoplasmic sequestration of LATS2. However, we were unable to show significant relocalization of nuclear LATS2 to the cytoplasm following ASPP1 expression (data not shown). During the course of these studies, we noted an overall increase in LATS2 expression at both mRNA and protein level in cells depleted of ASPP1, an effect that is enhanced by HU treatment (Figure 5A and B). Conversely, overexpression of ASPP1 and YAP resulted in a reduction in LATS2 expression in both untreated and Nutlin-treated cells (Figure 5C). In the light of our data showing that YAP mediates the activity of cytoplasmic ASPP1, we explored the role of YAP in regulating LATS2 expression. Interestingly, strong binding of YAP on the LATS2 promoter was detected and as predicted by our previous studies, this binding of YAP was reduced by ASPP1 depletion (Figure 5D). By contrast, the binding of p53 to a p53-binding site of the LATS2 promoter was not affected by ASPP1 depletion (Figure 5E). Interestingly, YAP was not found at the p53-binding site, suggesting that the binding of YAP to the LATS2 promoter is not through p53 or p73, but potentially through another transcription factor such as TEAD. These results indicate that the ability of ASPP1 to promote nuclear translocation of YAP results in YAP-dependent inhibition of LATS2 expression. Since LATS2 has been shown to cooperate with p53 to promote p21 expression, we predicted that this effect of ASPP1 in lowering LATS2 levels would help to limit p21 expression following p53 activation. Consistently, ChIP analysis showed a clear increase in LATS2 at the p21 promoter in response to ASPP1 depletion in HU-treated HCT116 cells (Figure 5F). These results therefore describe an indirect mechanism through which cytoplasmic ASPP1 can control p53-dependent p21 expression.
Figure 5.
ASPP1/YAP down-regulation increased LATS2 expression and binding to p21 gene. (A) HCT116 cells were transfected with control or ASPP1-directed siRNA and treated 24 h with 400 μM of HU, or 150 ng/ml of Doxorubicin (Doxo). mRNA expression of LATS2 was analysed by RT–qPCR using specific primers. The results were normalized against two different standard genes, and the graphs represent the mean of three independent experiments. (B) LATS2 protein expression was measured by western blot in HCT116 cells treated as in (A). Actin was used as a loading control. (C) LATS2 protein expression in U2OS cells overexpressing ASPP1 and YAP, with or without Nutlin treatment. CDK2 was used as a loading control. (D, E) HCT116 cells transfected with siRNA control or directed against ASPP1 and treated 24 h with 400 μM of HU, were fixed and chromatin immunoprecipitated with antibodies against YAP or p53. YAP and p53-bound DNA was then analysed by qPCR with specific primer amplifying indicated region of LATS2 gene. The results are expressed as a percentage of input and the mean of three experiments. (F) HCT116 cells were treated as in (D). LATS2-bound DNA was analysed by qPCR with specific primer amplifying indicated region of p21 gene. The results are expressed as a percentage of input and the mean of three experiments.
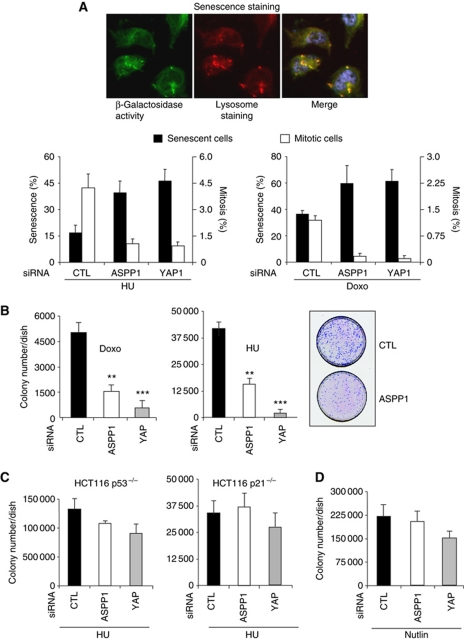
Our results suggest that cytoplasmic ASPP1 can decrease the expression of LATS2 through regulation of YAP, resulting in lower p21 activation in response to p53 induction. Both p21 and LATS2 expression have been linked with the induction of senescence. p21 functions to inhibit cyclin-dependent kinases that normally act to inhibit pRB (Chang et al, 1999; Fang et al, 1999). LATS2 helps to drive the p53-induced expression of p21, and has recently been shown to cooperate with pRB by phosphorylating and activating DYRK1A, which helps to assemble the DREAM complexes (Tschop et al, 2011). The ability of ASPP1 to reduce expression of LATS2 and p21 should therefore impede the induction of senescence. Consistent with this suggestion, we found that depletion of either ASPP1 or YAP increased the number of senescent cells in response to HU, as characterized by an increase of β-galacotosidase activity, lysosomal mass, a reduction of the mitotic index and an inhibition of cell-cycle-related genes. These results were further confirmed in cells treated with Doxorubicin, which also inhibits DNA replication and efficiently promotes senescence (Figure 6A; Supplementary Figure S7). The increase in senescence was also reflected in a reduction in the ability of cells transiently treated with HU or Doxorubicin to recover and form colonies (Figure 6B). Importantly, this effect of ASPP1 or YAP depletion was largely abrogated in p53 or p21 null cells (Figure 6C). Furthermore, the colony forming ability of cells treated with Nutlin, which has been shown to efficiently induce p21 but result in a reversible cell-cycle arrest rather than senescence (Korotchkina et al, 2009), was not strongly affected by ASPP1 or YAP depletion (Figure 6D). Interestingly, although depletion of either ASPP1 or YAP induced similar numbers of senescent cells (Figure 6A), depletion of YAP was somewhat more effective in inhibiting colony formation that the depletion of ASPP1 in both Doxorubicin and HU-treated cells (Figure 6B). A slight (albeit not statistically significant) effect of ASPP1 or YAP manipulation was potentially retained in the absence of p53, possibly reflecting our previous observation that YAP down-regulation can induce Bim-dependent apoptosis independently of p53 (Vigneron et al, 2010).
Figure 6.
ASPP1/YAP down-regulation increased senescence induction by DNA replication inhibitors. (A) HCT116 cells transfected with control, ASPP1 or YAP-directed siRNA were treated for 36 h with 400 μM of HU, or 150 ng/ml of Doxorubicin (Doxo). Cells were washed and incubated for another 48 h in normal medium and senescence analysed by measuring β-galactosidase activity and lysosomal mass using fluorescent markers. The mitotic index was assessed by immunofluorescence with an antibody directed against the phosphorylated residue S10 of Histone H3. More than 10 000 cells were analysed using an Operetta screening system, and the graph represents the percentage of cells with more than three-fold the average β-galactosidase activity of control cells and more than five-fold the average lysosomal mass, or expressing the mitotic phosphorylated Histone H3. (B) Cells treated as in (A) were replated at low density and ability of cells to form new colony was quantified after Giemsa staining and counting of the colonies. **P<0.01 and ***P<0.001. A representative picture of cells treated with HU is shown. (C) p53−/− and p21−/− HCT116 treated and analysed in the same way as in (B). (D) HCT116 cells transfected as in (A) were treated 36 h with 10 μM of Nutlin and analysed as in (B). The differences in (C, D) did not reach significance (P>0.05).
Discussion
Our data further delineate the complex relationship between ASPP1, p53 and the YAP/LATS pathway (Supplementary Figure S8). We showed previously that cytoplasmic ASPP1 promotes the activity of YAP and YAP-dependent transcriptional regulation, leading to growth in low serum, protection from anoikis and an increase in anchorage independent cell growth (Vigneron et al, 2010). In this study, we show that another consequence of the activation of YAP is decreased expression of LATS2, which results in a decreased ability of p53 to drive the expression of p21 and so impedes the induction of cell-cycle arrest and senescence. These data are consistent with the observation that other embryonic transcription factors such as TWIST are able to inhibit the induction of senescence by oncogenes such Ras, in part through the inhibition of p53 (Ansieau et al, 2010). Whether LATS2 down-regulation also participates in these tumourigenic pathways will need further investigation, but recent publications showing that LATS2 is key in the induction of senescence (Tschop et al, 2011) make this an attractive hypothesis.
Previous studies have shown that ASPP1 is phosphorylated by LATS2 in response to H-RasV12 activity, resulting in the nuclear localization of ASPP1 (Aylon et al, 2010). In our cell model, ASPP1 was mainly cytoplasmic despite the constitutive expression of the K-Ras mutant G13D by the HCT116 cells. Whether only certain forms of Ras mutant are able to induce LATS2 activity, or whether other signals need to be present to drive LATS2 activity will need more investigation, but an interesting possibility is that an additional signal such as mitotic stress is required for ASPP1 nuclear translocation in response to LATS2 activity. Mitotic stress has been shown to be a key event in LATS2 activation outside of the centrosome where it resides normally (Aylon et al, 2010) and only polyploid cells appear to be targeted by the LATS2–ASPP1–p53 pathway during oncogenic stress induced by H-RasV12 activity (Aylon et al, 2010). It seems likely that tumour cells expressing activated Ras, such as those used in the present study, will have developed mechanisms to circumvent the activation of this pathway, explaining why the engagement of nuclear ASPP1 and p53 is not longer observed. Such a mechanism that results in the selection for cells that show reduced LATS2 expression and so escape the oncogenic H-Ras-induced checkpoint has been described previously (Aylon et al, 2009). Finally, YAP/TAZ expression itself could inhibit LATS2 activity towards ASPP1 through a number of mechanisms; YAP/TAZ may sequester LATS2 so preventing the nuclear accumulation of ASPP1 or, as we have shown in this study, YAP can directly inhibit the transcription of LATS2 by binding to the LATS2 promoter.
Finally, it is important to mention the ability of YAP1 to drive p73-dependent apoptosis in some situations, particularly in the presence of a DNA strand crosslinking drug such as cisplatin (Strano et al, 2005). Although we failed to see a clear contribution of p73 in our cells, in this context the increase of nuclear YAP/TAZ driven by cytoplasmic ASPP1 is likely to be pro-apoptotic. Signals that shift YAP activity towards p73 will probably modify the effect of YAP on the LATS2 promoter and may favour increased LATS2 expression and so nuclear translocation of ASPP1. In this context, a coordinated activation of LATS2–ASPP1–p53 and YAP–p73 could strongly induce apoptosis.
Taken together, the results point to a complex network of mutual regulation of YAP/TAZ, LATS2 and ASPP with p53 and p73 that will result in different and potentially opposing cell fates depending on the balance and control of different components within the network. Ultimately, these responses may contribute to either the suppression or enhancement of tumour development—concepts that may complicate the targeting of these pathways for tumour therapy.
Materials and methods
Plasmids
Plasmids and retroviral constructs expressing human ASPP1 and human YAP have been described previously (Vigneron et al, 2010).
Cell culture
All cell lines were cultured in DMEM 10% FCS in a 37°C incubator at 5% CO2. Actinomycin D, Nutlin, Doxorubicin, thymidine and HU (all from Sigma) were used at the indicated concentrations.
siRNA
siRNA oligonucleotides were transfected using Hiperfect (Qiagen). The siRNAs targeting ASPP1 and YAP has been described previously (Vigneron et al, 2010). The siRNA against iASPP and p21 were purchased as a pool of four siRNA from Dharmacon.
Antibodies and immunoblotting
Cell extracts were prepared in either NP-40 buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% NP-40) or RIPA buffer (50 mM Tris–HCl, pH 8.0, 120 mM NaCl, 1% NP-40, 1% SDS 0.5% DOC, protease inhibitor cocktail (Roche)). Proteins were resolved by SDS–PAGE and transferred to nitrocellulose membrane (Millipore). Antibodies were purchased from Sigma (ASPP1 for immunofluorescence), Abcam (ASPP1 for western blot ab71163), Santa Cruz (p21 goat C-19, p53 DO1, YAP 63.7), Millipore (Actin C4) and Novus biological (Lats2 NEB200-199). Immunoblots were quantitated using ImageJ software or the Licor Odyssey system.
mRNA extraction and qRT–PCR
Analysis of mRNA expression was carried out using qRT–PCR, as previously described (Vigneron et al, 2010). Standard genes used for the normalization were β2 microglobulin and the ribosomal subunit RPLP0. Primer sequences used are available upon request.
Chromatin immunoprecipitation
1 × 106 cells were fixed with 1% formaldehyde and 1.5 mM EGS (Sigma) for 10 min before adding glycine at 250 mM final concentration to stop the reaction. After washing with PBS, cells were resuspended in RIPA buffer supplemented with 1% SDS and sonicated to obtain cell lysate containing nucleosomal fragments of DNA. Lysates were diluted with RIPA buffer to obtain a final concentration of 0.25% SDS, then incubated with the desired antibody (2 μg) and 20 μl of magnetic beads (Dynabeads Invitrogen) overnight. Beads were washed five times with low salt buffer, high salt buffer, LiCl buffer and twice in TE buffer. DNA was eluted from the beads in 200 μl of elution buffer (1% SDS, 0.1 M NaHCO3) supplemented with 250 μg/ml of proteinase K for 1 h at 45°C. In all, 8 μl 5 M NaCL was added, followed by overnight incubation at 67°C to reverse the crosslinking. DNA was purified (PCR purification kit (Qiagen)) in 150 μl of EB buffer (Qiagen) and used for quantitative PCR with specific primer recognizing indicated region (primer sequences available upon request). Results are expressed as a percentage of input (each PCR was repeated with 0.5% of DNA input treated as the samples) and represent the mean of three experiments.
Cell-cycle and apoptosis analysis
For cell-cycle analysis and S-phase quantification, cells were pulsed with 20 mM BrdU for 90 min, harvested in PBS/EDTA (2.5 mM EDTA) and fixed in cold methanol. Cells were then treated for 20 min with 2 M HCl, washed two times in PBS containing 0.5% BSA and 0.1% Tween20, then incubated in the same buffer with an antibody against BrdU (Santa Cruz: sc-51514) and 1 mg/ml of RNAaseA for 30 min. Cells were washed twice, incubated 20 min with a FITC-coupled secondary antibody and 1 mg/ml of RNAaseA, then washed once. Cells were then analysed by flow cytometry (FACScan, Becton Dickinson) for BrdU incorporation and DNA content using propidium iodide (PI) at 50 mg/ml in PBS 0.1% Tween20. Cells positive for BrdU with a DNA content between 2 N and 4 N were identified as cell actively replicating their DNA. Results are expressed of a percentage of cells in the whole population.
For apoptosis quantification, cells were harvested, fixed in methanol, treated 1 h with 1 mg/ml of RNAaseA (Sigma) and analysed by flow cytometry (FACScan, Becton Dickinson) for DNA content using PI staining at 50 mg/ml in PBS 0.1% Tween20. Cells with a sub-G1 DNA content were identified as apoptotic. Results are expressed of a percentage of apoptotic cells in the whole population.
Immunofluorescence, mitotic index, senescence quantification
Cells were plated in 12-well borosilicate glass plates at low confluence (20%). For ASPP1 immunofluorescence and mitotic index, cells were fixed with ice-cold 4% paraformaldehyde in PBS for 10 min at room temperature, then permeabilized in PBS containing 0.2% Triton X-100 for 5 min. The cells were washed three times with the blocking solution (PBS, 0.5% BSA, 0.1% Tween) and incubated for 30 min at room temperature with ASPP1 or phosphoS10 Histone H3 antibody at 2 μg/ml in blocking solution. After three washes in PBS, the cells were incubated for 30 min at room temperature with a donkey anti-mouse Alexa488-conjugated antibody (Molecular Probes) in blocking solution containing 1 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) (Sigma). The cells were washed three times with PBS and fluorescence was monitored by the high-content screening Operetta system (Perkin-Elmer). At least 10 000 cells were evaluated for the determination of the mitotic index. For β-galactosidase activity and lysosomal mass assessment, living cells were incubated 45 min with C12FDG (Invitrogen) compounds and lysosomal tracker (Invitrogen) according respectively to previously described protocol (Kurz et al, 2000) and the manufacturer's recommendation. Cells were washed and fluorescence immediately analysed by the high-content screening Operetta system (Perkin-Elmer). At least 10 000 cells were evaluated and the cells expressing more than four-fold the average β-galactosidase fluorescence intensity of control cells and more than five-fold the average lysosomal mass fluorescence intensity of control cells were counted as senescent.
Colony forming assay
250 000 cells were treated for 36 h with the indicated drugs, then replated at low density in a 150-mm dish. Cells were allowed to grow for 1 week and colonies were counted in 20 different microscopic fields. The number of colonies was then extrapolated to the area of the whole dish.
Statistical analysis
Error bars represent the standard error of the mean between the number of independent experiments detailed in the individual figure legends.
Supplementary Material
Acknowledgments
We acknowledge funding from Cancer Research UK.
Author contributions: AMV and KHV conceived, designed and interpreted the experiments. AMV performed the experiments.
The authors declare that they have no conflict of interest.
01/18/2012
This article has had the additional paragraph 'Statistical analysis' added at the end of the Materials and methods section since Advance Online Publication.
References
- Ahn J, Byeon IJ, Byeon CH, Gronenborn AM (2009) Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem 284: 13812–13822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S, Morel AP, Hinkal G, Bastid J, Puisieux A (2010) TWISTing an embryonic transcription factor into an oncoprotein. Oncogene 29: 3173–3184 [DOI] [PubMed] [Google Scholar]
- Aylon Y, Ofir-Rosenfeld Y, Yabuta N, Lapi E, Nojima H, Lu X, Oren M (2010) The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes Dev 24: 2420–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Yabuta N, Besserglick H, Buganim Y, Rotter V, Nojima H, Oren M (2009) Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation. Oncogene 28: 4469–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X (2004) ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol 24: 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, Del Sal G, Syed N, Smith P, Gasco M, Crook T, Lu X (2006) iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 38: 1133–1141 [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels-Lev Y, O’Neil NJ, Trigiante G, Crook T, Hseih JK, O’Conner DJ, Zhong S, Compargue I, Tomlinson ML, Kuwabara PE, Lu X (2003) iASPP oncoprotein is a key inhibitor of p53 conserved from worms to humans. Nat Genet 33: 162–167 [DOI] [PubMed] [Google Scholar]
- Brady CA, Attardi LD (2010) p53 at a glance. J Cell Sci 123: 2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W (2008) A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res 68: 2592–2598 [DOI] [PubMed] [Google Scholar]
- Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB (1999) Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18: 4808–4818 [DOI] [PubMed] [Google Scholar]
- Chen J, Xie F, Zhang L, Jiang WG (2010) iASPP is over-expressed in human non-small cell lung cancer and regulates the proliferation of lung cancer cells through a p53 associated pathway. BMC Cancer 10: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, Ohno S (2010) ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol 20: 1408–1414 [DOI] [PubMed] [Google Scholar]
- Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 18: 2789–2797 [DOI] [PubMed] [Google Scholar]
- Fogal V, Kartasheva NN, Trigiante G, Llanos S, Yap D, Vousden KH, Lu X (2005) ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ 12: 369–376 [DOI] [PubMed] [Google Scholar]
- Hershko T, Chaussepied M, Oren M, Ginsberg D (2005) Novel link between E2F and p53: proapoptotic cofactors of p53 are trasncriptionally upregulated by E2F. Cell Death Differ 12: 377–383 [DOI] [PubMed] [Google Scholar]
- Hirashima M, Sano K, Morisada T, Murakami K, Rossant J, Suda T (2008) Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Dev Biol 316: 149–159 [DOI] [PubMed] [Google Scholar]
- Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K, Padiernos E, Boonmark N, Zhu Z, Fan AC, Bailey AS, Fleming WH, Corless C, Felsher DW, Naumovski L, Lopez CD (2009) Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds. Proc Natl Acad Sci USA 106: 4390–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotchkina LG, Demidenko ZN, Gudkov AV, Blagosklonny MV (2009) Cellular quiescence caused by the Mdm2 inhibitor nutlin-3A. Cell Cycle (Georgetown, Tex) 8: 3777–3781 [DOI] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113 (Part 20): 3613–3622 [DOI] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28: 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL (2010) The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24: 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Lv X, Li T, Xu Y, Zhou X, Zhao S, Xiong Y, Lei QY, Guan KL (2011) PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J Biol Chem 286: 5558–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Vousden KH (2011) Metabolic regulation by p53. J Mol Med (Berl) 89: 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O’Neill E (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27: 962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia M, Gottifredi V, McKinney K, Prives C (2007) p53-Dependent p21 mRNA elongation is impaired when DNA replication is stalled. Mol Cell Biol 27: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, An CH, Kim SS, Yoo NJ, Lee SH (2010) Mutational analysis of ASPP1 and ASPP2 genes, a p53-related gene, in gastric and cololorectal cancers with microsatellite instability. Gut Liver 4: 292–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaly G, Chemnitz J, Dehde S, Nunez AM, Heukeshoven J, Deppert W, Dornreiter I (2005) A novel human p53 isoform is an essential element of the ATR-intra-S phase checkpoint. Cell 122: 21–32 [DOI] [PubMed] [Google Scholar]
- Samuels-Lev Y, O’Conner DJ, Bergamaschi D, Trigiante G, Hsieh J-K, Zhong S, Campargue I, Naumovski L, Crook T, Lu X (2001) ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8: 781–794 [DOI] [PubMed] [Google Scholar]
- Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, Ratnayaka I, Shipman M, Cheung A, Gaston-Massuet C, Ferretti P, Molnar Z, Lu X (2010) ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell 19: 126–137 [DOI] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, Del Sal G, Levrero M, Blandino G (2005) The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell 18: 447–459 [DOI] [PubMed] [Google Scholar]
- Sullivan A, Lu X (2007) ASPP: a new family of oncogenes and tumour suppressor genes. Br J Cancer 96: 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop K, Conery AR, Litovchick L, Decaprio JA, Settleman J, Harlow E, Dyson N (2011) A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev 25: 814–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron AM, Ludwig RL, Vousden KH (2010) Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev 24: 2430–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives V, Su J, Zhong S, Ratnayaka I, Slee E, Goldin R, Lu X (2006) ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev 20: 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408: 307–310 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137: 413–431 [DOI] [PubMed] [Google Scholar]
- Wang XD, Lapi E, Sullivan A, Ratnayaka I, Goldin R, Hay R, Lu X (2011) SUMO-modified nuclear cyclin D1 bypasses Ras-induced senescence. Cell Death Differ 18: 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Smolen GA, Haber DA (2008) Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res 68: 2789–2794 [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL (2010a) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, Tong X, Lu X, Wu M, Guo Y (2010b) Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology 51: 142–153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.