Abstract
Wnts are required for cardiogenesis but the role of specific Wnts in cardiac repair remains unknown. In this report, we show that a dynamic Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. Acute ischaemic cardiac injury upregulates Wnt1 that is initially expressed in the epicardium and subsequently by cardiac fibroblasts in the region of injury. Following cardiac injury, the epicardium is activated organ-wide in a Wnt-dependent manner, expands, undergoes epithelial–mesenchymal transition (EMT) to generate cardiac fibroblasts, which localize in the subepicardial space. The injured regions in the heart are Wnt responsive as well and Wnt1 induces cardiac fibroblasts to proliferate and express pro-fibrotic genes. Disruption of downstream Wnt signalling in epicardial cells decreases epicardial expansion, EMT and leads to impaired cardiac function and ventricular dilatation after cardiac injury. Furthermore, disruption of Wnt/βcatenin signalling in cardiac fibroblasts impairs wound healing and decreases cardiac performance as well. These findings reveal that a pro-fibrotic Wnt1/βcatenin injury response is critically required for preserving cardiac function after acute ischaemic cardiac injury.
Keywords: epicardium, fibrosis, ischaemia-reperfusion, repair, Wnt
Introduction
Heart disease is a leading cause of mortality and morbidity and an emerging public health problem in the developing world. Acute injury to the heart commonly occurs following occlusion of a culprit blood vessel with subsequent death of dependent cardiac muscle. The mammalian heart does not possess a robust ability for cardiac muscle regeneration after acute injury and the lost myocardium is replaced by fibrous tissue to form a scar. The inability of the mammalian heart to regenerate cardiac muscle coupled with a predominantly fibrotic response to acute myocardial injury remains a fundamental biological problem to the therapy of heart disease. Loss of cardiac muscle mass along with a non-functional scar increases the haemodynamic burden on remaining cardiac muscle. The cardiac chambers dilate and eventually, cardiac contractile function declines leading to heart failure. Heart failure remains the most common hospital discharge diagnosis and more than a half a million people are diagnosed with this condition every year in the United States alone (Hunt et al, 2005).
The Wnt signalling system, comprising 19 lipophilic proteins in mammals (Gordon and Nusse, 2006), plays a critical role in wound repair and regeneration from simple systems such as planaria and hydra (Gurley et al, 2008; Petersen and Reddien, 2008) to mammalian hair follicle regeneration after skin wounding (Ito et al, 2007). Wnts are developmentally important for cardiogenesis (Eisenberg and Eisenberg, 2006) but there is conflicting evidence about the role of Wnts in cardiac repair. Wnt antagonists such as secreted frizzled-related protein (Sfrp2) exert anti-apoptotic effects on cardiac myocytes and when injected into the injured heart reduce fibrosis (Mirotsou et al, 2007; Zhang et al, 2009; He et al, 2010). However, Kobayashi et al (2009) demonstrated a pro-fibrotic role of Sfrp2 with mice deficient in Sfrp2 exhibiting decreased fibrosis after myocardial injury. Interestingly, in each of these studies, investigators noted that Sfrp2 appeared to interact with the BMP pathway rather than the Wnt signalling pathway in affecting fibrosis. Such discrepant results, likely related to differences in genetic models, interaction between Wnt and other signalling pathways and known biphasic effects of Wnts highlight the complexity of Wnts in regulating repair. In this regard, little is known about the pathophysiological role of specific Wnts in cardiac repair. We investigate the role of Wnts in cardiac repair and unexpectedly observe a dynamic role of Wnt1 in orchestrating early repair events involving the epicardium and cardiac fibroblasts. Wnt1, a canonical Wnt and a marker of neural crest cells (McMahon and Bradley, 1990), is upregulated ∼eight-fold in the heart within 48 h of acute ischaemic cardiac injury and activates the epicardium and cardiac fibroblasts. Wnt1 is initially expressed in the epicardium and subsequently by cardiac fibroblasts in the region of cardiac injury. The epicardium becomes Wnt responsive, undergoes widespread activation and expands, undergoing epithelial–mesenchymal transition (EMT) to generate fibroblasts that localize in the subepicardial space. The injured regions of the heart are Wnt responsive as well and Wnt1 induces cardiac fibroblast proliferation and expression of pro-fibrotic genes. Using loss of function approaches, we demonstrate that interruption of downstream Wnt/βcatenin signalling in epicardial cells impairs epicardial expansion, EMT and severely compromises cardiac function following cardiac injury. Furthermore, disruption of downstream Wnt signalling in cardiac fibroblasts leads to a rapid decline in cardiac function, impaired wound healing and dilatation of cardiac chambers within a few days of cardiac injury. Taken together, our findings suggest a pro-fibrotic Wnt/βcatenin-dependent injury response activates the epicardium and cardiac fibroblasts, and is important for preserving cardiac function after acute cardiac injury.
Results
Dynamic Wnt1 expression from epicardium to injury region
We screened changes in expression of all 19 mammalian Wnts by quantitative PCR (qPCR) at different time points following acute ischaemic cardiac injury (Supplementary Figure S1A). The left anterior descending artery which supplies blood flow to the majority of the left ventricle of the heart is temporally occluded to induce cardiac muscle ischaemia and released 30 min later to further induce reperfusion injury to cardiac muscle cells. Sham-injured animals served as controls at each time point. Expression of most Wnts in the heart was low and did not change significantly following injury (Supplementary Figure S1A), while some Wnts were not expressed at all (Supplementary Figure S1D). Wnt1, Wnt4 and Wnt7A expression significantly increased following injury (Figure 1A; Supplementary Figure S1B and C). Wnt7A exhibited a transient increase and sharp decline (Supplementary Figure S1C), while Wnt4 expression peaked at 14 days following injury (Supplementary Figure S1B). In contrast, Wnt1 expression increased by seven-fold within 2 days of acute cardiac injury and remained persistently elevated even at 14 days albeit at lower levels (Figure 1A). As cardiac fibroblast recruitment, proliferation and an acute repair response occur within the first several days following myocardial injury (Sun and Weber, 2000), we focused on the possible role of Wnt1 in contributing to an active cardiac repair response. To determine the anatomical region of expression of Wnt1, we performed in-situ hybridization (ISH) to Wnt1 mRNA following acute cardiac injury. Wnt1 was not expressed in sham-injured animals (Figure 1B). However, 2 days following injury, we surprisingly observed intense Wnt1 expression in the epicardial and subepicardial space (Figure 1C). Masson-trichrome staining to determine areas of injury and early fibrosis demonstrated spotty Wnt1 expression in the area of injury as well (Figure 1C). At 4 days following injury, we again observed Wnt1 expression in the epicardium that had now expanded (Figure 1Di). We saw pockets of Wnt1 expression contiguous with the epicardium extending into the adjoining myocardial interstitium (Figure 1Dii and iii). Wnt1 expression in the region of injury was more intense compared with expression of Wnt1 in the injury region at day 2 (Figure 1Div). By 10 days following injury, the area of injury strongly expressed Wnt1 (Figure 1E). Sense and scrambled controls for ISH did not show any staining and the Wnt1 ISH probe was verified by staining regions of the mouse embryo known to express Wnt1 (Supplementary Figure S1Ei–iv).
Figure 1.
Wnt1 expression in the injured heart. (A) qPCR of Wnt1 expression in whole hearts following injury (n=8 animals/group, *P<0.05 versus sham; mean±s.e.m.). (B) Sham-injured heart with ISH for Wnt1 and Masson-trichrome (MT) staining of same (C) Wnt1 ISH day 2 post injury (arrows show epicardium and arrowhead region of injury) and MT staining of same (D) Wnt1 ISH day 4 post injury: (i) Wnt1 expression in epicardial cells (arrow) (ii) early invasion of Wnt1 expression (arrow) (iii) contiguous Wnt1 expression into adjoining myocardium (arrows) (iv) Wnt1 expression in region of injury (arrowhead). (E) Wnt1 ISH day 10 post injury; Wnt1 expression in area of injury (arrowhead) with MT staining of same. (F) qPCR of Wnt1 expression in cardiac fibroblasts isolated following injury (n=3 animals/group, *P<0.05 compared with sham; mean±s.e.m.). Scale bar: 100 μm.
Given the localization of Wnt1 expression from the epicardium to the region of injury by 10 days, we speculated that Wnt1 expression progressively increases in cardiac fibroblasts. We isolated cardiac fibroblasts by standard methods of differential attachment (Ieda et al, 2010) and consistent with ISH observed significant upregulation of Wnt1 by qPCR (Figure 1F). Western blotting on the injured region of the heart demonstrated upregulation of Wnt1 as well (Supplementary Figure S1F) confirming the qPCR shown in Figure 1A. Taken together, these observations demonstrating upregulation as well as localization of Wnt1 expression from the epicardium to the region of injury suggest a dynamic role of Wnt1 in activating the epicardium and cardiac fibroblasts following acute ischaemic cardiac injury.
Epicardial cells and cardiac fibroblasts express Wnt1 after cardiac injury
To confirm the phenotype of Wnt1-expressing cells, we crossed Wnt1Cre transgenic mice with the lineage reporter Rosa26RlacZ mice (Wnt1 cells express lacZ). We induced acute cardiac injury in Wnt1Cre/R26RlacZ mice and analysed lacZ expression in injured hearts.
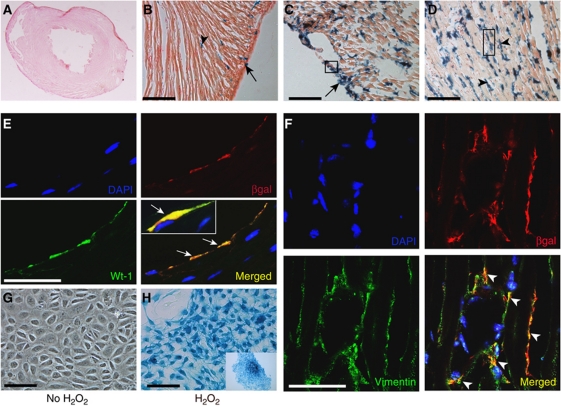
Sham-injured animals exhibited lacZ expression in the proximal aortic arch (Jiang et al, 2000) as well as in cardiac nerves (Nakamura et al, 2006; Supplementary Figure S2A), consistent with a neural crest origin of these structures. Xgal staining of cryosections showed minimal lacZ expression (Figure 2A) with only rare epicardial and interstitial cells expressing lacZ (Figure 2B). Wnt1 was expressed within 2 days of cardiac injury with strong lacZ expression observed in the epicardium (Figure 2C) and myocyte interstitium in the region of injury (Figure 2D). To identify the phenotype of Wnt1-expressing cells in Wnt1Cre/R26RlacZ mice after injury, we performed double immunostaining with high-resolution confocal microscopy with epicardial and cardiac fibroblast markers. As shown in Figure 2E, cells in the epicardium (day 2 post injury Wnt1Cre/R26RlacZ hearts) that stained with βgal antibody also expressed Wilms tumour 1 (Wt-1), a marker of epicardial cells (Wilm et al, 2005; Zhou et al, 2008). Moreover, cells in the injured region double stained for βgal and fibroblast marker vimentin (Krenning et al, 2010; Figure 2F) confirming that fibroblasts in the area of injury express Wnt1. To further corroborate the fibroblast phenotype of Wnt1-expressing cells in the area of injury, we digested the heart 2 days after injury and observed that βgalactosidase-expressing cells co-stained with the fibroblast marker, vimentin (Supplementary Figure S2B). As inflammatory cells are known to express Wnts (Lobov et al, 2005), we also stained for inflammatory markers (CD11b) but did not observe inflammatory cells to be a significant source of Wnt1 (Supplementary Figure S2C). Taken together, these findings support our in-situ data of Wnt1 being expressed in the epicardium and region of injury.
Figure 2.
Cardiac fibroblasts and epicardial cells express lacZ in Wnt1Cre/R26RlacZ mice after cardiac injury. (A) Xgal staining of sham-injured heart and (B) higher magnification (arrow points to rare lacZ-expressing epicardial cell and arrowhead to lacZ-expressing cell in myocyte interstitium). (C, D) Xgal staining of heart day 2 post injury with abundant staining of epicardial cells (arrow) as well as cells (arrowhead) in myocyte interstitium in region of injury. (E, F) Staining of (E) epicardium (shown in boxed area in C) with βgal (red) and Wt-1 (green) antibodies shows cells in the epicardium expressing both markers (arrows in merged panel) and (F) area of injury (boxed area in D) with antibodies against βgal (red) and fibroblast marker vimentin (green) showing cells that express both markers (arrowheads). Nuclei stained with DAPI (blue). (G, H) Epicardial cells isolated from E12.5 dpf Wnt1Cre/R26RlacZ mice demonstrating Xgal staining of (G) untreated control cells (H) cells treated with H2O2 (H, inset) epicardial colony in lower magnification (scale bar: A–D, G and H: 100 μm; E and F: 50 μm).
We next determined the mechanism of Wnt1 upregulation in epicardial cells following ischaemia-reperfusion cardiac injury. Acute ischaemia-reperfusion injury of the heart is associated with generation of free radicals and we investigated whether reactive oxygen species (ROS) could switch on Wnt1 transcription. We isolated epicardial cells from E12.5 days post fertilization (dpf) Wnt1Cre/R26RlacZ embryos and treated epicardial cells with hydrogen peroxide (10−4M) for 10 min and stained them for lacZ expression 24 h later. We chose to perform experiments on embryonic epicardial cells as epicardial cells isolated from adult hearts exhibit significant decline in trophic and migratory abilities (Smart et al, 2007b). Untreated control epicardial cells after 24 h did not express lacZ (Figure 2G); however, upon brief treatment with hydrogen peroxide, the epicardial colony stained with Xgal, associated with change in morphology of these cells to a spindle shaped phenotype (Figure 2H, inset). To further corroborate this observation, we stained epicardial cells with Wnt1 antibody and observed Wnt1 expression in epicardial cells treated with hydrogen peroxide (Supplementary Figure S2D). Taken together, this suggests that increased ROS directly or indirectly upregulates Wnt1 at least in culture.
The epicardium and cardiac fibroblasts in the region of injury are Wnt responsive
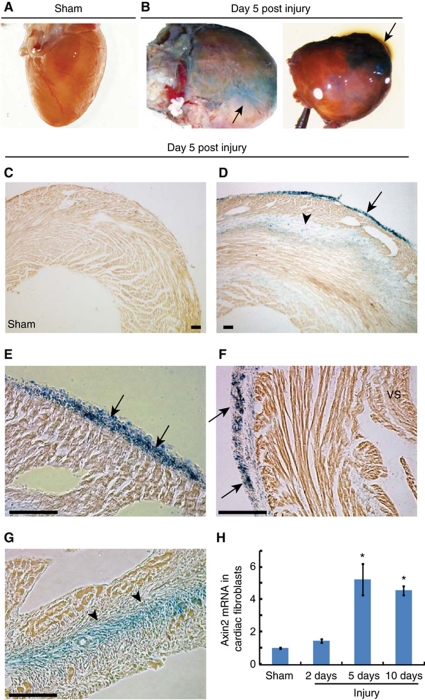
As the epicardium and cardiac fibroblasts express Wnt1 following cardiac injury, we investigated whether they are also Wnt responsive following injury. We used TOPGAL transgenic mice that express lacZ driven by TCF4 response elements, TCF4 being a downstream mediator of canonical Wnt signalling (DasGupta and Fuchs, 1999). We induced acute cardiac injury in 8-week-old TOPGAL transgenic mice and analysed hearts of mice for lacZ expression. Sham-injured hearts did not express lacZ (Figure 3A). However within 5 days of cardiac injury, intense lacZ expression was visible on the surface of injured hearts in agreement with recent reports of canonical Wnt activation post cardiac injury (Figure 3B; Aisagbonhi et al, 2011). Xgal staining of cryosections demonstrated widespread epicardial activation with epicardial lacZ expression present over the injured left ventricle (Figure 3D) but not in sham-injured hearts (Figure 3C). The lacZ-expressing epicardium over the left ventricle had also expanded (Figure 3E). Moreover, we observed that the epicardium over the uninjured portion of the left ventricle and ventricular septum were Wnt responsive as well (Figure 3F). LacZ expression was also observed in the region of injury (Figure 3G) consistent with our observations of cardiac fibroblasts in the injured region expressing Wnt1. To confirm this observation that cardiac fibroblasts were also Wnt responsive, we isolated cardiac fibroblasts from hearts of wild-type mice at 2, 5, and 10 days following injury and observed 5–6-fold upregulation of Axin2 expression in isolated cardiac fibroblasts (Figure 3H), Axin2 being a marker of Wnt responsive cells (Gordon and Nusse, 2006). It is interesting to note that Wnt responsiveness of cardiac fibroblasts closely correlates with the temporal pattern of Wnt1 expression in cardiac fibroblasts following injury (Figure 1F), suggesting that cardiac fibroblasts may be specifically responding to Wnt1. Taken together, these observations demonstrate that the epicardium and fibroblasts in the injury region express Wnt1 and respond to Wnts, suggesting a role of Wnt-dependent regulation of these cardiac cell components in repair.
Figure 3.
The epicardium and cardiac fibroblasts are Wnt responsive following acute cardiac injury. Hearts of TOPGAL transgenic mice (n=4 animals/group) stained with Xgal in whole mount (A) sham-injured (B) day 5 post injury with staining on cardiac surface (arrows). Xgal staining of frozen cryosections of (C) sham and (D–G) day 5 post injury heart shows (D) lacZ expression in epicardium over left ventricle (arrow) and area of injury (arrowhead). (E) Higher magnification of epicardium shows expansion of lacZ-positive epicardial cells (arrows). (F) LacZ-expressing epicardium (arrow) over the uninjured region of the left ventricle (arrow). (G) Presence of lacZ-expressing cells in the region of injury (arrowhead). (H) qPCR for Axin2 expression in cardiac fibroblasts isolated at different time points following injury (n=3, *P<0.05 versus sham; mean±s.e.m.). Scale bar: 100 μm. VS=ventricular septum.
Wnt1 activates cardiac fibroblasts and induces epicardial cells to undergo EMT
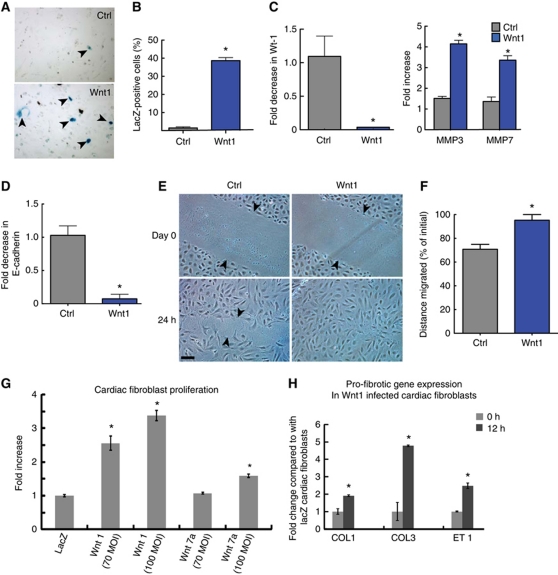
The epicardium during cardiac development gives rise to cardiac fibroblasts by undergoing EMT and Wnts are known to regulate EMT in the developing heart (Gessert and Kuhl, 2010). During EMT in development, the epicardial cells adopt a mesenchymal phenotype and migrate into the developing ventricle. Furthermore, a recent report suggests that epicardial cells undergo EMT after cardiac injury (Zhou et al, 2011). We next investigated the effects of Wnt1 on epicardial cells. We isolated epicardial cells from E12.5 dpf embryos of Col1a2CreER(T)/R26RlacZ mice and treated freshly isolated epicardial colonies with Wnt1 or PBS for 7 days. These mice carry a tamoxifen-inducible Cre-recombinase element under the fibroblast-specific regulatory sequence of proα2 type 1 collagen gene (Kapoor et al, 2008). Tamoxifen was concomitantly added to all epicardial colonies. We observed that 35% of epicardial cells following Wnt1 treatment expressed lacZ suggestive that Wnt1 treatment induced epicardial cells to undergo EMT and adopt a collagen-expressing fibroblastic phenotype. In contrast, only 2% of cells treated with PBS expressed lacZ after a week (Figure 4A and B). Wt-1 is downregulated following EMT in the developing embryo (Wilm et al, 2005) and we observed that Wnt1 treatment of epicardial cells for 6 h led to 95% downregulation of Wt-1 (Figure 4C). Moreover, EMT leads to increased expression of metalloproteinases (MMPs; Kalluri and Weinberg, 2009; Thiery et al, 2009) and enhanced migratory ability and we saw that epicardial cells upregulated MMP3 and MMP7 expression within 6 h of Wnt1 treatment (Figure 4C). By 24 h of Wnt1 treatment, expression of epithelial marker E-cadherin was downregulated by 90% in epicardial cells (Figure 4D), a critical event in EMT (Thiery et al, 2009). Taken together, our observations are thus consistent with Wnt1 inducing epicardial cells to undergo EMT. Wnt1 is known to mediate its effects through the canonical βcatenin-dependent pathway. To determine whether Wnt1 was mediating EMT in epicardial cells in a βcatenin-dependent manner, we infected epicardial cells isolated from Col1a2CreER(T)/R26Rtdtomato (Madisen et al, 2010) embryonic mice hearts with lentivirus shRNA targeting βcatenin. Epicardial cells were treated with Wnt1 for 7 days and the degree of tomato fluorescence analysed, as a readout on the degree of EMT. We observed that epicardial cells infected with a βcatenin shRNA lentivirus had a 60% reduction in the number of cells expressing tomato fluorescence compared with cells infected with a lentivirus expressing a scrambled shRNA construct (Supplementary Figure S3A and B). These observations suggest that Wnt1 mediates its effects on epicardial EMT predominantly via the canonical βcatenin pathway. EMT confers cells with enhanced migratory properties and Wnt1 also significantly increased migration of epicardial cells by ∼25% consistent with epicardial cells adopting a mesenchymal phenotype (Figure 4E and F). As cardiac fibroblasts in the region of injury, expressed Wnt1 and were Wnt responsive as well, we next investigated the effects of Wnt1 on cardiac fibroblast function. We observed that Wnt1 overexpressing cardiac fibroblasts proliferate two-to-three times greater than lacZ-expressing control cardiac fibroblasts (Figure 4G; Supplementary Figure S3C). In this respect, Wnt7A had a modest effect compared with that of Wnt1 (Figure 4G; Supplementary Figure S3C). Wnt1 overexpressing cardiac fibroblasts also increased expression of genes known to promote cardiac fibrosis (Figure 4H) compared with control cardiac fibroblasts expressing lacZ. These observations demonstrate that Wnt1 signalling promotes epicardial EMT, increases cardiac fibroblast activity, and enhances a pro-fibrotic cardiac injury response.
Figure 4.
Wnt1 induces epicardial cells to undergo EMT and activates cardiac fibroblasts. (A) LacZ expression in Col1a2CreER(T)/R26RlacZ epicardial cells (E12.5 dpf) treated with tamoxifen and PBS (Ctrl) or Wnt1 for 7 days. (B) Quantitation of lacZ-positive cells in Wnt1-treated epicardial cells (n=3, *P<0.05; mean±s.e.m.). (C) qPCR for Wt-1, MMP3 and MMP7 in epicardial cells treated with Wnt1 for 6 h (n=3, *P<0.05; mean±s.e.m.). (D) qPCR for E-cadherin in epicardial cells treated with Wnt1 for 24 h (n=3, *P<0.05; mean±s.e.m.). (E) Effects of Wnt1 on epicardial cell migration in a scratch wound assay. (F) Distance migrated by the cells (expressed as % of initial separation, n=3, *P<0.05; mean±s.e.m.). (G) Change in cardiac fibroblast proliferation following lentiviral infection of cardiac fibroblasts with Wnt1, Wnt7A or lacZ gene using a Cyquant assay (n=5, *P<0.05; mean±s.e.m.; MOI multiplicity of infection). (H) Expression of pro-fibrotic genes (qPCR) following transient lentiviral infection of Wnt1 in cardiac fibroblasts (n=3, *P<0.05; mean±s.e.m. Col1, Col3: Collagen type 1 & 3 ET-1: Endothelin 1). Scale bar: 100 μm.
Interruption of Wnt/βcatenin signalling in epicardial cells disrupts EMT and compromises cardiac function after acute cardiac injury
Our data so far demonstrate that Wnt1 is upregulated in the heart after cardiac injury and is expressed by both epicardial cells and cardiac fibroblasts. Epicardial cells and cardiac fibroblasts are also Wnt responsive and Wnt1 at least in vitro induces βcatenin-dependent epicardial EMT and enhances cardiac fibroblast proliferation. To further determine the functional significance of Wnt signalling in the epicardial and fibroblast response to injury, we performed loss of function experiments in each cell type.
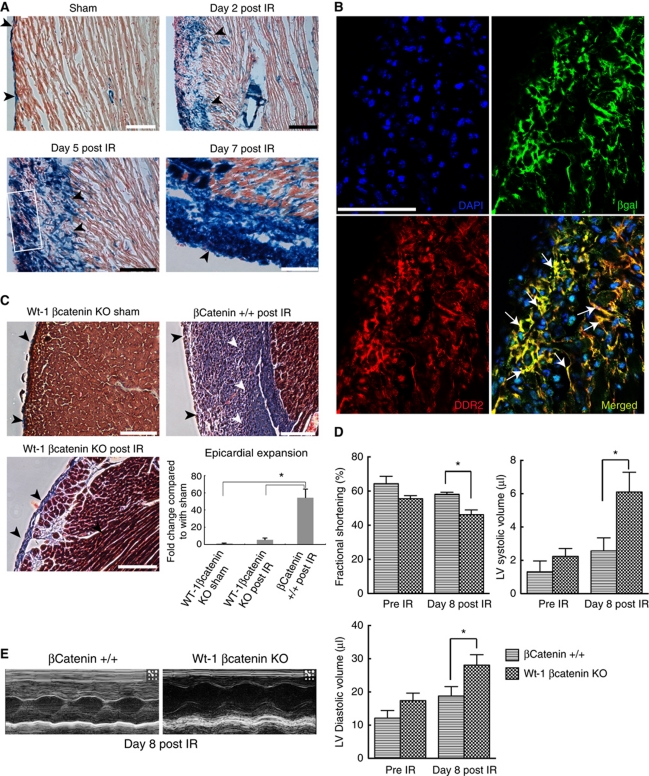
We crossed Wt-1Cre mice with the lineage reporter Rosa26RlacZ mice to create progeny Wt-1Cre/R26RlacZ mice. Wt-1 is expressed in epicardial cells in the embryonic heart and labels the adult epicardium (Zhou et al, 2008; Martinez-Estrada et al, 2010). Uninjured hearts demonstrated Xgal staining in majority of the epicardial cells around the heart over the left ventricle as well as right ventricle (Figure 5A; Supplementary Figure S4A). To confirm epicardial cell expansion after cardiac injury, we induced acute cardiac injury in 8-week-old Wt-1Cre/R26RlacZ mice and analysed their hearts for lacZ expression at 2, 5 and 7 days following acute cardiac injury (Figure 5A; Supplementary Figure S4B). We observed progressively increasing expansion of the epicardium (Figure 5A) within the first week after cardiac injury with peak epicardial expansion seen at 7 days (Figure 5A). These findings support our observations made in Figure 1Di–iii that the epicardium rapidly expands after acute cardiac injury.
Figure 5.
Interruption of downstream Wnt signalling in epicardial cells decreases epicardial expansion, EMT and worsens cardiac function after cardiac injury. (A) Xgal staining of the epicardium and subepicardial space in Wt-1Cre/R26RlacZ mice at 2, 5 and 7 days after cardiac injury (IR); arrowheads point to lacZ-expressing cells. A single layer of epicardial cells is seen overlying the left ventricle of sham-injured animals (top left) with peak epicardial expansion seen at 7 days (lower right). (B) Staining of expanded epicardium (boxed area in A) of Wt-1Cre/R26RlacZ mice with βgalactosidase and DDR2 antibodies shows abundance of cells that express both antigens (arrows). (C) Masson-trichrome staining shows minimal epicardial expansion in Wt-1-specific βcatenin-deficient mice 14 days following IR injury. Littermate βcatenin+/+ mice following injury serve as controls; epicardium (black arrowheads) and subepicardial space (white arrowheads). Quantitation of epicardial expansion using J image software. (D) Echocardiographic measurements of heart function pre injury and 8 days after acute ischaemia-reperfusion (IR) injury in mice having Wt-1-specific βcatenin deletion compared with littermate controls (βcatenin+/+) at 8 days following injury (n=6, *P<0.05, two-way ANOVA, mean±s.e.m.). (E) Representative M-mode echocardiograms of Wt-1-specific βcatenin-deficient mice and littermate βcatenin+/+ controls 8 days after IR injury. No statistical difference was observed in measurements between Wt-1-specific βcatenin-deficient mice and littermate controls before injury.
Epicardial cells are activated during heart development when the epicardial cells delaminate, undergo EMT and migrate into the adjoining myocardium through fascial planes (Männer et al, 2001). We next investigated whether the epicardial cells after acute cardiac injury adopted a fibroblastic phenotype. We performed double immunostaining along with high-resolution confocal microscopy of the expanded epicardium of Wt-1Cre/R26RlacZ mice hearts 5 days following injury. Double staining of the epicardium with βgalactosidase and fibroblast antibodies demonstrated that βgal-positive cells in the expanded epicardium stained for fibroblast marker DDR2 (Figure 5B) and vimentin (Supplementary Figure S4C), demonstrating that epicardial cells adopted a fibroblast phenotype. These observations suggest that the epicardium following injury not only gets activated and expands but also adopts a mesenchymal phenotype.
To determine the functional significance of Wnt signalling in regulating epicardial EMT after cardiac injury, we generated mice harbouring epicardial-specific deletion of βcatenin (downstream mediator of canonical Wnt signalling) by crossing Wt-1Cre mice with mice having both βcatenin alleles floxed. Live pups survived into adulthood had normal body weights compared with control littermates and phenotypically normal hearts with no significant difference in cardiac function (Figure 5D) compared with control littermates with intact βcatenin. We induced ischaemia-reperfusion injury and harvested their hearts 14 days later to detect changes in epicardial expansion and subepicardial collagen deposition. Masson-trichrome staining of heart cryosections demonstrated greatly expanded epicardium with abundant collagen deposition in the epicardial/subepicardial space in mice with intact epicardial βcatenin (Figure 5C). In mice harbouring epicardial deletion of βcatenin, the epicardium expanded minimally following injury and was similar to the epicardium in sham-injured animals. Consistent with deficient epicardial EMT, we observed minimal collagen deposition in the epicardium/subepicardium (Figure 5C). Quantitative measurements of maximal epicardial expansion demonstrated a near 10-fold decreased epicardial expansion in hearts of animals lacking epicardial βcatenin (Figure 5C). To further determine whether epicardial-derived cardiac fibroblasts are dependent on βcatenin signalling in vivo, we subjected Wt-1Cre/R26RlacZ/βcateninfl/fl mice to ischaemia-reperfusion, harvested and digested the heart and then analysed for expression of the fibroblast marker DDR2. As shown in Supplementary Figure S4D, Cre-expressing cells (lacZ positive) did not express the fibroblast marker DDR2 with the exception of rare cells. These observations suggest that epicardial EMT to generate cardiac fibroblasts is predominantly βcatenin dependent. Furthermore, mice with epicardial-specific βcatenin deletion exhibited cardiac dysfunction with increased cardiac volumes and depressed fractional shortening within 8 days of cardiac injury compared with littermates with preserved epicardial βcatenin (Figure 5D and E; Supplementary Table S1). Double immunostaining for βcatenin and troponin demonstrated that myocytes in the injured heart of Wt-1Cre/βcateninfl/fl mice had preserved βcatenin while the epicardial region of these animals demonstrated minimal βcatenin expression compared with wild-type hearts (Supplementary Figure S4E). Western blotting demonstrated a near 90% reduction in βcatenin levels in Wt-1-positive cells isolated from the adult heart (Supplementary Figure S4F). TTC and Evans blue staining demonstrated that there were no significant differences in initial infarct size between the βcatenin mutant and wild-type groups (Supplementary Figure S4G). Deletion of βcatenin in GATA5 expressing embryonic epicardium is known to impair the ability of epicardial progenitors to form coronary vasculature (Zamora et al, 2007). Although the reasons behind an inability of the epicardium of Wt-1Cre/βcateninfl/fl mice to respond following cardiac injury are unclear in our study, deletion of βcatenin in epicardial cells during development could have led to a less trophic and responsive epicardium. A significant fraction of intracellular βcatenin resides in the cell membrane in association with cell adhesion molecules and it is possible that deletion of βcatenin in epicardial cells could have disrupted epicardial-myocyte junctions. Previous studies have noted that deletion of cell adhesion molecule N-cadherin in Wnt1-expressing cells induces epicardial blebbing, cardiac muscle atrophy, and thinning of ventricular walls during cardiac development (Luo et al, 2006). We did not observe any changes in N-cadherin expression in the epicardium following epicardial deletion of βcatenin (data not shown) but deletion of βcatenin could have led to disruption of other βcatenin–cell adhesion complexes and contributed partially to cardiac dysfunction. Notwithstanding, our observations underscore the importance of a βcatenin-driven response for epicardial expansion, EMT and preservation of cardiac function after acute cardiac injury.
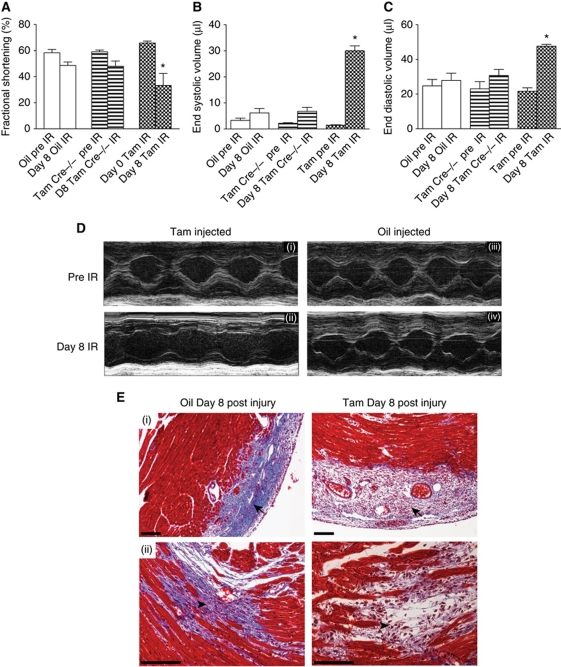
Interruption of Wnt/βcatenin signalling in cardiac fibroblasts leads to cardiac dysfunction after acute cardiac injury
Finally using a loss of function approach, we investigated the effects of interrupting downstream Wnt signalling in fibroblasts after cardiac injury. We first crossed Col1a2CreER(T) with the lineage reporter Rosa26RlacZ mice to identify cardiac fibroblasts following injury. Collagen type 1 is an important component of cardiac scar, is expressed by cardiac fibroblasts and serves as an useful marker of cardiac fibroblasts (Kapoor et al, 2008). We injected mice with tamoxifen for 10 days (stopped 5 days before injury), and harvested their hearts for Xgal staining 11 days after injury. Animals following cardiac injury demonstrated prominent lacZ expression in the area of injury compared with sham-injured animals (Supplementary Figure S5A). Double immunostaining demonstrated that lacZ-expressing cells in Col1a2CreER(T):R26RlacZ mice are of a fibroblast phenotype (Supplementary Figure S5D) and there was no lacZ expression in cardiac myocytes (Supplementary Figure S5C), consistent with previous reports of Col1a2CreER(T)/R26RlacZ mice (Zheng et al, 2002; Florin et al, 2004).
We next confirmed that Wnt1 activates the canonical pathway in cardiac fibroblasts and observed 3.5-fold upregulation of βcatenin, in cardiac fibroblasts overexpressing Wnt1 (Supplementary Figure S5E). To interrupt downstream canonical Wnt signalling specifically in cardiac fibroblasts, we generated tamoxifen-inducible Col1a2CreER(T)/βcateninfl/fl mice by injecting mice with tamoxifen for 10 days prior to injury. Western blotting demonstrated ∼75% reduction in βcatenin in cardiac fibroblasts isolated from the adult heart (Supplementary Figure S5F). Echocardiographic analysis demonstrated decline in cardiac performance associated with left ventricular dilatation within 8 days of injury, in contrast to oil-injected animals that did not exhibit cardiac dilatation at identical time points (Figure 6A–D; Supplementary Table S2). Animals lacking Cre recombinase but injected with tamoxifen had very similar cardiac function as oil-injected controls (Figure 6A–D; Supplementary Table S2). There were no differences in cardiac parameters in any of the groups prior to the procedure (Figure 6A–C; Supplementary Table S2). Infarct size to area at risk ratios estimated by TTC and Evans Blue staining did not demonstrate any differences between the βcatenin mutant and control groups (Supplementary Figure S5B). Histological analysis of hearts of animals with fibroblast-specific βcatenin deletion showed loose granulation tissue and minimal collagen deposition in the injury region at 8 days compared with vehicle-injected controls (Figure 6Ei and ii). Given the effects of Wnt1/βcatenin signalling in enhancing cardiac fibroblast proliferation, we hypothesized that cardiac fibroblast proliferation in vivo was dependent on Wnt/βcatenin signalling. We isolated cardiac fibroblasts from hearts of Col1a2CreER(T)/βcatenin mice 8 days after cardiac injury (tamoxifen injected in similar fashion), seeded them on 10 cm dishes and counted fibroblast numbers in six random fields 48 h after seeding. We observed nearly 80% reduction in fibroblast numbers isolated from mice lacking fibroblast βcatenin compared with control animals post injury (Supplementary Figure S6A and B). Furthermore, immunohistochemistry showed decreased expression of the fibroblast marker DDR2 in subepicardial regions of βcatenin mutant hearts compared with controls (Supplementary Figure S6C) further supporting our observations of the pro-proliferative effects of βcatenin on cardiac fibroblasts. Staining for Ki67 (marker of proliferation) demonstrated decreased Ki67 expression in the area of injury and subepicardial regions in fibroblast-specific βcatenin mutant hearts compared with controls (Supplementary Figure S6D and E). These observations strongly suggest that Wnt/βcatenin signalling plays an important role in regulating cardiac fibroblast proliferation and response to injury after cardiac injury. Cardiac fibroblast proliferation is a critical early repair response for the injured heart and impaired fibroblast response is known to undermine wound healing. Our observations reveal an unexpected role of Wnt/βcatenin signalling in mediating this critically required pro-fibrotic injury response for cardiac homoeostasis after acute ischaemic injury.
Figure 6.
Interruption of downstream Wnt signalling in cardiac fibroblasts after cardiac injury leads to acute cardiac dilatation and cardiac dysfunction. Echocardiographic M-Mode measurement of (A) fractional shortening (B) end systolic volume and (C) end diastolic volume (*P<0.05 of D8 Tam IR compared with D8 Oil IR and Tam Cre (−/−) mice IR; mean±s.e.m., n=15/group). (D) M-mode echo of the cardiac chambers 8 day after injury in tamoxifen-injected animals (Cre+/−) compared with oil-injected ones. (E) Masson-trichrome staining of heart of Col1a2CreER(T)/βcateninfl/fl animals 8 days following injury: (i) compact collagen deposition (arrow) in oil-injected animal but loose granulation tissue and little collagen deposition in similar region of injury in tamoxifen-injected animals (arrow); (ii) collagen deposition in subendocardial area of injury in oil-injected animals (arrowhead) but disorganized granulation tissue with scant collagen deposition in similar regions in tamoxifen-injected animals (arrowhead). Scale bar: 100 μm (IR: ischaemia-reperfusion; D8 refer to days following injury).
Discussion
Wnt1 is a cardiac ‘response to injury’ gene activating the epicardium and cardiac fibroblasts
Several Wnts are expressed during cardiac development in a spatio-temporal manner and participate in cardiogenesis but expression of most Wnts in the adult heart is low (Eisenberg and Eisenberg, 2006). In our screen for Wnts upregulated following injury, Wnt1 and Wnt7a were the only Wnts that are significantly elevated early after cardiac injury. Although a potential contribution of Wnt7a to fibrosis cannot be excluded, Wnt7A exhibited a transient spike in expression while Wnt1 expression peaked early after injury and was sustained albeit at lower levels days following injury. We noted Wnt4 expression to increase after 7 days with a peak at 14 days after injury. Wnt4 is known to contribute to renal fibrosis (Surendran et al, 2002) and it is possible that Wnt4 plays a role in later stages of cardiac fibrosis. However, critical repair responses in the heart such as fibroblast proliferation occur within the first few days after cardiac injury and we observed Wnt1 to be dynamically expressed from the epicardium to the area of injury early after cardiac injury. Wnt1 is not known to directly contribute to cardiac development but neural crest cells express Wnt1 and contribute to the proximal portion of the aortic arch, formation of cardiac nerves as well as to valve leaflets (Nakamura et al, 2006). Our experiments demonstrate a novel function of Wnt1 as a ‘cardiac response to injury’ gene in driving early repair events in the heart. The epicardium is an epithelial layer surrounding the myocardium and is derived from the pro-epicardium during cardiac development. The epicardium undergoes EMT during cardiac development and gives rise to cardiac fibroblasts, contributes to formation of coronary arteries and a small subset of cardiac myocytes as well (Cai et al, 2008; Zhou et al, 2008). The mammalian epicardium has been recently described to have mesenchymal and vascular progenitors and thymosin β4 identified as a molecule that activates and mobilizes epicardial progenitors and induces EMT (Limana et al, 2007, 2010; Smart et al, 2007a; Bock-Marquette et al, 2009). Moreover, a recent report suggests that the epicardium can modulate cardiac repair by paracrine mechanisms (Zhou et al, 2011). Our results reveal that the mammalian heart possesses an endogenous ability to activate its epicardium in a Wnt-dependent manner after acute cardiac injury. The epicardium is dynamically activated in a widespread manner, expands, undergoes EMT and generates subepicardial fibroblasts. Although the mechanisms of widespread activation of the epicardium are unclear from our studies, it may be related to intercellular gap junctions or redox conditions generated secondary to ischaemia-reperfusion injury. The precise depth of migration as well as the role of epicardial-derived fibroblasts compared with non-epicardial-derived fibroblasts is not clear from our experiments; however, the importance of epicardial activation is underscored by the fact that disruption of epicardial Wnt signalling decreased epicardial EMT, impaired subepicardial collagen deposition, and led to ventricular dilatation and worsening cardiac performance.
Fibroblasts in the region of injury respond to Wnts as well and Wnt1 enhances the pro-fibrotic function of cardiac fibroblasts, inducing fibroblast proliferation and expression of pro-fibrotic genes. Disruption of downstream Wnt signalling in cardiac fibroblasts leads to a precipitous decline in cardiac function. Hearts lacking fibroblast βcatenin (Col1a2CreER(T)/βcateninfl/fl) exhibited dramatically reduced fibroblast numbers compared with control mouse hearts. Fibroblast numbers are known to peak in the heart within the first 7 days of injury and the fibroblast response to injury is a critical element of cardiac repair (Camelliti et al, 2005). A disruption of Wnt/βcatenin signalling abrogated this critically required fibroblast mediated injury response and induced a rapid decline in cardiac function. Consistent with this observation, interruption of downstream Wnt signalling in epicardium and cardiac fibroblasts led to severely decreased collagen deposition and loosely organized granulation tissue in the injured region as well as in the subepicardial region. We propose that gross disorganization of wound healing along with little collagen deposition leads to an adverse rapid cardiac remodelling that results in acute ventricular dilatation and heart failure. Cardiac fibroblasts are known to generate tensile forces (Eastwood et al, 1998) and consistent with Laplace’s law, an interruption of cardiac fibroblast activation will lead to decreased tensile strength of the cardiac wall and predispose the cardiac chambers to dilate from the pressure of the blood within the chamber.
Our conclusions supporting a pro-fibrotic role of Wnt1 is consistent with a similar role of Wnts in promoting skin and pulmonary fibrosis in different pathologic conditions (Cheon et al, 2002; Konigshoff et al, 2009). Our observations about epicardial activation bear a striking similarity to the response of the zebrafish heart (Lepilina et al, 2006) after acute cardiac injury and suggest that the activation of the epicardium after cardiac injury is an evolutionary conserved response. However, the outcome is fibrotic in contrast to a regenerative one in fish. Further studies may help delineate Wnt-dependent strategies to manipulate epicardial activation after cardiac injury to decrease fibrosis and enhance regeneration.
Materials and methods
Myocardial injury
Mice are anaesthetized with isoflurane and a left thoracotomy is performed under mechanical ventilation using a volume cycled Harvard Rodent ventilator. Under direct visualization, the pericardial sac is opened and the LAD artery is temporally occluded close to its origin with 8-0 suture. Myocardial ischaemia is confirmed by myocardial blanching as well as by ST elevation on continuous ECG monitoring. Following 30 min of ischaemia, the suture is released to induce reperfusion injury and this is confirmed by decreased ST segment elevation on ECG. For sham injury, an identical procedure is followed and a ligature is passed under the LAD without occluding it. Body temperatures of the animals are monitored with a rectal probe and maintained by using a heated surgical platform. The chest wall is closed in layers and the mice are transferred onto a temperature-controlled pad for recovery. The surgeon was blinded to the genotype of the mice or tamoxifen treatment.
In-situ hybridization
For ISH, a mouse Wnt1 cDNA fragment (460–1377 bp) was subcloned into pKanascript vector, linearized with SalI and XbaI to prepare probes by in-vitro transcription. Digoxigenin probes were synthesized using DIG-UTP labeling kit (Roche). Probes were validated by staining areas of mouse embryo known to express Wnt1 and sense strand controls were used in parallel. Harvested hearts were perfused with RNAse-free PBS, and frozen in OCT. ISH was performed on 7–15 μM cryosections.
Generation of Col1a2CreER(T)/βcateninfl/fl and Wt-1Cre/βcateninfl/fl mice
Col1a2CreER(T)/0 mice carry a tamoxifen-inducible Cre-recombinase (CreER(T)) element under the control of a fibroblast-specific regulatory sequence from the proα2(I) collagen gene (Kapoor et al, 2008). Col1a2CreER(T)/0 mice were crossed with βcateninfl/fl mice (Jackson Labs) to generate mice heterozygous for both alleles. The second cross between βcateninfl/fl mice and heterozygous mice from the first cross produced Col1a2CreER(T)/βcateninfl/fl mice, which were used in experiments. Mice lacking βcatenin in Wt-1 cells were generated by a similar strategy following mating of Wt-1Cre and βcateninfl/fl mice. To delete βcatenin in tamoxifen-inducible Col1a2Cre/βcateninfl/fl mice, a stock solution of tamoxifen (Sigma-Aldrich) in ethanol (100 mg/ml) was diluted in corn oil to 10 mg/ml. Adult mice (age, 6–7 weeks) were given intraperitoneal injections of the tamoxifen suspension (0.1 ml of 10 mg/ml) for 10 days and injections were stopped 5 days before surgery. Mice lacking the Cre transgene were injected in an identical fashion. All surgical procedures were approved by Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, NC.
Quantitative RT–PCR
Total RNA from mouse heart, or cultured cells was isolated with SV Total RNA Isolation system by following the manufacturer′s protocols (Promega). qPCR (including no ‘RT control’) were performed at least in triplicate. The reactions were run at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Fold changes in gene expression were calculated using the ΔΔCt method after normalizing to GAPDH. Wnt primer sequences were used as previously described (Kemp et al, 2005).
Xgal staining
For whole mount Xgal staining, tissues were harvested and fixed with (0.2%. glutaraldehyde, 5 mM EGTA, pH 7.3, PBS) at 4°C for 2 h (heart) or 1 h (embryo E11.5–E13.5). Following fixation, samples were washed three times for 30 min in Xgal wash buffer (2 mM MgCl2, 0.1% Triton-100 in PBS) and then stained overnight with Xgal staining solution (1 mg/ml Xgal, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide in washing buffer) at 37°C.
For Xgal staining of cryosections, whole hearts were harvested and fixed 0.2% glutaraldehyde solution (as mentioned above) at 4°C for 4 h. The fixed hearts were embedded in Tissue-Tek OCT compound and frozen. Cryosections (15 and 7 μm) were prepared and stained for βgalactosidase activity. Prior to staining, sections were refixed in cold PBS containing 0.2% glutaraldehyde for 10 min. Sections were washed three times for 5 min in lacZ wash buffer (2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet-P40 (NP-40) in PBS) and then stained in Xgal staining solution at 37°C overnight. Sections were rinsed in PBS, counterstained, moved through a graded ethanol series for dehydration and then mounted.
Echocardiography
Cardiac trans-thoracic echocardiography was performed (blinded to mouse genotype and treatment) on conscious mice using a VisualSonics Vevo 770 ultrasound biomicroscopy system (VisualSonics, Inc., Toronto, Ontario, Canada) using the 707B scan head (30 MHz) as previously described (Li et al, 2007; Willis et al, 2007). Briefly, two-dimensional guided M-mode was performed in the parasternal long axis and short axis at the level of the papillary muscle. Epicardial to endocardial leading edges were used to measure anterior wall (IVSd, IVSs) and posterior (PWTd, PWTs) wall thicknesses, as well as the left ventricular internal diameters (LVEDD, LVESD). Left ventricular systolic function was assessed by ejection fraction (LV EF%=((LV Vol (d)−LV Vol (s)/LV Vol (d) × 100)) and fractional shortening (%FS=((LVEDD−LVESD)/LVEDD) × 100). M-mode measurements represent three average consecutive cardiac cycles from each mouse.
Construction of plasmids and lentiviruses
Mouse Wnt1 full-length cDNA clone (Open Biosystems) was cloned into pLenti6/V5-DEST Gateway® Vector system following the manufacturer's protocol (Invitrogen). pLenti6.2/V5-GW/lacZ was used as a control vector. The plasmid was verified by sequencing and restriction enzyme digestion. Lentiviruses were produced from Vector Core Facility at the University of North Carolina at Chapel Hill.
Lentiviral shRNA
Epicardial cells were harvested from mouse embryonic hearts (Col1a2CreER(T)/R26Rtdtomato) and infected with lentiviral shRNA (Santa Cruz Biotechnology, Santa Cruz, CA) targeting βcatenin or a control lentivirus (similar MOI) encoding a scrambled sequence according to the manufacturer's instructions. GFP co-expression on the lentiviral construct was used to determine efficiency of viral transduction. Epicardial EMT was determined by the number of tomato fluorescent cells in each high power field.
Epicardium cell isolation and wound migration assay
Timed matings were performed to harvest E12.5 dpf embryos for isolation of epicardial cells as described (Dong et al, 2008). Briefly, the ventricle following dissection was placed ‘apex down’ on 12- or 24-well plates pre-coated with gelatin or collagen type I gel. After 24 h, the ventricle was removed and the epicardial cells used for the experiment within 24 h. For in-vitro wounding (migration) experiments, epicardial cells were harvested on gelatin-coated 24-well plates. The monolayer was injured by scratching across the epicardial colony with a 20-μl pipette tip. The wells were washed two times to remove detached cells or cell debris. The cells were then cultured in 3% FBS medium with/without Wnt1 protein (25 nM) (Abcam, MA, USA). After 24 h, images of the scratched areas under each condition were photographed. Scratch wound distance was measured using WCIF image J software.
Measurement of epicardial thickness
Photographs were taken of cryosections of hearts stained with Masson trichrome. Epicardial thickness was measured with J image software at five regions around the ventricular surface by dropping a perpendicular line from the epicardial surface to edge of the viable myocardium. The thickening of the epicardium and subepicardial regions were not uniform in all sections. To circumvent this issue, and obtain a true representation of differences in epicardial activation following injury, we measured epicardial thickness in sections where we observed injury. Second for each heart section examined, we measured maximal epicardial thickness in 3–5 regions around the heart section. Finally, epicardial thickness in three different heart regions (i.e. base, mid ventricle and apex) was averaged to obtain a mean epicardial thickness (Supplementary Figure S7).
Cardiac fibroblast isolation and proliferation assays
Briefly, 8-week-old adult mouse hearts were excised, minced, and placed in a solution containing 50 U/ml Collagenase II and 0.1% trypsin. The hearts were subjected to periods of digestion at 37°C for 10 min with gentle shaking; cells from the second to sixth digestion were pooled, filtered by 40 μm nylon cell strainer, centrifuged, and suspended in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin (100 U/ml). The cell suspension was seeded into uncoated plastic culture dishes (100 mm) for 90 min to allow for the preferential attachment of fibroblasts, after which unattached cells were rinsed off. The fibroblasts became 60–80% confluent within 4 days and were subsequently passaged with trypsin. All cells used in the experiments were from passages 2 or 3. The purity of these cultures was determined by immunofluorescent staining with vimentin and DDR2 antibodies. The proliferation of isolated cardiac fibroblasts was assayed by CyQUANT cell proliferation assay kit (Invitrogen) and FITC BrdU Flow Kit (BD Biosciences) as described by the manufacturer's protocols. For proliferation assays, cardiac fibroblasts were seeded at a density of 4 × 103 cells/well in 48-well plates a day prior to Wnt1 treatment.
Cytoplasmic β-catenin western blotting
Cardiac fibroblasts were infected with a Wnt1 lentivirus and cytoplasm extracted 48 h later using a FractionPREP Cell Fractionation Kit (Biovision, USA). Protein lysates were separated by SDS–PAGE followed by electrotransfer onto nitrocellulose membrane (Millipore). Membranes were incubated for 1 h at room temperature with primary antibodies: anti-βcatenin (Cell Signaling Technology; 1:1000), anti-GAPDH (Millipore; 1:1000), and then with peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized using Amersham ECL western blotting detection reagents (GE Healthcare). Membranes were analysed with the UVP gel Imaging System.
Histology and immunohistochemistry
Whole hearts were harvested and fixed with 2% paraformaldehyde at 4°C for 24 h. Fixed tissues were dehydrated, embedded in paraffin and 5 μM thickness sections prepared. Haematoxylin-Eosin (H/E) staining and Masson's trichrome staining were performed using standard methods. For immunohistochemistry, sections were subjected to antigen retrieval by treating them with boiling citrate buffer (pH 6.0) for 15 min followed by staining according to the manufacturer's instructions (Vector Labs ABC kit). Ki67 and CD11b antibodies (Abcam) were used at 1:100 dilution.
TTC and Evans blue staining and estimation of infarct area/area at risk
TTC combined with Evans blue staining was done to determine infarct area and area at risk. In brief, a subgroup of mice 48 h following ischaemia-reperfusion injury were subjected to thoracotomy and religation of the left anterior descending artery. A suture was left in place during initial ischaemia to mark the site of arterial ligation. Following ligation of the left anterior descending artery, the aorta of the mice was injected with 1% Evans blue. The infusion was discontinued as soon as epicardial blush was observed. Excess Evans Blue was washed off and the heart snap frozen and cut into sections, stained with TTC for 20 min followed by fixation with PFA. After 20 min, the sections were washed lightly and photographs of both sides of each heart section were taken with a microscope. Analysis was done with Image J software as described (Inagaki et al, 2003).
Immunofluorescence
Cells were fixed in 2% paraformaldehyde for 15 min and permeabilized with PBS buffer containing 0.1% Triton-100. Cryosections were fixed in acetone for 10 min at −20°C and treated with fresh 1 mg/ml sodium borohydride in PBS for 10 min, three times before applying primary antibody. Sections were incubated with anti-vimentin (Millipore, 1:100), anti-DDR2 (Santa Cruz Biotechnology, 1:50), anti-β-galactosidase (MP Biomedicals, 1:200), anti-Wt-1 (DAKO, 1:100), anti-Wnt1 (Abcam, 1:100), and anti-CD11b (Abcam, 1:100) primary antibodies followed by addition of biotinylated secondary antibodies and Avidin D FITC or Texas Red (Vector Biolabs). For heart tissue staining, sections were further treated with 1% Sudan B for 5 min to quench auto-fluorescence. Sections were washed, mounted with ProLong Gold anti-fade reagent (Molecular Probes Inc.) on glass slides, and photographed using a Leica SP2 AOBS upright laser scanning confocal microscopy.
Statistical analysis
Student's t-test, one-way and two-way ANOVA with Bonferroni's post-test analysis were used as appropriate. All statistical calculations were computed using GraphPad Prism software.
Supplementary Material
Acknowledgments
We thank the In-situ Hybridization, Histology Research, Michael Hooker Microscopy and Animal Histology core facilities at UNC, Chapel Hill for assistance with techniques. We thank Jackie Kylander and Taylor Kopple in the Mouse Cardiovascular core lab at UNC, Chapel Hill for assistance with echocardiography and Dr Gobinda Sarkar (Mayo Clinic, Rochester, MN) for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health and Ellison Medical Foundation to Arjun Deb.
Author contributions: JD, CG, DL, EH, LS, LR, JR, MR, MW and AD performed experiments. AL and MW provided vital reagents and aided in experimental design. JD, MW and AD analysed data. JD and AD wrote the manuscript. AD conceptualized the project.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK (2011) Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 4: 469–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock-Marquette I, Shrivastava S, Pipes GC, Thatcher JE, Blystone A, Shelton JM, Galindo CL, Melegh B, Srivastava D, Olson EN, DiMaio JM (2009) Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol 46: 728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65: 40–51 [DOI] [PubMed] [Google Scholar]
- Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA (2002) beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA 99: 6973–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568 [DOI] [PubMed] [Google Scholar]
- Dong XR, Maguire CT, Wu SP, Majesky MW (2008) Chapter 9. Development of coronary vessels. Methods Enzymol 445: 209–228 [DOI] [PubMed] [Google Scholar]
- Eastwood M, McGrouther DA, Brown RA (1998) Fibroblast responses to mechanical forces. Proc Inst Mech Eng H 212: 85–92 [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA (2006) Wnt signal transduction and the formation of the myocardium. Dev Biol 293: 305–315 [DOI] [PubMed] [Google Scholar]
- Florin L, Alter H, Grone HJ, Szabowski A, Schutz G, Angel P (2004) Cre recombinase-mediated gene targeting of mesenchymal cells. Genesis 38: 139–144 [DOI] [PubMed] [Google Scholar]
- Gessert S, Kuhl M (2010) The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res 107: 186–199 [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281: 22429–22433 [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Alvarado AS (2008) {beta}-Catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319: 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ (2010) Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci USA 107: 21110–21115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Abraham W, Chin M, Feldman A, Francis G, Ganiats T, Jessup M, Konstam M, Mancini D, Michl K (2005) ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult—Summary Article A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 46: 1116–1143 [DOI] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D (2003) Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 108: 2304–2307 [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320 [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cardiac neural crest. Development 127: 1607–1616 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Liu S, Shi-wen X, Huh K, McCann M, Denton CP, Woodgett JR, Abraham DJ, Leask A (2008) GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest 118: 3279–3290 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L (2005) Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn 233: 1064–1075 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes D, Ge G, Grieskamp T, Yamada C, Liu T, Huang G, Basson C, Kispert A, Greenspan D, Sato T (2009) Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O (2009) WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R (2010) The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD (2006) A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127: 607–619 [DOI] [PubMed] [Google Scholar]
- Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C (2007) Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest 117: 3211–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Bertolami C, Mangoni A, Di Carlo A, Avitabile D, Mocini D, Iannelli P, De Mori R, Marchetti C, Pozzoli O, Gentili C, Zacheo A, Germani A, Capogrossi MC (2010) Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol 48: 609–618 [DOI] [PubMed] [Google Scholar]
- Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC (2007) Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res 101: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA (2005) WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437: 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, High FA, Epstein JA, Radice GL (2006) N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol 299: 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männer J, Pérez-Pomares JM, Macías D, Muñoz-Chápuli R (2001) The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169: 89–103 [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P, Hosen N, Hill RE, Munoz-Chapuli R, Hastie ND (2010) Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet 42: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Bradley A (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62: 1073–1085 [DOI] [PubMed] [Google Scholar]
- Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V (2007) Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104: 1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J (2006) Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res 98: 1547–1554 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW (2008) Smed-{beta}catenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319: 327–330 [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR (2007a) Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445: 177–182 [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR (2007b) Thymosin beta-4 is essential for coronary vessel development and promotes neovascularization via adult epicardium. Ann NY Acad Sci 1112: 171–188 [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT (2000) Infarct scar: a dynamic tissue. Cardiovasc Res 46: 250–256 [DOI] [PubMed] [Google Scholar]
- Surendran K, McCaul SP, Simon TC (2002) A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol 282: F431–F441 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890 [DOI] [PubMed] [Google Scholar]
- Willis MS, Ike C, Li L, Wang DZ, Glass DJ, Patterson C (2007) Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circ Res 100: 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM (2005) The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132: 5317–5328 [DOI] [PubMed] [Google Scholar]
- Zamora M, Manner J, Ruiz-Lozano P (2007) Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci USA 104: 18109–18114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Deb A, Pachori A, He W, Guo J, Pratt R, Dzau VJ (2009) Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol 46: 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP (2002) Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol 160: 1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT (2011) Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 121: 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.