Abstract
Nature 481 7379, 85–89 (2012); published online December 07 2011
Metastatic niches support the survival and fitness of disseminated tumour cells (DTCs) in otherwise inhospitable tissue environments. The components of metastatic niches have remained a matter of conjecture, but recent reports, including one in a current issue of Nature, point at the extracellular matrix (ECM) proteins periostin and tenascin C (TNC) as key metastatic niche molecules. By enhancing Wnt and Notch signalling in cancer cells, these proteins provide physical as well as signalling support for metastasis-initiating cells. These findings underscore the importance of the ECM environment in cancer and provide potential drug targets against metastasis.
In many cancers, tumour cells start spreading through the body long before the primary tumour is detected and removed (Pantel et al, 2009). Although cancer cells may enter the circulation and egress into distant tissues by the millions, only a few of these cells manage to form overt metastases. This rate is far too low to be explained solely by a scarcity of metastasis-initiating cells, but it rather suggests that the tissue environment in the target sites is generally inhospitable to DTCs. Some DTCs do thrive nonetheless, and form metastases, implying that these metastasis-initiating cells found exceptional spots that provided support to resist the new environment and remain fit for eventual outgrowth. A context that provides DTCs with this kind of support is referred to as a ‘metastatic niche’, by analogy to the niches that support stem cells in healthy tissues.
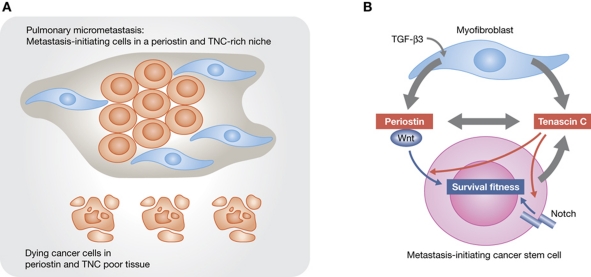
In recent years, much attention has been devoted to stromal cells that rally to tumours and secrete enzymes, growth factors and angiogenic cytokines for tumour growth and metastasis (Joyce and Pollard, 2009). Another important source of regulatory signals in normal tissues and tumours is the ECM (Hynes, 2009). Owing to the complex composition and interactions of the ECM components, and the rarity of oncogenic ECM mutations in cancer, the specific roles of these components in metastasis have remained elusive. However, several reports have recently revealed that the ECM proteins periostin and TNC play key roles as metastasis niche components for tumour-initiating cells that invade the lungs (Figure 1A; Malanchi et al, 2011; Oskarsson et al, 2011; O’Connell et al, 2011).
In the most recent of these reports, Malanchi et al (2011) show that the ECM protein periostin, is expressed in the end buds of mammary glands. The authors also detect periostin expression in myofibroblasts of mouse mammary tumours and their metastases in the lungs and demonstrate a role for periostin in metastasis initiation by means of periostin null mice. These mice can develop mammary tumours driven by a polyoma virus middle T antigen (PyMT) transgene. However, the ability of these tumours to metastasize to the lungs is significantly diminished compared with PyMT-driven tumours in wild-type mice. The in vitro growth of tumour cell populations in suspension oncospheres (an assay that enriches for tumour-initiating cells) could be blocked by anti-periostin antibodies. The authors show that stromal fibroblasts increase periostin production in response to TGF-β3, and periostin acts by presenting Wnt to the cancer cells leading to enhanced colonization of the lungs (Figure 1B). Moreover, they demonstrate that the only cancer cells able to benefit from periostin, respond to Wnt, and initiate metastasis are contained within a subpopulation defined by Thy-1 and CD24 markers. This population comprises ∼3% of the PyMT mammary tumour cells, and has been shown to represent cells enriched with tumour-initiating capacity in mouse models (Cho et al, 2008). Based on this, Malanchi et al propose that the role of periostin in progression of lung metastasis is to concentrate Wnt ligands in the metastatic niche for the stimulation of stem-like metastasis-initiating cells. These findings provide an exciting example of the role of the ECM in metastasis outgrowth.
Figure 1.
(A) The ECM components periostin and TNC in the metastatic niche help activate developmental pathways for the viability of metastasis-initiating cells in the lungs. (B) In the pulmonary parenchyma, TGF-β3 stimulates myofibroblasts to produce periostin, which binds stromal Wnt factors for presentation to stem-like metastasis-initiating cells (Malanchi et al, 2011). Myofibroblasts and the cancer cells themselves also produce TNC, which promotes the intracellular functioning of the Wnt and Notch pathways (Oskarsson et al, 2011). A direct biochemical connection between these functions is likely, as periostin binds TNC and anchors it to ECM components including fibronectin and type I collagen (Kii et al, 2010).
These new findings have striking parallels with recent findings on the role of TNC in breast cancer metastasis to the lungs (Oskarsson et al, 2011). TNC forms radial hexamers (hexabrachions) and interacts with various membrane receptors and ECM proteins. TNC is present in stem cell niches and tumour invasive fronts, and its expression in breast tumours is clinically associated with lung metastasis. TNC is expressed not only in cancer-associated fibroblasts but also in breast cancer cells. Stem-like human breast cancer cells expressing TNC showed a superior ability to form lung metastases when implanted as orthotopic tumours in mice (Oskarsson et al, 2011). TNC was found to support the survival and fitness of metastasis-initiating cells by enhancing their responsiveness to Wnt and Notch (Figure 1B). This effect was mediated by TNC-dependent signalling to components of the Notch pathway (Musashi) and the Wnt pathway (LGR5). Although cancer cell-derived TNC provides an advantage in metastasis initiation, stromal TNC is important too. Indeed, TNC-deficient mice implanted with mammary cancer cells show resistance to the formation of lung metastases, suggesting a significant role for stromal TNC, which is produced by S100A4+ fibroblasts (O’Connell et al, 2011).
The functional similarities between periostin and TNC as ECM components of the metastatic niche may not be coincidental. Earlier biochemical studies have shown that these two proteins bind tightly, with periostin additionally binding type I collagen and fibronectin, and thereby anchoring TNC to these general ECM components (Kii et al, 2010) (Figure 1B). The recent findings suggest that the collaboration between periostin and TNC in the metastasis niche and, more generally, in stem cell niches, may extend beyond building a proper ECM architecture. Periostin may gather Wnt for stem cells while TNC may enhance the ability of these cells to respond to Wnt and Notch (Figure 1B). Thus, periostin and TNC may represent two sides of the same metastasis niche coin.
The role of these molecules in promoting metastasis initiation raises several interesting questions. Why are stem-like cancer cells the only population that can respond to Wnt ligand presented by periostin? Are these cells uniquely capable of ‘reading’ periostin–TNC ECM units? And, what is the source of the TGF-β3 that induces periostin expression in myofibroblasts in the first place? Cancer cells and various stromal components can produce TGF-β, but the recent finding that cancer cell-associated platelets can act as carry-on source of TGF-β provides additional clues (Labelle et al, 2011).
The new roles of periostin and TNC as ECM components of the metastatic niche, and other recent studies, in turn underscore the importance of developmental and cell survival pathways in metastasis. The key roles of the Wnt, Notch and PI3K pathways in metastatic progression is increasingly evident, as is the nature of the molecules that metastasis-initiating cells resort to in order to maximize the activity of these pathways in difficult microenvironments (Chen et al, 2011; Malanchi et al, 2011; Oskarsson et al, 2011). This wave of newly identified molecular components of the metastatic niche provides exciting opportunities to develop novel therapies to target the survival and viability of DTCs, to complement and eventually replace adjuvant chemotherapy in the oncology clinic. This may be particularly relevant in cancer-like breast cancer where DTCs must survive in latency for long periods while they await a chance for outgrowth (Pantel et al, 2009). Targeting the signalling provided by the metastatic niche could reduce the probability of a relapse.
Footnotes
The authors declare that they have no conflict of interest.
References
- Chen Q, Zhang XH, Massagué J (2011) Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20: 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF (2008) Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells 26: 364–371 [DOI] [PubMed] [Google Scholar]
- Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A (2010) Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285: 2028–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2012) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481: 85–89 [DOI] [PubMed] [Google Scholar]
- O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R (2011) VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108: 16002–16007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J (2011) Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17: 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel K, Alix-Panabieres C, Riethdorf S (2009) Cancer micrometastases. Nat Rev Clin Oncol 6: 339–351 [DOI] [PubMed] [Google Scholar]