Abstract
Purpose
Age-adjusted cancer incidence and age-related penetrance studies have helped guide cancer risk assessment and management. PTEN Hamartoma-Tumor Syndrome (PHTS) is a term encompassing subsets of several clinical syndromes with germline mutations in the PTEN tumor suppressor gene. We conducted the first prospective study to clarify corresponding cancer risks to shed biological insights on human germline PTEN mutations, and to better inform current surveillance recommendations based on expert opinion.
Methods
A series of 3,399 individuals meeting relaxed International Cowden Consortium PHTS criteria were prospectively recruited; 368 individuals were found to have deleterious germline PTEN mutations. Age-adjusted standardized incidence ratio (SIR) calculations and genotype-phenotype analyses were performed.
Results
Elevated SIRs were found for carcinomas of the breast (25.4, 95%C.I. 19.8-32.0), thyroid (51.1, 38.1-67.1), endometrium (42.9, 28.1-62.8), colorectum (10.3, 5.6-17.4), and kidney (30.6, 17.8-49.4), and melanoma (8.5, 4.1–15.6). Estimated lifetime risks were, respectively, 85.2% (95%C.I. 71.4%-99.1%), 35.2% (19.7%-50.7%), 28.2% (17.1%-39.3%), 9.0% (3.8-%14.1%), 33.6% (10.4%–56.9%) and 6% (1.6%-9.4%). Promoter mutations were associated with breast cancer, while colorectal cancer was associated with nonsense mutations.
Conclusion
Lifetime risks for a variety of cancers, now extending to colorectal cancer, kidney cancer and melanoma, are increased in patients with PTEN mutations. The genotype-phenotype associations here may provide new insights on PTEN structure and function. We propose a comprehensive approach to surveillance of patients with PTEN mutations.
Keywords: PTEN, Cowden syndrome, lifetime cancer risk, cancer risk assessment and genetic counseling
Introduction
Individuals with germline mutations of the PTEN (MIM 601728) tumor suppressor gene on 10q23.3 have diverse phenotypic features affecting multiple systems, with the primary clinical concern of high lifetime risks of cancer of the breast, endometrium and thyroid. The PTEN tumor suppressor gene, located on 10q23.3, encodes a dual-specificity phosphatase that can dephosphorylate both protein (1) and phospholipid substrates (2). Somatic PTEN alterations are common and well-recognized in a variety of cancers, such as endometrial cancer, prostate cancer, breast cancer, thyroid cancer and kidney cancer. Germline PTEN mutations underpin the PTEN Hamartoma-Tumor Syndrome (PHTS), an umbrella term that includes a range of autosomal-dominant clinical syndromes mainly including Cowden syndrome (CS, [MIM 158350]), presenting in adulthood, and Bannayan-Riley-Ruvalcaba syndrome (BRRS [MIM 153480]) (3) in children. Inheritance of PHTS is autosomal dominant and age-related penetrance is believed to be high, around 80% (4). A primary clinical concern for affected individuals is the high lifetime risk of cancer, including cancers of the breast, endometrium, thyroid, colon and kidney. Consequently, clear evidence-based surveillance strategies for these individuals are required. To date, however, our understanding of cancer risks for these individuals have been gleaned from limited reports of retrospectively identified patients from single centers and on expert opinion. To address this, since 2000, the International Cowden Consortium (ICC) (5) has prospectively recruited patients from international centers (Mainly North America and Europe) for the purpose of studying PHTS, corresponding risks for cancer and other associated disorders, and genotype-phenotype correlation. Over this period, this study identified additional key features of PHTS, particularly polyposis (6) and autism (7, 8), which were eventually included in the operational criteria. We have recently developed a new diagnostic scoring system, permitting more accurate identification of individuals with PTEN mutations and hence genetic counseling over conventional NCCN criteria (9). We report here results from the first prospective international study conducted from 2000 to 2010. This study identified a consecutive series of adult and pediatric patients with PTEN mutation from North America, Europe (majority) and Asia, allowing us to investigate age-related cancer risks and genotype-phenotype correlations to gain biologic insights and to inform genetic counseling, cancer risk assessment and surveillance recommendations.
Patients and Methods
Research Participants
A total of 3,399 individuals meeting relaxed ICC criteria (pathognomonic criteria, or at least 2 criteria; either major or minor) (10, 11) were accrued prospectively into protocols approved by the respective institutions' Institutional Review Boards. These patients were recruited from both community and academic medical centers throughout North America, Europe (>85% originating from these two continents) and Asia using a standard protocol. Upon providing informed consent, checklists to document presence or absence of specific features were completed by specialist genetic counselors or physicians concurrently with submission of samples. Specialist genetics staff reviewed all checklists and corresponded with the enrolling center; if necessary, further primary documentation of medical records, particularly pathology reports, were obtained for phenotype confirmation with patient consent (9). For each mutation-positive individual, the diagnosis of cancer was obtained through referring physicians, and confirmed through primary records wherever possible. Relatives of mutation-positive probands were offered mutation testing where appropriate.
PTEN Mutation Analysis
Genomic DNA was extracted from peripheral blood leukocytes using standard methods, and scanned for PTEN mutations using methods and primers previously reported (9). In brief, genomic DNA samples for PTEN mutations was performed with a combination of denaturing gradient gel electrophoresis, high-resolution melting (HRM) curve analysis (Idaho Technology, Salt Lake City, Utah) and direct Sanger sequencing (ABI 3730xl) (12). Deletion analysis using the multiplex ligation-dependent probe amplification (MLPA) assay (13) was performed with the P158 MLPA kit (MRC-Holland, Amsterdam) according to manufacturer's protocol. All patients underwent re-sequencing of the PTEN promoter region (14), and promoter mutations were defined as previously reported based on individual characterization(9).
Statistical Methods
We calculated standardized incidence ratios (aka standardized incidence rates [SIR]) using incidence data from the Surveillance Epidemiology and End Results (SEER) database. For age-adjusted analysis, the projected U.S. population (year 2000) was used (15); 84% of the 3,399 individuals were white, justifying the use of the US SEER population. Age-adjusted SIRs and mid-p exact tests were calculated with OpenEpi using indirect standardization and age-specific SEER incidence rates (2003-2007). There were 38 categories based on two genders and nineteen age groups. Incidence was assumed to be 0 for categories where statistics were not provided. Person-years of observation (PYO) were calculated for each type of cancer from birthdate to the date of cancer for subjects who developed cancer, or to the date of most recent information for subjects without cancer. The expected number of cancers was calculated by multiplying SEER incidence rates in each of the 38 categories by PYO in each category (indirect standardization). For female-specific cancers, calculations were done using nineteen age categories among female subjects only. Prophylactic surgery was not considered in the analyses. Age-related penetrance of cancer was estimated using the Kaplan-Meier method. R 2.12.0 was used for additional analysis (16).
Genotype-phenotype correlation was performed using logistic regression, evaluating the association between mutation status/type and the corresponding clinical phenotypes. For evaluation of correlation between conservation and clinical phenotypes, conservation of bases was determined using PHYLOPS. For each base pair, a dichotomous classification for conservation was set up through classification of mammalian conservation scores at the median threshold. A similar logistic regression procedure was conducted, where substitution mutations (higher versus lower conserved bases) were analyzed with corresponding patient phenotypes, in order to determine whether the phenotypic profiles of these patients differed.
All statistical tests were two-sided, and p-values<0.05 were deemed significant.
Results
PTEN Mutation Spectra
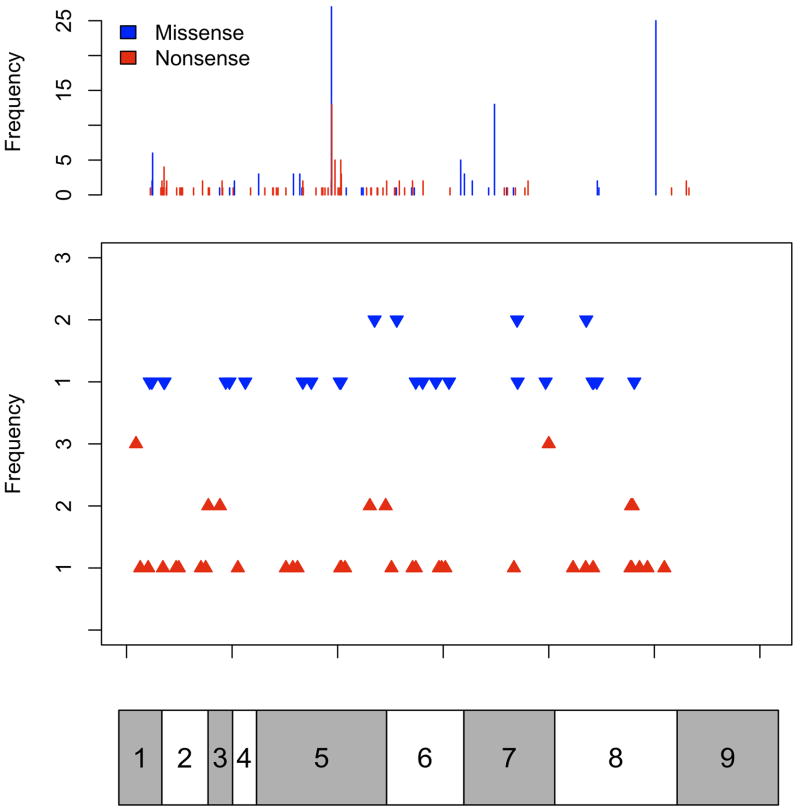
Of the 3,366 individuals tested, 295 probands (8.8%) were found to carry germline pathogenic PTEN mutations. An additional 73 individuals with germline PTEN mutations were identified following screening of the relatives of the probands. Baseline clinicopathologic information is presented (Table 1). The PTEN mutation spectra demonstrate clear hot-spots in exons 5, 7 and 8, corresponding to three truncation mutations R130X, R233X and R335X (Figure 1). All types of mutations, including insertions, deletions, indels, splice site mutations and large deletions were represented.
TABLE 1. Cohort baseline data for 368 research participants with germline deleterious PTEN mutations.
| Variable | Frequency counts (%) | |
|---|---|---|
| Gender (%) | Male | 163 (44) |
| Female | 205 (56) | |
|
| ||
| Age (Years) | Median | 39 |
| Range | 0.4 - 83 | |
|
| ||
| Pediatric Subjects (%) | <18 | 98 (27) |
|
| ||
| Proband Status (%) | 295 (80) | |
|
| ||
| Mutation Type (%) | Missense | 102 (28) |
| Nonsense | 109 (30) | |
| Small insertion | 33 (9) | |
| Small deletion | 47 (13) | |
| Small indel | 5 (1) | |
| Splice Junction | 35 (10) | |
| Promoter | 20 (5) | |
| Large Deletion | 17 (5) | |
Figure 1.
Consolidated PTEN mutation spectrum. Distribution and number of substitutions (missense and/or nonsense), small insertion mutations, and small deletion mutations across the gene. In the top panel, blue bars represent missense mutations and red bars represent nonsense mutations. In the second panel, the blue arrowheads represent small insertions and the red arrowheads represent small deletions along the gene. Complex mutations (indels, splice-site mutations, and large deletions) and promoter mutations are not depicted. For both panels, frequencies of both the substitution mutations and the insertion/deletion mutations are shown on the left. The bar below corresponds to the multiple exons of the PTEN cDNA molecule, with exon 1 on the left to exon 9 on the right, allowing for matching of mutation to exon.
Cancer Risks
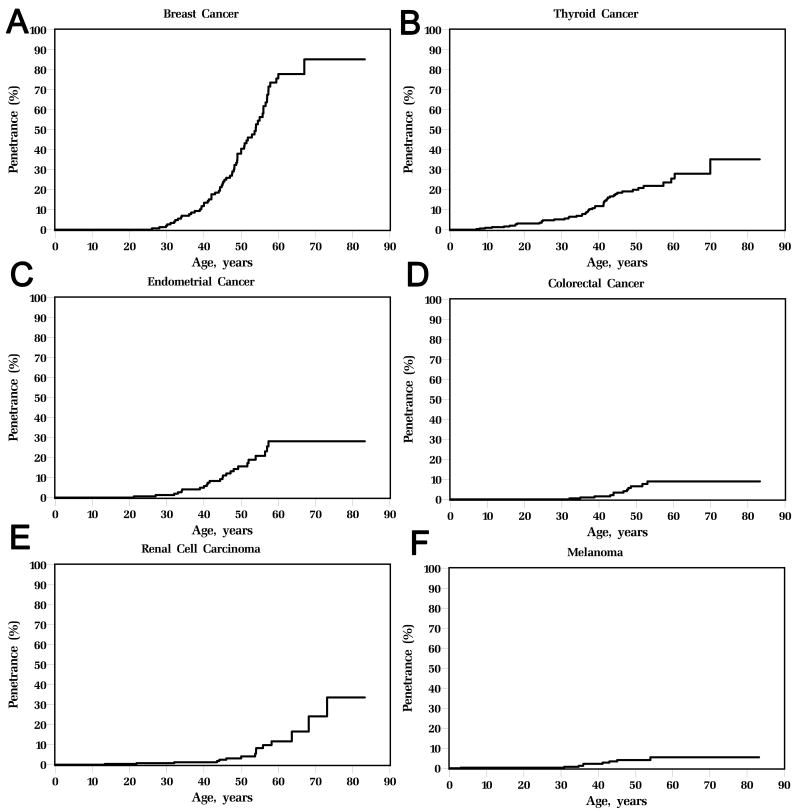
Elevated risks of breast (age-adjusted standardized incidence rate (SIR) 25.4, 95% C.I. 19.8-32.0), thyroid (SIR 51.1, 95%C.I. 38.1-67.1), endometrial (SIR 42.9, 95% C.I. 28.1-62.8), colorectal (SIR 10.3, 95%C.I. 5.6-17.4), and kidney cancers (SIR 30.6, 95% C.I. 17.8-49.4), and melanoma (SIR 8.5, 95% C.I. 4.1 – 15.6) were found (Table 2). Age-related penetrance estimates (Figure 2) reveal 85.2% (95% C.I. 71.4-99.1%) lifetime risk for invasive female breast cancer, 35.2% (19.7%-50.7%) for epithelial thyroid cancer, 28.2% (17.1-39.3%) for endometrial cancer, 9.0% (3.8-14.1%) for colorectal cancer and 33.6%(10.4–56.9%) for kidney cancer and 6% (1.6-9.4%) for melanoma. The particularly elevated penetrance of breast cancer in females with PTEN mutations is noted, beginning around age 30 and rising to an estimated 85% lifetime risk. PTEN-related endometrial cancer-risk begins at age 25 rising to 30% by age 60, whereas for thyroid cancer, risk begins at birth and continues lifelong (Figure 2). Risks of colorectal and kidney cancers begin around age 40, with a lifetime risk of 9% and 34% respectively. For melanoma, the earliest reported age of onset was 3 years.
TABLE 2. Standardized Incidence Rates of Cancer in the Patient Population.
| Cancer | Number of Cancers | SIR | 95% C.I. | P value | |
|---|---|---|---|---|---|
| Observed | Expected | ||||
| Breast* | 67 | 2.64 | 25.4 | 19.8 – 32.0 | < 0.001 |
| Thyroid | 48 | 0.94 | 51.1 | 38.1 – 67.1 | < 0.001 |
| Endometrium* | 24 | 0.56 | 42.9 | 28.1 – 62.8 | < 0.001 |
| Colorectal | 12 | 1.17 | 10.3 | 5.6 – 17.4 | < 0.001 |
| Kidney | 15 | 0.49 | 30.6 | 17.8 – 49.4 | < 0.001 |
| Melanoma | 9 | 1.06 | 8.5 | 4.1 – 15.6 | < 0.001 |
Female Subjects Only
Figure 2.
Age-related penetrance curves for (A) female breast cancer; (B) thyroid cancer; (C) endometrial cancer; (D) colorectal cancer; (E) kidney cancer; and (F) melanoma. The highest age-related penetrance is observed in female breast cancer, with an estimated 85% lifetime risk.
Genotype-Phenotype Correlation
We analyzed genotype-phenotype associations, finding significant correlations between promoter mutations and breast cancer and between nonsense mutations and colorectal cancer (Table 3). No correlation between any cancer risk and mutations within the catalytic core motif of the N-terminal phosphatase domain (aa 123-131) were noted (data not shown), nor was any correlation between mutations upstream and within the phosphatase core motif and involvement of all major organ systems (central nervous system, thyroid, breast, skin and gastrointestinal tract) found. Analysis by conservation of bases (more versus less conserved bases) did not yield any correlation with cancer incidence.
TABLE 3. PTEN mutation spectra and corresponding cancers.
| Mutation Type | Nonsense | Missense | Deletion | InDel | Insertion | Splice Junction | Large Deletion | Promoter | |
|---|---|---|---|---|---|---|---|---|---|
| Female Breast Cancer | Cases | 21 | 19 | 7 | 0 | 5 | 3 | 3 | 9 |
| % Case Total | 31.34 | 28.36 | 10.45 | 0 | 7.46 | 4.48 | 4.48 | 13.43 | |
| Odds Ratio | 1.11 | 1.05 | 0.76 | 0 | 0.78 | 0.39 | 0.95 | 4.04 | |
| P value | 0.77 | 0.88 | 0.69 | 0.59 | 0.81 | 0.17 | 1 | <0.01 | |
| Endometrial Cancer | Cases | 7 | 8 | 2 | 0 | 5 | 1 | 0 | 1 |
| % Case Total | 29.17 | 33.33 | 8.33 | 0 | 20.83 | 4.17 | 0 | 4.17 | |
| Odds Ratio | 0.98 | 1.34 | 0.6 | 0 | 2.94 | 0.39 | 0 | 0.74 | |
| P value | 1 | 0.75 | 1 | 0.05 | 0.72 | 0.62 | 1 | ||
| Thyroid Cancer | Cases | 14 | 13 | 8 | 1 | 4 | 3 | 1 | 4 |
| % Case Total | 29.17 | 27.08 | 16.67 | 2.08 | 8.33 | 6.25 | 2.08 | 8.33 | |
| Odds Ratio | 0.98 | 0.97 | 1.43 | 1.67 | 0.91 | 0.6 | 0.4 | 1.71 | |
| P value | 1 | 1 | 0.36 | 0.51 | 1 | 0.6 | 0.71 | 0.31 | |
| Kidney Cancer | Cases | 5 | 3 | 3 | 0 | 1 | 1 | 1 | 1 |
| % Case Total | 33.33 | 20 | 20 | 0 | 6.67 | 6.67 | 6.67 | 6.67 | |
| Odds Ratio | 1.2 | 0.65 | 1.74 | 0 | 0.71 | 0.67 | 1.49 | 1.25 | |
| P value | 0.77 | 0.77 | 0.42 | 1 | 1 | 1 | 0.52 | 0.58 | |
| Colorectal Cancer | Cases | 6 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| % Case Total | 66.67 | 11.11 | 11.11 | 0 | 0 | 11.11 | 0 | 0 | |
| Odds Ratio | 4.97 | 0.32 | 0.85 | 0 | 0 | 1.19 | 0 | 0 | |
| P value | 0.02 | 0.45 | 1 | 1 | 1 | 0.6 | 1 | 1 |
Discussion
We have reported elevated risks of a protean variety of solid tumors in patients with germline PTEN mutations, testimony to the key role of the PTEN tumor suppressor in regulating cell proliferation in a wide range of tissues (4). Multiple mechanisms have been identified to underpin this effect, chief among which is the concept of reduced PTEN protein dose (17). The effect of reduced PTEN protein dose on cancer susceptibility has been demonstrated both in animal models (18) and recently, in humans (9).
Cancer Risks
Our study highlights that three additional cancers (colorectal, kidney and melanoma) should be considered as members of the cancer spectra arising from germline mutations of PTEN. Our results also yield new insights on the classic features of breast, endometrial and thyroid cancers, where a much higher estimated lifetime risk of female breast cancer (85%) is reported relative to the traditional estimates of 25-50% that were previously used for clinical risk discussion and counseling (4). Individuals with promoter mutations are at particular risk. Strikingly, this risk is even higher than the best estimates for individuals with BRCA1 or BRCA2 mutations (19). Previously, endometrial cancer was noted while performing a genotype-phenotype analysis (20) and expert opinion believed that risk was only mildly elevated over that of the population (4% lifetime risk). Here, we show that endometrial cancer follows a similar age-of-onset as breast cancer with 28% lifetime risk. For thyroid cancer, the early onset of elevated risk from birth, which is sustained throughout life, is of key clinical interest especially for pediatric surveillance. The onset of colorectal cancer and renal cell carcinoma occurs at about age 40, with a lifetime risk of 9% and 34% respectively. In terms of the new additions of melanoma and kidney cancer to the cancer spectrum, several individual case reports have previously noted melanoma in patients with Cowden syndrome (21). This is of particular interest, given that there has been conflicting evidence in the somatic setting (as compared to the germline setting here) of the involvement of the PTEN signaling pathway in melanoma (22, 23). While the penetrance of melanoma is relatively low, ease in detection should mean that regular dermatologic surveillance is helpful for patients with PTEN mutations. For kidney cancer, while somatic PTEN mutations are relatively rare (24), reduced PTEN expression has been associated with renal carcinogenesis (25) and poorer prognosis (24, 26). The very high lifetime risk of kidney cancer (34%) in these PTEN mutation carriers, however, strongly supports the inclusion of PHTS as a hereditary RCC syndrome as well.
In terms of genotype-phenotype analysis, we demonstrated interesting genotype-phenotype associations between truncating mutations and colorectal cancer, as well as between promoter mutations and breast cancer. Given that these associations do not have absolute predictive value one way or the other, these should not directly inform counseling, at this time. Nonetheless, these associations would be of biologic interest. In an early study over ten years ago, we reported an exploratory association between mutations upstream and within the phosphatase core motif, and the involvement of 5 major organ systems (central nervous system, thyroid, breast, skin and gastrointestinal tract) versus 4 or fewer (20), recommending that this finding be validated in a larger number of patients. Following the prospective accrual of a much larger number of patients over ten years for this study, this association was no longer demonstrated, most likely due to the increasing number of organs and phenotypes that have been formally associated with PHTS in the intervening 12 years. For example, we found that >90% of mutation carriers have polyps (6) and >74% have macrocephaly(27), then it is almost certain that we would not find such an association.
Ascertainment bias is always a potential limitation when evaluating patients with rare syndromes. We have sought to minimize this through inclusion of asymptomatic family members with pathogenic PTEN mutations identified through screening.
Surveillance
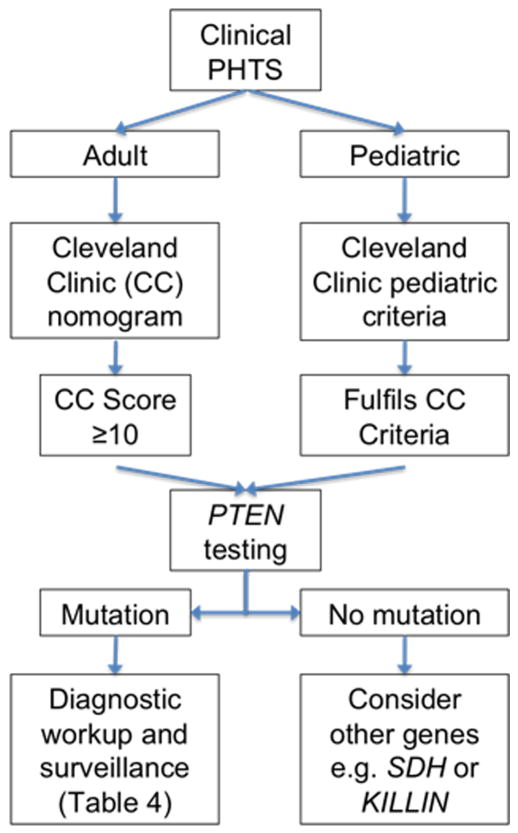
We aim to improve existing recommendations for surveillance on the basis of our prospective study. The NCCN recommendations for cancer surveillance are largely based on retrospective data accrued by the International Cowden Consortium (5), which we started 14 years ago. We present recommendations for management of patients with PTEN mutations (Figure 3, Table 4) supported by our analyses and extensive clinical experience from this prospective series of patients, by far the largest in the literature, all of whom have been clinically reviewed by a single author (C.E.). Our recommendations deviate from the current NCCN guidelines in several ways: (i) annual renal imaging is proposed based on the relatively high incidence of RCC; (ii) endometrial sampling as a routine surveillance procedure in our patients based on the high incidence of endometrial cancer; (iii) we are able to pinpoint a starting age for breast and endometrial screening; (iv) surveillance for colorectal cancers is now included based on accrued data showing an increase of colorectal cancers (6). It is true that none of these procedures have been demonstrated in randomized trials to prolong survival; it is however impractical, and some would consider unethical, to conduct such a procedure in patients with PTEN mutations. It should be noted that conclusive randomized data demonstrating the benefits of surveillance and prophylactic surgery in patients with BRCA1 and BRCA2 mutations took more than a decade to accrue (28). It should be noted that we are not recommending the use of specific mutation types to guide surveillance; while the genotype-phenotype analysis is very interesting, and may shed light on biologic correlations, its use to directly inform surveillance recommendations currently may be premature due to the relatively low specificity. Our current data will prove critical for informing new comprehensive surveillance recommendations, which should also take into account clinically significant but non-malignant features of PHTS, such as arteriovenous malformations and autism.
Figure 3.
Schematic showing a flowchart of recommendations for the evaluation, workup and screening for a patient with a potential PTEN mutation.
TABLE 4. Recommendations for Diagnostic Workup and Cancer Surveillance in Patients with PTEN mutations.
| Pediatric (<18 years) | Adult Male | Adult Female | |
|---|---|---|---|
| Baseline Workup | Targeted History and Physical Examination Baseline Thyroid Ultrasound Dermatologic Examination Formal neurologic and psychological testing |
Targeted History and Physical Examination Baseline Thyroid Ultrasound Dermatologic Examination |
Targeted History and Physical Examination Baseline Thyroid Ultrasound Dermatologic Examination |
| Cancer Surveillance | |||
| From diagnosis | Annual Thyroid Ultrasound and Skin Examination | Annual Thyroid Ultrasound and Skin Examination | Annual Thyroid Ultrasound and Skin Examination |
| From age 30* | As per adult recommendations | Annual mammogram (for consideration of breast MRI instead of mammography if dense breasts) Annual endometrial sampling or transvaginal ultrasound (or from 5 years before age of earliest endometrial cancer) |
|
| From age 40* | As per adult recommendations | Biannual colonoscopy** Biannual renal ultrasound / MRI |
Biannual colonoscopy** Biannual renal ultrasound / MRI |
| Prophylactic Surgery | Nil | Nil | Individual discussion of prophylactic mastectomy or hysterectomy. |
Surveillance may begin 5 years before the earliest onset of a specific cancer in the family, but not later than the recommended age cutoff.
The presence of multiple non-malignant polyps in patients with PTEN mutations may complicate non-invasive methods of colon evaluation. More frequent colonoscopy should be considered for patients with a heavy polyp burden.
Statement of Translational Relevance.
Germline mutation of PTEN underlies the PTEN Hamartoma-Tumor Syndrome (PHTS), which is manifested by increased lifetime risks of a wide variety of cancers. As PTEN plays a role in suppressing tumor growth in multiple tissues, the extent and magnitude of these risks are of interest, particularly since no prospective studies have been previously conducted. Here, we report estimated lifetime risks of PHTS patients for breast, colorectal, thyroid, endometrial, skin (melanoma) and kidney cancer from the only international prospective study accruing PHTS patients, noting that PTEN mutation is associated with an estimated lifetime breast cancer of 85%. Additionally, genotype-phenotype analysis demonstrates several associations, including an association between promoter mutation and breast cancer, allowing for potentially better understanding of PTEN structure and function. Our data here provide a basis for cancer risk assessment and counseling. We also suggest a comprehensive surveillance approach for these patients based on this collective experience.
Acknowledgments
We would like to thank the Genomic Medicine Biorepository of the Cleveland Clinic Genomic Medicine Institute, as well as members of the Eng lab, genetic counselor and research coordinators, and database managers over the last 14 years who have contributed technical assistance, technical advice and helpful discussions. We would also like to express our gratitude to all the patients and clinical collaborators from all the centers around the world who have contributed their time and specimens over the last ten years.
Financial Support: National Cancer Institute, Bethesda, MD (P01CA124570 and R01CA118989, to CE); American Cancer Society (RPG-02-151-01-CCE to CE); William Randolph Hearst Foundations and Doris Duke Distinguished Clinical Scientist Award (to CE). MHT is the Lee Foundation (Singapore) Fellow and an Ambrose Monell Foundation Cancer Genomic Medicine Clinical Fellow at the Cleveland Clinic Genomic Medicine Institute. JN is a SingHealth (Singapore) Fellow and an Ambrose Monell Foundation Cancer Genomic Medicine Clinical Fellow at the Cleveland Clinic Genomic Medicine Institute. CE is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic, and is the recipient of an American Cancer Society Clinical Research Professorship, generously funded, in part, by the F.M. Kirby Foundation.
Footnotes
Conflict of interest disclosure: The authors have no conflict of interest to declare
The authors assume full responsibility for analyses and interpretation of these data. No funders of the study had any involvement in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–9. [PubMed] [Google Scholar]
- 2.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorlin RJ, Cohen MM, Jr, Condon LM, Burke BA. Bannayan-Riley-Ruvalcaba syndrome. Am J Med Genet. 1992;44:307–14. doi: 10.1002/ajmg.1320440309. [DOI] [PubMed] [Google Scholar]
- 4.Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11:687–94. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- 5.Nelen MR, Padberg GW, Peeters EA, Lin AY, van den Helm B, Frants RR, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13:114–6. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- 6.Heald B, Mester J, Rybicki L, Orloff MS, Burke CA, Eng C. Frequent Gastrointestinal Polyps and Colorectal Adenocarcinomas in a Prospective Series of PTEN Mutation Carriers. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, et al. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3:137–41. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 8.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan MH, Mester J, Peterson C, Yang YR, Chen JL, Rybicki L, et al. A Clinical Scoring System for Selection of Patients for PTEN Mutation Testing is Proposed on the Basis of a Prospective Study of 3,042 Probands. Am J Hum Genet. 2011 doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–30. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet. 2004;41:323–6. doi: 10.1136/jmg.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Stoep N, van Paridon CD, Janssens T, Krenkova P, Stambergova A, Macek M, et al. Diagnostic guidelines for high-resolution melting curve (HRM) analysis: an interlaboratory validation of BRCA1 mutation scanning using the 96-well LightScanner. Hum Mutat. 2009;30:899–909. doi: 10.1002/humu.21004. [DOI] [PubMed] [Google Scholar]
- 13.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teresi RE, Zbuk KM, Pezzolesi MG, Waite KA, Eng C. Cowden syndrome-affected patients with PTEN promoter mutations demonstrate abnormal protein translation. Am J Hum Genet. 2007;81:756–67. doi: 10.1086/521051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2000. Stat Notes. 2001:1–9. [PubMed] [Google Scholar]
- 16.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 17.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, et al. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet. 2010;42:454–8. doi: 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalfe K, Lubinski J, Lynch HT, Ghadirian P, Foulkes WD, Kim-Sing C, et al. Family history of cancer and cancer risks in women with BRCA1 or BRCA2 mutations. J Natl Cancer Inst. 2010;102:1874–8. doi: 10.1093/jnci/djq443. [DOI] [PubMed] [Google Scholar]
- 20.Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7:507–15. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- 21.Greene SL, Thomas JR, 3rd, Doyle JA. Cowden's disease with associated malignant melanoma. Int J Dermatol. 1984;23:466–7. doi: 10.1111/ijd.1984.23.7.466. [DOI] [PubMed] [Google Scholar]
- 22.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, et al. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–32. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velickovic M, Delahunt B, McIver B, Grebe SK. Intragenic PTEN/MMAC1 loss of heterozygosity in conventional (clear-cell) renal cell carcinoma is associated with poor patient prognosis. Mod Pathol. 2002;15:479–85. doi: 10.1038/modpathol.3880551. [DOI] [PubMed] [Google Scholar]
- 25.Brenner W, Farber G, Herget T, Lehr HA, Hengstler JG, Thuroff JW. Loss of tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer. 2002;99:53–7. doi: 10.1002/ijc.10303. [DOI] [PubMed] [Google Scholar]
- 26.Shin Lee J, Seok Kim H, Bok Kim Y, Cheol Lee M, Soo Park C. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol. 2003;84:166–72. doi: 10.1002/jso.10302. [DOI] [PubMed] [Google Scholar]
- 27.Mester JL, Tilot AK, Rybicki LA, Frazier TW, 2nd, Eng C. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet. 2011;19:763–8. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–75. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]