Summary
Overwhelming evidence suggests that the JNKs are a set of key stress responsive kinases that mediate cell apoptosis, which is an important process for tumor suppression. However, the JNKs have also been implicated in the malignant transformation and tumorigenesis of cells. This review attempts to reconcile these two contradictory functions of the JNKs with the recent discoveries on the role of the JNKs in the compensatory growth of the neighboring cells and stem cells, which may provide new mechanistic understanding on the role of the JNKs in the regulation of cancer stem cells and the pathogenesis of cancers.
In the past few years, a new concept in tumorigenesis, the cancer stem cell (CSC), has emerged (1). It is believed that CSCs are a population of rare cells that are capable of initiating and maintaining the tumor, differentiating into endothelial cells for tumor vascularization and allowing the propagation and colonization of the tumor cells at sites distant from the original tumor location. Similar to normal stem cells, CSCs retain the properties of self-renewal and multi-lineage differentiation. However, these cells distinguish themselves from normal stem cells by maintaining their malignant potentials such as a loss of both the genomic integrity and epigenetic identity of the normal stem cells. An unsolved issue in CSC theory is whether CSCs are truly stem cells or if they are non-stem cells in which the self-renewal is activated by oncogenic mechanisms.
C-Jun N-terminal kinases (JNKs) are protein kinases involved in the cellular stress response, apoptosis and malignant transformation (2–4). They regulate a wide spectrum of intracellular signaling pathways that converge to regulate both gene expression and the homeostasis of macromolecules including mRNAs and proteins (5). In the human genome, three genetic loci encode JNK1, JNK2 and JNK3, each of which has 2 to 4 isoforms that result from the alternative splicing of the corresponding pre-mRNAs. Both JNK1 and JNK2 are ubiquitously expressed, while JNK3 is expressed predominantly in the brain and to a lesser extent in the heart and testis (2, 4). The JNKs have a well-documented functional redundancy to phosphorylate their cognate and non-cognate substrates, which include c-Jun, JunD, ATF2, PRC1 subunit Bmi1 (6), Akt (7) FoxO4, PPARγ1, c-Myc, p53, NFATc2, STATs (8), IRS-1, Itch, 14-3-3, histone H3 (9), SIRT1 (10), and other proteins (5). However, there is also evidence implying that JNK1, rather than JNK2 or JNK3, is the key JNK family kinase responsible for the phosphorylation of c-Jun on serines 63 and 73 and for the expression of RNA polymerase III (11, 12). In myoblast cells, JNK1, but not JNK2, mediates TNFα-induced cell proliferation by inhibiting myoblast cell differentiation and promoting the generation of the inflammatory cytokines such as IL-6 and LIF (13). In addition, the importance of JNK1 over JNK2 had been demonstrated in the pathogenesis of several human diseases including diabetes, lung fibrosis, and cancer (14). Furthermore, gene knockout studies in mice revealed that JNK1 is the most important JNK family kinase for the proliferation of the CD8+ T cells (15) and for neural development (16, 17).
JNK1 and JNK2 in carcinogenesis
Although the JNKs are primarily attributed to pro-apoptotic cell death or tumor suppression in response to a variety of stress, inflammatory or oncogenic signals (18), emerging evidence suggests that the JNKs, especially JNK1, play a role in the malignant transformation of cells and in tumorigenesis. For example, the genetic disruption of the jnk1 locus in mice decreased the susceptibility to a Bcr-abl-induced lymphoma (19). In UV-induced tumorigenesis, activation of JNK1 is essential for the cell transformation and proliferation in response to the oncogenic Ras signal (20). In cells derived from the soft tissue of a childhood sarcoma, silencing of JNK1 but not of JNK2 by siRNA repressed the growth of these tumor cells, indicating that JNK1 is pro-proliferative, while JNK2 might be pro-apoptotic (21, 22). JNK1 has been viewed as a pivotal kinase that promotes the development of tobacco smoke-induced lung tumors because the ablation of JNK1 alone reduced the effect of tobacco smoke on both the lung tumor multiplicity and the tumor size (23). Animal models of gastric cancer also showed that JNK1 contributes to the development of gastric tumors that are induced by the chemical carcinogen N-methyl-N-nitrosourea (24). The most compelling evidence for the role of JNK1 in cancer initiation is from studies of hepatocellular carcinoma (HCC) in both human and animal models. By using human HCC tissue samples that were case-matched with the adjacent non-cancerous liver tissues, two independent studies found that more than 50% of the HCC samples exhibited a higher activation of JNK1 but not of JNK2 (25, 26). Additional studies further demonstrated that higher JNK1 activation was associated both with a poorer prognosis of the patients and with the overexpression of several hepatic stem cell or progenitor cell markers, such as EpCAM, CD24, CD133, KRT19, and AFP (27). In mouse HCC models, genetic disruption of the jnk1 locus substantially reduced the number and size of HCCs that were induced by diethylnitrosamine (DEN) (25). JNK1 has also been shown to be an essential kinase for mediating the development of HCC due to a hepatocyte-specific deficiency of IKKβ or IKKγ, which are the key subunits of the IKK kinase complex for NF-κB signaling in mice (28–30).
JNK-induced compensatory proliferation links apoptosis to carcinogenesis
Overwhelming evidence has unequivocally unraveled the role of the JNKs, especially JNK1, in cell apoptosis or tumor suppression (31–33). The pro-apoptotic or tumor suppressor-like function of JNK1 was revealed even in studies that showed an oncogenic effect of the sustained JNK1 activation in animal cancer models (25, 28). How can we reconcile these two contradictory functions of JNK1? A growing consensus is that the evasion of apoptosis is one of the hallmarks of cancer (34, 35). Accordingly, it is tempting to attribute this defective in apoptosis to the oncogenic role of the JNKs, despite reports suggesting that the major apoptotic signaling pathways, CD95 (Fas) and CD95 (FasL), are required for the optimal growth of ovarian cancer, liver cancer and glioblastoma in animal models (36–38). In addition to the possibility that the JNKs can directly induce growth signals at the same time of inducing apoptosis, it is also possible that a compensatory proliferation of the neighboring cells might be triggered by the apoptotic, stressed cells. In other words, the compensatory growth might be an essential linker to bridge apoptosis and carcinogenesis.
a. JNK-induced compensatory growth in Drosophila
How JNK1-mediated cell death triggers the compensatory proliferation of neighboring cells is not fully understood. The key evidence for the compensatory proliferation induced by JNK-activated cell death is from studies in Drosophila (39, 40). After apoptosis was initiated by disrupting the anti-apoptotic signal from diap1 (XIAP in mammals) and the activation of the effector caspase was blunted to create “undead” cells in the Drosophila larval imaginal discs, an overgrowth of the neighboring, normal cells was observed (39). Biochemical studies found that these undead cells were able to secrete wg and dpp mitogens (the Wnt and BMP orthologues of mammalian cells, respectively) in a JNK-dependent manner.
The Wnt and BMP proteins have long been viewed as key signaling proteins involved in embryonic development, cell proliferation, oncogenesis, and stem cell maintenance (41). Thus, it is very likely that wg (Wnt) and dpp (BMP), the secreted glycoproteins from the stressed cells in which JNK is activated, are the master regulators for the JNK-induced compensatory proliferation of the neighboring cells. The affected neighboring cells can be either the same lineage as the stressed cells or a different lineage. The degree of the compensatory proliferation might be dictated by both the intrinsic Wnt- and BMP-responding pathways and the differentiation states of the affected cells. A number of reports have suggested that Wnt and BMP stimulate cell growth and tissue regeneration in vertebrates and insects by cooperating with or inducing the JAK/Start and/or the β-catenin/TCF signaling pathways (42–44). Additionally, wg signaling is capable of repressing Notch activity, which leads to the expression of dmyc and the microRNA bantam, which both promote cell growth by affecting the G1-S phase transition of the cell cycle (45).
In addition to wg and dpp, the JNK-dependent activation or induction of the JAK/STAT pathway might also be involved in the undead-cell- or tissue-damage-induced compensatory proliferation of normal cells in Drosophila (46, 47), which could explain the compensatory growth in Drosophila with the loss-of-function mutations of wg, dpp, or both wg and dpp (48). Unlike its mammalian counterparts that contain multiple isoforms of all of the major JAK/STAT pathway components, the Drosophila genome encodes only one JAK (HOP) and one STAT (STAT92E) molecule (49). The evidence suggesting that the constitutive activation of JAK/STAT signaling causes cancer has long been established in both human and Drosophila. A gain-of-function mutation of the Drosophila HOP (JAK) protein resulted in the over proliferation of the larval blood cells and subsequent melanotic tumors (50). In the midgut of Drosophila, a tissue injury induced by bleomycin activates JNK, which in turn induces a rapid translocation of the Yorkie (Yki, the mammalian Yap homologue) protein from the cytoplasm to the nucleus. As a co-factor for transcriptional regulation, the nuclear translocated Yki is capable of up-regulating the expression of the Unpaired family of cytokines (Upd, Upd2 and Upd3, the IL-6 orthologues of mammalian cells) and the activation of JAK/STAT signaling (51). In resting cells, Yki is predominantly localized in the cytoplasm due to its phosphorylation by the tumor suppressor Hippo (Hpo)/Wts. It is unclear how JNK impinges upon Hpo/Wts to activate Yki. One of the potential mechanisms might be that JNK directly phosphorylates and inactivates Hpo or Wts. However, it is also possible that JNK may phosphorylate Yki to antagonize the phosphorylation and inactivation of Yki by Hpo/Wts. In apoptotic conditions, JNK-dependent activation of Yki and the consequent release of the Upd cytokines from the stressed cells are pivotal factors for the compensatory overgrowth of the non-apoptotic compartment (52).
Both the wg/dpp and JAK/STAT signaling pathways are essential factors for the self-renewal of intestinal stem cells (ISCs) in the midgut of Drosophila (42, 51, 53–55). This finding raises an interesting question about whether the compensatory growth is a result of the over proliferation of the stem cells to replenish the damaged cells in response to stress or tissue injury. It is well-recognized that adult stem cells are responsible for replenishing the dead cells to maintain the homeostasis of the normal tissues. Earlier studies showed a contribution of JNK activation in the intestinal absorptive enterocytes (ECs) to the compensatory division and/or differentiation of ISCs in circumstances such as infection, chemical or mechanical damage (56, 57). Through asymmetrical division, Drosophila ISCs give rise to an ISC and an enteroblast (EB) cell, which can then further differentiate into two major types of intestinal epithelial cells: ECs and secretory enteroendocrine cells (EEs). The activation of JNK in ECs by silencing of the JNK suppressor, puckered (puc), or expression of the active form of hemipterous (Hep, Drosophila JNK kinase, DJNKK) resulted in a substantial increase in both the Upd cytokines and in the number of ISCs (51, 56). It is believed that upon the JNK activation in the ECs, the released Upd cytokines engaged with the IL-6R-type receptor, Domeless (dome), on the surface of ISCs, which led to the activation of the JAK/STAT signaling in ISCs followed by a dramatic increase in the mitotic index of the ISCs. Similarly, paracrine wg from the circular muscles next to the ISCs had been implicated as an external niche signal that is important for the self-renewal of ISCs (54). In addition to the paracrine role of wg/dpp and the Upd cytokines from the stressed ECs that is induced by the JNK activity on ISCs, JNK activity within the ISCs themselves appeared to be critical for the ISC proliferation when the Drosophila were challenged with paraquat or bleomycin (58). In this scenario, JNK- and ERK-dependent phosphorylation of the FOS protein within the ISCs is sufficient to promote the stress-induced ISC proliferation, which may occur through the AP-1 dependent transcriptional regulation of several genes that drive the cell cycle transition.
b. JNK-induced compensatory growth in animal disease models
Whereas the majority of studies on JNK-regulated compensatory proliferation were performed in Drosophila, there are reports suggesting that JNK is a key contributor to the compensatory proliferation of the hepatocytes in a mouse HCC model with an IKKβ deficiency (28, 30). Mice with a hepatocyte specific disruption of the IKKβ gene exhibited a substantial increase in cell apoptosis, reactive oxygen species production and JNK activation in the hepatocytes in response to DEN treatment. Meanwhile, these mice also showed a marked enhancement in the hepatocyte proliferation and carcinogenesis induced by DEN. Such effects were prevented in the progenies from the cross breeding of these mice with JNK1 knockout mice, suggesting that the JNKs, especially JNK1, play a central role in the hepatocyte apoptosis and in the compensatory proliferation of the non-apoptotic cells. Similar findings were observed in murine liver tumor models with a hepatocyte-specific IKKγ/NEMO or TAK1 deficiency (29, 59). It was originally hypothesized that this compensatory proliferation was induced by the growth factors released from the Kupffer cells. Alternatively, it is possible that mitogens released from the apoptotic hepatocytes in which JNK is activated induce the compensatory proliferation of the non-apoptotic hepatocytes.
A potential role of JNK in cancer stem cells
As links were revealed between the JNK activation and wg/dpp or JAK/STAT signaling in the tissue damage- or stress-induced compensatory proliferation, it is plausible to hypothesize that some human cancers are formed as a result of the compensatory overgrowth of stem cells (Fig. 1). Either wg/dpp or JAK/STAT, which are both regulated by JNK activation, can provide a suitable niche for the dynamic proliferation of stem cells. Sustained activation of JNK will cause the aberrant generation of the wg/dpp and JAK/STAT signals, which will be potentially dangerous for either the over-compensatory proliferation of tissue stem cells or, alternatively, for the oncogenic transformation of stem cells. It is also possible that a prolonged activation of such signals in non-stem cells might cause trans-differentiation of these cells into cancer stem cells. Although the specific contributions of JNK, Yap (Yki in Drosophila), Wnt/BMP (wg/dpp in Drosophila), and JAK/STAT signaling to stem cell overgrowth and cancer in vertebrates remains to be established, we expect that similar signaling pathways and their regulatory effects on stem cells might be involved in the development of murine or human cancers. Indeed, the augmentation of the JNK signaling via the transgenic gut-specific expression of constitutively active JNK1 in mice significantly increases the ISC proliferation and villus length (60). Remarkably, there appears to be a convergence between JNK signaling and Wnt signaling in which the activation of JNK not only induces the expression of c-Jun, cyclinD1 and CD44, the classic JNK target genes (Fig. 1), but also up-regulates the mRNAs of some of the Wnt target genes, including tcf4, axin2 and lgr5 in crypt base columnar (CBC) cells, a group of intestinal cells with stem cell-like properties (61). In the case of the JNK dependent expression of lgr5, a CSC marker of colon cancer, it was suggested that the phosphorylation of c-Jun by JNK prevents c-Jun from recruiting the Mbd3/NuRD transcription repressor complex at the promoter region of the lgr5 gene (62). Furthermore, in a mouse Apc mutation model, JNK activation not only was associated with enhanced Wnt signaling from the loss of Apc, but it also promoted mTORC1 activation, which led to a translational upregulation of the proteins necessary for intestinal tumorigenesis (63). Thus, these data clearly indicate that JNK signaling, compensatory overgrowth and stem cell proliferation are shared mechanisms for tumorigenesis between invertebrates and vertebrates.
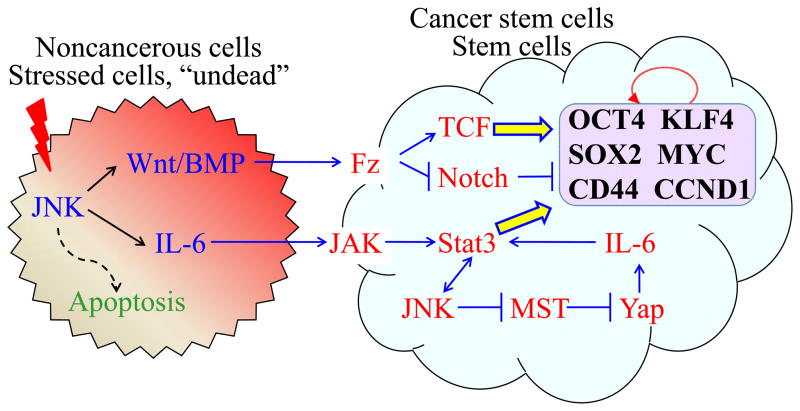
Fig. 1.
JNK signaling enhances the compensatory proliferation of the neighboring cells, stem cells or cancer stem cells (CSCs). In response to stress signals, activated JNK induces the release of Wnt/BMP and IL-6 from the stressed cells in which an apoptotic response might be initiated but not yet completed, thus inducing a state of “undead” cells. The released Wnt/BMP and IL-6 interact with Fz and JAK complexes, respectively, on the surface of the neighboring cells, stem cells or CSCs, which is followed by the activations of the β-catenin/TCF and Stat3 signaling pathways in these cells. Both β-catenin/TCF and Stat3 are capable of enhancing the expression of the genes such as CCND1, OCT4, Sox2, KLF4, c-Myc, CD44, and others that are important for the cell proliferation and self-renewal of the stem cells or CSCs. There is a reciprocal positive feedback between Stat3 and JNK signaling in the non-stressed neighboring cells or stem cells. Alternatively, JNK can affect Stat3 through the suppression of Hpo/Wts (MST/LATS in mammals) to alleviate Yki (YAP in mammals) that can induce Stat3 through IL-6 signaling. Similarly, in addition to the regulation of the β-catenin/TCF pathway, the Wnt signaling can regulate the cell growth of stem cells by suppressing Notch, a repressor of c-Myc and other cell cycle genes. Circled arrow indicates a group of genes important for the self-renewal of the stem cells or CSCs.
Several lines of evidence also support the notion that JNK and its regulation of Wnt and JAK/STAT signaling are critical for cancer development in mammals, although the stem cell hypothesis in this JNK-mediated process has not been tested directly. First, a number of human cancers exhibit enhanced activation and/or increased expression of JNK, Yap, IL-6, STAT3, Wnt, or TGFβ (2, 64). Second, the JNK1-dependent compensatory proliferation has been viewed as a key mechanism in the mouse model of HCC with a hepatocyte-specific deletion of the IKKβ or IKKγ gene (28, 29). Lastly, both the STAT3 and Wnt signaling pathways have been viewed as important regulators for maintaining the self-renewal of CSCs in some experimental cancer models (65, 66). Both Wnt and BMP, the downstream targets of JNK signaling, have been shown to be important for the self-renewal of many stem cells including embryonic stem cells (ESCs), lineage specific stem cells, or cancer stem cells (67–70). The transgenic overexpression of wnt1 in mice induces a mammary tumorigenesis with an increased number of cancer stem cells (71). In a cell culture model, the addition of the exogenous Wnt protein is sufficient for the expansion of mammary stem cells for many generations (72). The importance of Wnt signaling in mouse or human ESCs also provided complementary support for the potential of JNK and Wnt in cancer stem cells. Wnt signaling is believed to maintain the self-renewal of the stem cells by cooperating with or enhancing the function of several stem cell transcription factors such as Oct3/4, Sox 2 and Nanog (73). In contrast, BMP signaling induces the differentiation of the human ESCs by limiting the activity of Nanog (74).
The notion that JNKs might be involved in the regulation of CSCs in human cancer is reinforced by findings indicating an association between JNK or IL-6 and the CSC markers in human HCC (27, 75, 76). Accumulating evidence suggests that the most common etiological factors in HCC are the chronic inflammation of the liver due to HBV or HCV infection or the exposure to environmental carcinogens. IL-6, the key inflammatory cytokine, had been viewed as a central molecular linker between chronic liver inflammation and HCC. Clinical data clearly show an elevated blood IL-6 level in male HCC patients (77). Animal studies using IL-6 knockout mice demonstrated a nearly complete inhibition of HCC development in mice treated with DEN (78). Positive feedback between JNK and IL-6 has been observed in an obesity-induced HCC model (79). As a preferential activator of STAT3 signaling, IL-6 is capable of inducing the expression of the JAK-STAT3 target genes such as VEGF, Bcl-xl, cyclin D1, MMP, and others for the sustained proliferation of hepatocytes and hepatic CSCs (Fig. 1) (76, 80). Notably, genes downstream of IL-6 were enriched in the surrounding noncancerous liver tissue of the HCC patients with the poorest survival rates (81), which might indicate a compensatory proliferation of HCC cells or CSCs induced by IL-6 from adjacent tissues with chronic inflammation.
A potential link between JNK1 and HCC progenitor cells or CSCs was revealed in a gene profiling study based on collected human HCC tissues that were stratified by their JNK1 activation levels (26, 27). The genes with signatures corresponding to both poor HCC prognosis and hepatoblastoma, an embryonic liver tumor that features liver progenitor cells or CSCs, were enriched in HCCs that had higher JNK1 activation (27). A re-analysis of the gene profiling data in previous studies (26, 27) indicates that many important genes for the CSCs are highly expressed in the HCCs with higher JNK1 levels including CD24, CD44, CD133, Stat3, GPC3, EpCAM, KRT19, KRT7, SOX4, Tet1, Runx1, Runx2, Wdr, Seme6A, JARID1a, and JARID1b. These data clearly suggest an important role for the JNK1 regulation of HCC progenitor cells or CSCs.
JNK activation in the stemness of embryonic stem cells
The data derived from studies in Drosophila and some human cancers indicate that JNK might be a regulator of stem cells or CSCs. The embryonic lethality of JNK1 and JNK2 double-knockout mice suggests that JNK kinases are essential for embryonic development (16). Because of the central role of embryonic stem cells (ESCs) in the development of the embryo, an important concept to examine is whether JNKs play a role in the establishment, maintenance and differentiation of ESCs. Several protein kinase pathways had been implicated as pivotal regulators for the self-renewal, proliferation or differentiation of the ESCs, including PI3K (82), receptor and non-receptor tyrosine kinases (83), and others. However, to date, a systematic study of the role of JNKs in certain aspects of ESCs such as self-renewal, maintenance of stem cell totipotency and differentiation has not been performed. Uncertainties and controversies remain regarding whether JNKs are required for the proliferation or differentiation of mouse ESCs (mESCs). In testing the toxicity of the carcinogenic metal chromium [Cr(VI)], Xia and associates demonstrated that JNKs protect mESCs from Cr(VI)-induced cytotoxicity and suppress the differentiation of mESCs or the derived embryonic bodies (EBs) (84). Similarly, JNK signaling appears to be important for the proliferation of mESCs by collaborating with the Akt-mTOR pathway in response to zinc stimulation (85). Furthermore, in a recent study to determine how an essential amino acid, L-threonine, regulates mESCs, Ryu and Han showed that JNK is one of the key kinases necessary for the self-renewal and proliferation of mESCs (86). The addition of the JNK inhibitor, SP600125, blocked the L-threonine-induced expression of the stem cell marker OCT4 and several cell proliferative molecules such as cyclin D1, cyclin E and c-Myc (86).
In contrast, an earlier study that investigated the neurogenesis of JNK1-deficient mESCs had found that a deficiency of either JNK1 or JNK2 had no effect on the expression of the mESC markers or the self-renewal of the mESCs (87). However, JNK1 deficiency clearly impaired the neural differentiation of mESCs because JNK1 was required for the transcriptional expression of a neural-specific gene, the neurofilament light chain, in response to nerve growth factor. That study also suggested that JNK1 might facilitate mESC differentiation by inhibiting Wnt-4 and Wnt-6, which are two key Wnt signaling molecules in vertebrates. The concept that the JNKs are involved in the differentiation of mESCs was supported by another study showing that the JNKs are required for lineage specific differentiation but are dispensable for the self-renewal of mESCs (88). These results appear to contradict what had been found in the intestinal cells of Drosophila and of the mouse (39, 60).
Unlike what has been found in mESCs, the potential role of JNKs in the self-renewal of human ESCs (hESCs) appears to be straightforward (89–91). Interrogation of the phosphoproteomes of the hESC line, WiCell’s H1, by identifying phosphorylated peptides via multidimensional liquid chromatography-based mass spectrometry (LC-MS), Ding and colleagues observed significantly elevated JNK activity in undifferentiated hESCs (89). This observation was further supported by the treatment of undifferentiated hESCs with the JNK inhibitor SP600125 or with a JNK inhibitor III polypeptide. Inhibition of JNK by either SP600125 or JNK inhibitor III caused the differentiation of the hESCs and the substantially reduced expression of NANOG and OCT4, which are two important markers of undifferentiated hESCs. The possible contribution of JNK signaling to the maintenance and/or self-renewal of hESCs was additionally confirmed in a different hESC line, Harvard’s HUES-7, by the stable isotope labeling of amino acids in cell culture (SILAC) combined with LC-MS/MS (91). JNK1 activity and the activity of other kinases including CDK1/2 and MAPK14 (p38α) was overrepresented in hESCs. In response to the BMP-induced differentiation, a transient elevation of c-Jun phosphorylation was observed, which indicated both the competence of the basal JNK pathway to maintain the stemness of the hESCs and the possible involvement of JNK activation in the initiation of hESC differentiation. Furthermore, as determined by electron transfer dissociation-based large scale tandem mass spectrometry, the MAPK pathway appears to be the one of the top three signaling pathways in another hESC line, although it was not defined which MAPK pathway among the ERK, JNK or p38 pathways were activated (92).
The comprehensive analyses of the hESC transcriptome provided corroborating evidence for the role of JNK signaling in the self-renewal and/or pluripotency of hESCs (93, 94). Both jun and fos, two JNK target genes, have been found to be signature genes in several tested hESC lines (95). Additionally, analysis of the gene expression dynamics of the hESCs demonstrated that the expression of some of the JNK signaling molecules was significantly higher in the undifferentiated hESCs than in the differentiated hESCs (94). These JNK signaling molecules include the JNK target gene Jun and two upstream kinases of JNK: MAP4K1 (MEKKK1) and MAP3K7 (TAK1). Both MAP4K1 and MAP3K7 are preferential upstream kinases for the activation of JNK (96, 97). Differentiation of the hESCs by removal of both the feeder cells and bFGF resulted in the down-regulation of these JNK signaling molecules (94).
In accordance with these observations, recent genome-wide RNAi screening in hESCs showed that the genes of several of the JNK signaling molecules, such as MEKK3, MEKK4, MEKK8, JNK3, and Fos, contain binding sites in their promoter or enhancer regions for PRDM14, which is a stem-cell-specific transcription factor (98). ChIP-seq analysis showed that there is direct binding of PRDM14 to the regulatory regions of these genes. PRDM14 not only up-regulates the expression of Fos but also inhibits DUSP10 and DUSP12, the negative regulators of JNK signaling. In mESCs, PRDM14 overexpression can enhance the activity of NANOG to prevent the mESC differentiation of the extraembryonic endoderm (99), which provided complementary evidence indicating a possible role for JNK signaling in the maintenance of the ESCs.
JNKs in adult stem cells
While the function of JNKs in the proliferation and/or self-renewal of hESCs is noteworthy, it is also of interest to investigate the role of JNKs on the proliferation of human adult stem cells, such as adipose-derived stem cells (ASC) (100) and mesenchymal stem cells (MSCs) (101). There is evidence indicating that JNK activation is essential for the injury-induced proliferation of the ASCs and the release of several angiogenic factors and growth factors such as PDGF, VEGF and HGF. The inhibition of JNK activity using a chemical JNK inhibitor not only repressed the release of those growth factors but also reduced the number of the cells harboring the stem cell marker CD34 (100). MSCs can differentiate into mesenchymal lineage cells such as osteoblasts, chondrocytes, and adipocytes. MSCs have also been thought to be the progenitor cells for some human cancers. In an attempt to determine the contribution of MAP kinases to the growth factor FGF-induced MSC proliferation, studies by Ahn et al. showed that JNK but not ERK or p38 is critical for the proliferation of the MSCs in response to FGF (101).
Conclusion and perspective
Despite the mixed sentiments among researchers in the field, revealing the role and regulation of intracellular signaling pathways is undoubtedly the most important task in understanding how the capacity for both the self-renewal and multipotency of a given stem cell is maintained. It is known that epigenetic modifications, especially modifications of the histone proteins, determine the accessibility of the chromatin for the differentiation programs to produce divergent cell types. Accordingly, any signaling that occurs to maintain the stemness of a cell must be achieved by the epigenetic activation of the stemness programs and the termination of the differentiation programs. In addition to the JNK-regulated signaling pathways discussed above, the JNKs have also been implicated in the phosphorylation of histone H3 serine 10 and serine 28 (102), which affects the binding of the Trithorax (TRX) and Polycomb repressive complex 2 (PRC2) to the chromatin and, thus, the propagation of active and silent chromatin, respectively. Furthermore, the JNKs or JNK signaling molecules have been implicated in the antagonizing of the PRC complexes formation of a permissive chromatin structure on some of the genes that are involved in cell growth and lineage development (6, 103). Important issues concerning the mechanism by which the JNKs affect the balance between the stability and plasticity of stem cells must now be addressed. A critical question is whether JNKs are essential kinases for the multipotency of stem cells or whether the kinases are required for the earlier differentiation of stem cells. It might be overreaching to claim that the JNKs are the central kinases for the key properties of stem cells. However, it would be fair to state that the JNKs are critical kinases in concert with other key signaling molecules or transcription factors that govern the development and fate of stem cells and CSCs.
The achievement of effective cancer treatments remains a challenge. Some of the new treatment strategies, such as personalized medicine, are too cumbersome to be scaled up. Because cancers very frequently originate from CSCs, the targeting of a particular signaling pathway, such as JNK, in CSCs might circumvent some of the setbacks that are currently faced by conventional therapies, such as fast relapse and chemoresistance. The significance of stem cell research is its promise for the stem-cell-based treatment for some degenerative diseases or for cancer. The recently recognized tumorigenic nature of hESCs, adult stem cells and iPSCs has put stem-cell-based therapies in jeopardy. It is plausible to assume that this tumorigenicity of stem cells might be a consequence of aberrant JNK activation. Thus, the inhibition of JNK will not only force the differentiation of the stem cells to replace the damaged tissues but also reduce the tumor burden in cancer patients by eliminating CSCs. Recent evidence has shown that the administration of an inhibitor against the downstream target of JNK, JAK, is clinically beneficial in treating some forms of myeloproliferative neoplasm (104). Accordingly, a JNK-based therapeutic strategy that targets CSCs for cancers could be developed in the foreseeable future.
Acknowledgments
The author’s research is funded by NIH (RO1 ES017217).
References
- 1.Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Beezhold K, Castranova V. JNK1, a potential therapeutic target for hepatocellular carcinoma. Biochim Biophys Acta. 2009;1796(2):242–251. doi: 10.1016/j.bbcan.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 5.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70(4):1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voncken JW, Niessen H, Neufeld B, Rennefahrt U, Dahlmans V, Kubben N, et al. MAPKAP kinase 3pK phosphorylates and regulates chromatin association of the polycomb group protein Bmi1. J Biol Chem. 2005;280(7):5178–5187. doi: 10.1074/jbc.M407155200. [DOI] [PubMed] [Google Scholar]
- 7.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, et al. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98(1):111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Lee SC, Ro J, Kang HS, Kim HS, Yoon S. Jnk signaling pathway-mediated regulation of Stat3 activation is linked to the development of doxorubicin resistance in cancer cell lines. Biochem Pharmacol. 2010;79(3):373–380. doi: 10.1016/j.bcp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhong S, Zhang Y, Jansen C, Goto H, Inagaki M, Dong Z. MAP kinases mediateUVB-induced phosphorylation of histone H3 at serine 28. J Biol Chem. 2001;276(16):12932–12937. doi: 10.1074/jbc.M010931200. [DOI] [PubMed] [Google Scholar]
- 10.Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4(12):e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong S, Johnson DL. The JNKs differentially regulate RNA polymerase III transcription by coordinately modulating the expression of all TFIIIB subunits. Proc Natl Acad Sci U S A. 2009;106(31):12682–12687. doi: 10.1073/pnas.0904843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46(8):591–598. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter J, Rozentzweig D, Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem. 2008;283(34):23224–23234. doi: 10.1074/jbc.M801379200. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Lin A. Wiring the cell signaling circuitry by the NF-kappa B and JNK1 crosstalk and its applications in human diseases. Oncogene. 2007;26(22):3267–3278. doi: 10.1038/sj.onc.1210417. [DOI] [PubMed] [Google Scholar]
- 15.Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, et al. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation. J Exp Med. 2002;195(7):811–823. doi: 10.1084/jem.20011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89(1–2):115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 17.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22(4):667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15(1):36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 19.Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ. Survival signaling mediated by c-Jun NH(2)-terminal kinase in transformed B lymphoblasts. Nat Genet. 2002;32(1):201–205. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- 20.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 21.Durbin AD, Somers GR, Forrester M, Pienkowska M, Hannigan GE, Malkin D. JNK1 determines the oncogenic or tumor-suppressive activity of the integrin-linked kinase in human rhabdomyosarcoma. J Clin Invest. 2009;119(6):1558–1570. doi: 10.1172/JCI37958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durbin AD, Hannigan GE, Malkin D. Oncogenic ILK, tumor suppression and all that JNK. Cell Cycle. 2009;8(24):4060–4066. doi: 10.4161/cc.8.24.10093. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta-and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata W, Maeda S, Hikiba Y, Yanai A, Sakamoto K, Nakagawa H, et al. c-Jun NH2-terminal kinase 1 is a critical regulator for the development of gastric cancer in mice. Cancer Res. 2008;68(13):5031–5039. doi: 10.1158/0008-5472.CAN-07-6332. [DOI] [PubMed] [Google Scholar]
- 25.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118(12):3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, et al. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol. 2009;50(2):323–333. doi: 10.1016/j.jhep.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Q, Chen J, Beezhold KJ, Castranova V, Shi X, Chen F. JNK1 activation predicts the prognostic outcome of the human hepatocellular carcinoma. Mol Cancer. 2009;8:64. doi: 10.1186/1476-4598-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11(2):119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103(28):10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng SH, Wang CH, Lin SM, Chen CK, Huang HY, Chen Y. Activation of c-Jun N-terminal kinase 1 and caspase 3 in the tamoxifen-induced apoptosis of rat glioma cells. J Cancer Res Clin Oncol. 2004;130(5):285–293. doi: 10.1007/s00432-004-0546-y. [DOI] [PubMed] [Google Scholar]
- 32.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24(24):10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465(7297):492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13(3):235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, et al. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell. 2009;5(2):178–190. doi: 10.1016/j.stem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Morata G, Shlevkov E, Perez-Garijo A. Mitogenic signaling from apoptotic cells in Drosophila. Dev Growth Differ. 2011;53(2):168–176. doi: 10.1111/j.1440-169X.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 41.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129(7):1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2(1):37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and four-jointed. EMBO Rep. 2009;10(9):1051–1058. doi: 10.1038/embor.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106(15):6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herranz H, Perez L, Martin FA, Milan M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 2008;27(11):1633–1645. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1(2–3):144–154. doi: 10.1242/dmm.000950. discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463(7280):545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136(7):1169–1177. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- 49.Zeidler MP, Bach EA, Perrimon N. The roles of the Drosophila JAK/STAT pathway. Oncogene. 2000;19(21):2598–2606. doi: 10.1038/sj.onc.1203482. [DOI] [PubMed] [Google Scholar]
- 50.Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, et al. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17(3):1562–1571. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20(17):1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350(1):139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou SX. Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J Cell Physiol. 2010;224(3):581–584. doi: 10.1002/jcp.22194. [DOI] [PubMed] [Google Scholar]
- 54.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455(7216):1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 55.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327(5962):210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138(6):1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107(2):844–849. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28(13):1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 62.Aguilera C, Nakagawa K, Sancho R, Chakraborty A, Hendrich B, Behrens A. c-Jun N-terminal phosphorylation antagonises recruitment of the Mbd3/NuRD repressor complex. Nature. 2011;469(7329):231–235. doi: 10.1038/nature09607. [DOI] [PubMed] [Google Scholar]
- 63.Fujishita T, Aoki M, Taketo MM. JNK Signaling Promotes Intestinal Tumorigenesis Through Activation of mTOR Complex 1 in ApcDelta716 Mice. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao Z, Mishra L. Cancer stem cells and hepatocellular carcinoma. Cancer Biol Ther. 2009;8(18):1691–1698. doi: 10.4161/cbt.8.18.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5(6):597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14(10):1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 68.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 69.Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9(5):651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19(2):150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68(19):7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 72.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6(6):568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, et al. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13(7):762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3(2):196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci U S A. 2008;105(7):2445–2450. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 79.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 81.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15(11):1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 83.Anneren C. Tyrosine kinase signalling in embryonic stem cells. Clin Sci (Lond) 2008;115(2):43–55. doi: 10.1042/CS20070388. [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Ovesen JL, Puga A, Xia Y. Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ Health Perspect. 2009;117(7):1124–1130. doi: 10.1289/ehp.0800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryu JM, Lee MY, Yun SP, Han HJ. Zinc chloride stimulates DNA synthesis of mouse embryonic stem cells: involvement of PI3K/Akt, MAPKs, and mTOR. J Cell Physiol. 2009;218(3):558–567. doi: 10.1002/jcp.21628. [DOI] [PubMed] [Google Scholar]
- 86.Ryu JM, Han HJ. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J Biol Chem. 2011 doi: 10.1074/jbc.M110.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amura CR, Marek L, Winn RA, Heasley LE. Inhibited neurogenesis in JNK1-deficient embryonic stem cells. Mol Cell Biol. 2005;25(24):10791–10802. doi: 10.1128/MCB.25.24.10791-10802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu P, Davis RJ. c-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol Cell Biol. 2010;30(6):1329–1340. doi: 10.1128/MCB.00795-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, et al. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5(2):204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutchins AP, Robson P. Unraveling the human embryonic stem cell phosphoproteome. Cell Stem Cell. 2009;5(2):126–128. doi: 10.1016/j.stem.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Van Hoof D, Munoz J, Braam SR, Pinkse MW, Linding R, Heck AJ, et al. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5(2):214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 92.Swaney DL, Wenger CD, Thomson JA, Coon JJ. Human embryonic stem cell phosphoproteome revealed by electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A. 2009;106(4):995–1000. doi: 10.1073/pnas.0811964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY, et al. Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells. 2005;23(2):166–185. doi: 10.1634/stemcells.2004-0162. [DOI] [PubMed] [Google Scholar]
- 94.Kim CG, Lee JJ, Jung DY, Jeon J, Heo HS, Kang HC, et al. Profiling of differentially expressed genes in human stem cells by cDNA microarray. Mol Cells. 2006;21(3):343–355. [PubMed] [Google Scholar]
- 95.Mandal A, Bhowmik S, Patki A, Viswanathan C, Majumdar AS. Derivation, characterization, and gene expression profile of two new human ES cell lines from India. Stem Cell Res. 2010;5(3):173–187. doi: 10.1016/j.scr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26(4):1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boomer JS, Tan TH. Functional interactions of HPK1 with adaptor proteins. J Cell Biochem. 2005;95(1):34–44. doi: 10.1002/jcb.20401. [DOI] [PubMed] [Google Scholar]
- 98.Chia NY, Chan YS, Feng B, Lu X, Orlov YL, Moreau D, et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature. 2010;468(7321):316–320. doi: 10.1038/nature09531. [DOI] [PubMed] [Google Scholar]
- 99.Ma Z, Swigut T, Valouev A, Rada-Iglesias A, Wysocka J. Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat Struct Mol Biol. 2011;18(2):120–127. doi: 10.1038/nsmb.2000. [DOI] [PubMed] [Google Scholar]
- 100.Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, et al. IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells. 2009;27(1):238–249. doi: 10.1634/stemcells.2008-0261. [DOI] [PubMed] [Google Scholar]
- 101.Ahn HJ, Lee WJ, Kwack K, Kwon YD. FGF2 stimulates the proliferation of human mesenchymal stem cells through the transient activation of JNK signaling. FEBS Lett. 2009;583(17):2922–2926. doi: 10.1016/j.febslet.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 102.Lee K, Song K. Basal c-Jun N-terminal kinases promote mitotic progression through histone H3 phosphorylation. Cell Cycle. 2008;7(2):216–221. doi: 10.4161/cc.7.2.5155. [DOI] [PubMed] [Google Scholar]
- 103.Lee N, Maurange C, Ringrose L, Paro R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature. 2005;438(7065):234–237. doi: 10.1038/nature04120. [DOI] [PubMed] [Google Scholar]
- 104.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]