Abstract
Phostensin binds to the pointed ends of actin filaments and modulates actin dynamics. The genomic location of phostensin is between the HLA-C and HLA-E gene clusters on human chromosome 6, and the mRNA of this protein is predominantly distributed in the spleen, thymus, and peripheral leukocytes. However, the distribution of phostensin in leukocyte cell populations and the subcellular localization have not yet been determined. In this study, an anti-phostensin monoclonal antibody (PT2) that recognizes residues 89–124 of phostensin was prepared and used to examine the subcellular localization and distribution of phostensin in white blood cell populations and in lymphatic tissues. It was found that phostensin is mainly concentrated at the cell periphery and co-localizes with actin filaments. In addition, phostensin was abundant in helper T-lymphocytes, cytotoxic T-lymphocytes, mature monocytes, macrophages, B-lymphocytes, natural killer cells, and granulocytes as well as in the lymphatic tissues, such as the thymus, lymph nodes, and spleen. Phostensin is expressed in the mature lymphocytes of the thymic medulla but not in the immature lymphocytes of the thymic cortex. Taken together, phostensin is a ubiquitous protein in leukocytes, and it may play an important role in modulating the cellular functions of leukocytes.
Keywords: phostensin, actin filament, leukocytes, actin dynamics, protein phosphatase 1
Phostensin, which is encoded in humans by KIAA1949, consists of 165 amino acids with a consensus protein phosphatase 1 (PP1)–binding motif located at residues 91–94 (K91ISF94). Phostensin mRNA is predominantly localized to the peripheral leukocytes, thymus, and spleen (Kao et al. 2007; Su et al. 2010), whereas the phostensin protein is present in crude extracts of human peripheral leukocytes. In addition, phostensin associates with PP1 in a complex that localizes to the actin cytoskeleton, where this protein binds to the pointed ends of actin filaments but not to actin monomers, sides of filaments, or barbed ends of filaments (Lai et al. 2009). Phostensin retards the elongation and depolymerization rates of gelsolin-actin seeds, directly associates with pointed ends, and reduces the rate of G-actin addition at the pointed ends (Lai et al. 2009).
The swine phostensin gene is located between the non-classical and classical swine leukocyte antigen (SLA) class I gene clusters (Nagase et al. 2001; Shigenari et al. 2004). This genomic segment is 433 kb long and is orthologous to a 595-kb region within the human leukocyte antigen (HLA) complex. Nucleotide sequencing analysis indicated that 21 swine genes are present in this segment and that these genes exhibit sequence identity with those in the corresponding region on chromosome 6 between the HLA-C and HLA-E genes in humans. This unusual genomic location suggests that phostensin may play a role in immune activity. Although previous work demonstrated that phostensin is present in the spleen, thymus, and peripheral leukocytes (Kao et al. 2007), the distribution of phostensin in peripheral leukocyte cell populations and the immunolocalization of phostensin in lymphatic tissues have not been determined. In this study, we prepared a monoclonal antibody (PT2) that recognizes the sequence from residues 89–124 of phostensin. The distribution of phostensin in leukocyte cell populations and in lymphatic tissues and the subcellular localization of this protein were examined.
Materials and Methods
Materials
Blue Sepharose and metal-chelating Sepharose were obtained from Amersham Biosciences (Fairfield, CT). Tris, Luria-Bertani (LB) broth, dithiothreitol (DTT), ampicillin, phenylmethylsulfonyl fluoride, benzamidine, imidazole, and anti-thioredoxin (trx) polyclonal antibody were obtained from Sigma-Aldrich (St. Louis, MO). Anti-α-tubulin monoclonal antibody was purchased from Thermo Scientific (Fremont, CA).
Protein Purification
Full-length phostensin cDNA was amplified by PCR with gene specific primers: 5′-GCC CAT GGC TCT GGT GCC ACG CGG TTC TAT GAG CCG CCT GTT CTA TGGG-3′ and 5′-CGG AAT TCT CAC CGC CGG CAG GAC TCA TC-3′. The resulting product was digested with NcoI/EcoRI and subcloned into pET32a. Escherichia coli BL21(DE3) was transformed with the recombinant pET-32a plasmid, which encoded the trx-phostensin fusion protein. The transformed bacteria were grown in LB broth with ampicillin (0.1 g/L) and induced by Isopropyl-beta-D-1-thiogalactopyranoside (IPTG) (final concentration of 1 mM) for 4 hr at 37C. Cells were harvested by centrifugation and resuspended in 100 ml of 20 mM Tris-HCl buffer, pH 7.9, containing 0.5 M NaCl, 0.2 mM phenylmethylsulfonyl fluoride, 0.02% NaN3, 4 mM benzamidine, and 0.5 mM imidazole. Cells were then lysed using a French press. The phostensin fusion protein was purified from the crude lysate by nickel-chelating Sepharose and Blue Sepharose chromatography. The purified protein was pooled, concentrated by ultrafiltration, and dialyzed against 5 mM Tris-HCl with 0.2 mM CaCl2, pH 8.0. Purified phostensin was stored at 4C. The expression vectors for trx-phostensin residues 1–39, trx-phostensin residues 1–88, trx-phostensin residues 1–129, and trx-phostensin residues 125–165 were constructed using appropriate primers, and the trx-phostensin fragments were purified as described above.
Phostensin Monoclonal Antibody, PT2
Two mg of trx-phostensin fusion protein dissolved in 2 ml of phosphate-buffered saline (PBS) was used for monoclonal antibody production by Kelowna International Scientific Inc (Taipei, Taiwan). PT2, an anti-phostensin monoclonal antibody, was purified by protein A–Sepharose from ascites fluid. The stock concentration of purified PT2 was adjusted to 1 mg/ml. Conjugation of PT2 with Alexa-488 was carried out at room temperature for 1 hr with protection from light. Reaction components included 2 mg of PT2 and 160 µg of Alexa-488 tetrafluorophenyl ester in 1 ml of 500 mM carbonate buffer, pH 9.5. Upon completion of the reaction, excess reagents were removed by dialysis against PBS buffer. The stock concentration of Alexa-488–conjugated PT2 was adjusted to 1 mg/ml.
Western Blotting
Human peripheral mononuclear cells (PBMCs) were prepared as described (Lu et al. 2008). Cells, including PBMCs, Jurkat, HeLa, HL60, K562, and U937, were grown to confluence in 10-cm tissue culture dishes, harvested, and centrifuged at 1500 × g for 5 min. Pelleted cells were resuspended in 0.1 ml of 1% SDS and lysed by ultrasonication. Aliquots (100 µg) of cell extracts were analyzed by SDS-PAGE and then electrotransferred onto a polyvinylidene difluoride membrane. Western blot analysis was performed with the anti-phostensin monoclonal antibody PT2 (1:2000 dilution). HL-60 cells differentiated to granulocytes or to monocytes were prepared as described (Nakashima et al. 1999).
Immunocytochemistry
PBMCs were adhered onto poly-D-lysine–coated cover slips. Cells were washed twice with PBS, fixed for 15 min in 4% paraformaldehyde, and, when needed, permeabilized for 15 min with 0.5% Triton X-100 in PBS. The cells were then blocked for 1 hr with 5% skim milk in PBS, incubated with a 1:100 dilution of anti-phostensin monoclonal antibody PT2 for 60 min, and then incubated with a 1:400 dilution of goat-anti-mouse rhodamine-conjugated secondary antibody (Invitrogen, Carlsbad, CA). For staining polymerized actin, a 1:1000 dilution of Alexa-488–conjugated phalloidin (Cytoskeleton, Denver, CO) was used. Cells were analyzed with a Nikon 80i fluorescent microscope or with a Zeiss LSM 510 confocal microscope.
Flow Cytometry
For surface markers, whole blood cells were washed with staining buffer containing 0.2% bovine serum albumin and 0.1% sodium azide (BD Biosciences) and then stained with phycoerythrin-conjugated antibodies for CD4, CD8, CD14, CD19, CD56, or CD88 (BD Biosciences) at room temperature for 10 min. Red blood cells were lysed with a Lysis Kit (BD Bioscience). For intracellular staining of phostensin with Alexa-conjugated PT2, the Intracellular Staining Kit from BD Biosciences was used according to the manufacturer’s instructions. After several washes with PBS, the cells were analyzed using a FACScan flow cytometer (BD Biosciences).
Subcellular Fractionation
PBMCs were suspended in 200 µl of buffer (10 mM Tris-HCl, pH 7.9; 10 mM KCl; 0.05% Nonidet P-40; and 1.5 mM MgCl2) followed by incubation on ice for 5 min. Proteins in the cytoplasmic and nuclear fractions were separated by centrifugation at 760 × g at 4C for 10 min. The supernatant containing the cytoplasmic proteins was transferred into a new microcentrifuge tube. The pellet containing the nuclear proteins was resuspended in 50 mM Tris, pH 7.9; 300 mM KCl; 12.5 mM MgCl2; 1 mM EDTA; and 20% glycerol. Both protein fractions were subjected to Western blot analysis using the anti-phostensin monoclonal antibody PT2. The nuclear fraction was identified with an anti-lamin monoclonal antibody (BD Biosciences).
Immunohistochemistry
The human lymphatic tissue array (LN 801) was obtained from US Biomax, Inc. (Rockville, MD). Lymphatic tissue was deparaffinized by heating at 60C for 20 min, washing three times with xylene, rinsing with ethanol, and drying at room temperature. The array slide was rinsed with PBS and treated with 3% hydrogen peroxide for 5 min. After an additional PBS rinse, the array slide was incubated with anti-phostensin monoclonal antibody PT2 (1:500 dilution) for 2 hr followed by incubation with biotinylated anti-mouse secondary antibody for 30 min and streptavidin-conjugated HRP for 30 min (Dako, Glostrup, Denmark). Chromogen generation was achieved using DAB as the substrate (Dako). The array slide was counterstained with hematoxylin for 3 min and then dehydrated and sealed using Acrytol mounting medium. Image observations were performed using a Nikon 80i microscope.
Immunoprecipitation
HeLa cells were grown to confluence in 10-cm tissue culture dishes, harvested, and centrifuged at 1500 × g for 5 min. After being washed with PBS, cells were pelleted by centrifugation. The pelleted cells were ruptured by 1 ml of 1% NP40 buffer (20 mM Tris-HCl, pH:8.0, containing 137 mM NaCl, 10% glycerol, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, aprotinin [0.2 U/ml], and leupeptin [20 µg/ml]). PMBC isolated from 12 ml of blood was ruptured by 0.5 ml of 1% NP40 buffer. 1500 µg and 500 µg of proteins extracted from HeLa cells and PBMC, respectively, were subjected to immunoprecipitation by PT2 (10 µg) immobilized on protein G–Sepharose (12.5 µl). After incubation at 4C for 2 hours, all components were washed with 1% NP40 buffer four times and harvested by centrifugation (760 × g) at 4C for 1.5 min.
Results
Anti-phostensin Monoclonal Antibody PT2
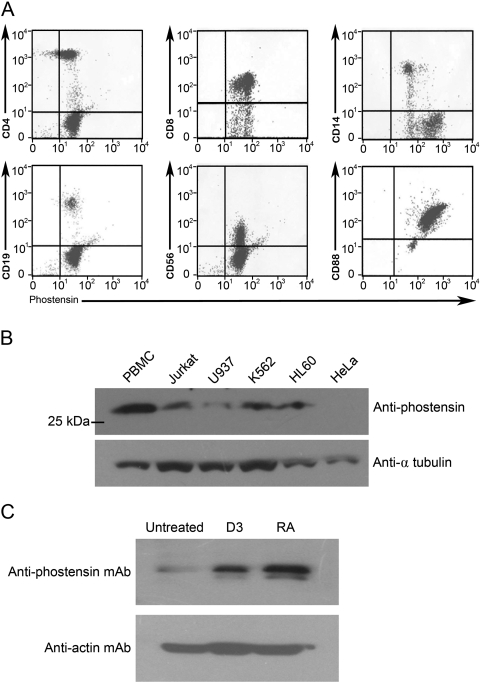
We have produced an anti-phostensin monoclonal antibody, PT2. To examine the recognition site of PT2, recombinant trx-phostensin mutants were prepared. All mutant proteins were purified to homogeneity of greater than 90%, as evidenced by SDS-PAGE gels stained with Coomassie Brilliant Blue (data not shown). In Western blot analysis, PT2 did not bind to residues 1–39, residues 1–88, or residues 125–165 but did bind to residues 1–129 and wild-type phostensin, suggesting that PT2 binds to the sequence between residues 89 and 124 of phostensin (Figure 1A). The PT2 was then stripped from the membranes using stripping buffer (0.5 M acetic acid with 0.5 M NaCl), and the membranes were reblotted with anti-trx polyclonal antibody. All prepared trx-phostensin fragments were detected by the anti-trx polyclonal antibodies (Figure 1B). Western blot analysis demonstrated that PT2 only recognizes phostensin in crude proteins extracted from human PBMCs (Figure 1C). Our analysis predicts that the molecular weight of phostensin is 26 kDa, which is identical to the determination in previous studies in which phostensin was extracted from human PBMCs and recognized by a polyclonal anti-phostensin antibody that recognizes residues 144-162 of the protein (Kao et al. 2007).
Figure 1.

Characterization of the anti-phostensin monoclonal antibody, PT2. (A) PT2 binds to full-length trx-phostensin and to residues 1–129 but not to residues 1–39, residues 1–88, or residues 125–165, as observed by Western blot analysis. An aliquot (100 ng) of each protein was loaded onto SDS-PAGE gels (12.5%) and analyzed by Western blot using the anti-phostensin monoclonal antibody, PT2. (B) PT2 antibodies bound to proteins on the membrane from (A) were stripped (in 0.5 M acetic acid plus 0.5 M NaCl) for 30 min, and the membranes were reblotted with anti-trx antibody. All loaded proteins contained a trx fragment. (C) Phostensin is present in human PBMCs. Cellular proteins were extracted by 1% SDS and ultrasonication. An aliquot (100 µg) of crude extract was analyzed by SDS-PAGE (12.5%), transferred onto polyvinylidene difluoride membranes, and blotted with PT2 (1:2000). The molecular weight of phostensin was observed to be 26 kDa.
Phostensin Is Widely Distributed in Human Leukocytes and Leukemic Cell Lines
PBMCs contain a mixture of cell populations (~90% lymphocytes, 5–8% monocytes, and 2–5% other cell populations) (Lu et al. 2010). Although phostensin is present in PBMCs, the exact cell population that contains PBMCs was unknown. In addition, the presence of phostensin in other cell populations was unknown. To determine the presence of phostensin in various cell populations, cells were stained with phycoerythrin-conjugated anti-cell surface marker antibodies and Alexa-488–conjugated PT2 anti-phostensin antibodies and analyzed by flow cytometry. Phostensin was present in many types of leukocytes, including CD4-, CD8-, CD14-, CD19-, CD56-, and CD88-positive cells (Figure 2A). These results suggested that phostensin is widely expressed in helper T-lymphocytes, cytotoxic T-lymphocytes, mature monocytes, macrophage, B-lymphocytes, natural killer cells, and granulocytes. Western blot analysis was used to determine whether phostensin was present in human leukemic cell lines, such as Jurkat, U937, K562, HL60, and HeLa cells. Crude protein extracts from these cell lines were separated by SDS-PAGE and analyzed by Western blot with PT2. Phostensin was detected in Jurkat, U937, K562, and HL60 cells but not in HeLa cells. HL60, U937, and K562 cells are of the myeloid lineage (Gallagher et al. 1979; Klein et al. 1976; Lozzio and Lozzio 1975), whereas HL60 cells are predominantly a neutrophilic promyelocyte. HL60 cells can differentiate into granulocytes and monocytes by induction with retinoic acid and 1,25-dihydroxyvitamin D3, respectively (Birnie 1988; Nakashima et al. 1999). In addition, phostensin expression was significantly increased when HL60 cells were differentiated into mature monocytes and granulocytes (Figure 2C).
Figure 2.
Phostensin is expressed in leukocytes and in leukemic cell lines. (A) Leukocytes were double stained for cell surface markers (CD4, CD8, CD14, CD19, CD56, or CD88) and phostensin expression and analyzed by flow cytometry. (B) Phostensin is present in leukemic cell lines. Cellular proteins were extracted by 1% SDS and ultrasonication. An aliquot (100 µg) of each crude extract was analyzed by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and blotted with PT2 (1:1000). The arrow indicates phostensin recognized by PT2. (C) Differentiation of HL60 cells was induced by retinoic acid and 1,25-dihydroxyvitamin D3, respectively. Cellular proteins were extracted by 1% SDS and ultrasonication. All components of extracted cells were analyzed by Western blot using the PT2 monoclonal antibody.
Cellular Localization
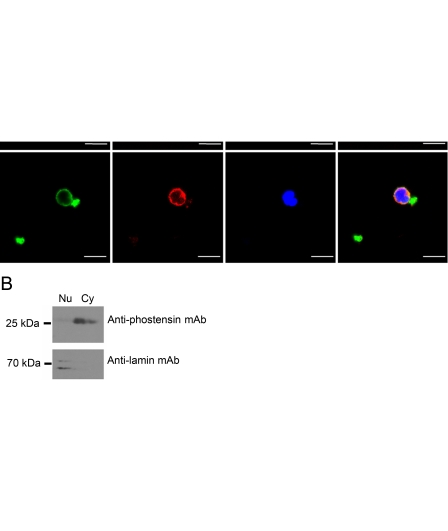
Indirect immunofluorescence microscopy demonstrated that overexpressed phostensin-enhanced green fluorescent protein (phostensin-EGFP) was conspicuously localized to the F-actin cytoskeleton at the cell periphery and was also localized to the nucleus of MDCK cells (Kao et al. 2007); however, the cellular localization of endogenous phostensin and actin filaments has not yet been determined. Actin filaments of PBMCs were stained with rhodamine-conjugated phalloidin. Endogenous phostensin was detected with PT2 and an Alexa-488–conjugated secondary antibody. Endogenous phostensin was mainly concentrated at the cell periphery and co-localized with actin-based structures (Figure 3A). Endogenous phostensin was sparse in the nucleus as observed with immunofluorescence microscopy (Figure 3A). To further confirm the low expression of phostensin in the nucleus, PBMCs were analyzed by immunoblot. Endogenous phostensin was found in both the cytoplasmic and nuclear fractions but at much higher levels in the cytoplasmic fraction (Figure 3B). Endogenous phostensin binding to the F-actin cytoskeleton was also verified by immunoprecipitation with PT2. Proteins extracted from PBMCs were immunoprecipitated by PT2. The precipitated components were separated by SDS-PAGE and immunoblotted with an anti-actin antibody. Indeed, actin was co-immunoprecipitated by PT2, suggesting that endogenous phostensin is associated with the actin cytoskeleton in PBMCs (Figure 3C). Endogenous phostensin was not identified in crude extracts from HeLa cells (Figure 2B), and actin was not co-immunoprecipitated by PT2 in these cells (Figure 3C).
Figure 3.
Endogenous phostensin is mainly co-localized with actin filaments at the cellular periphery of PBMCs. (A) Endogenous phostensin was detected with PT2 and stained with rhodamine-conjugated secondary antibody (red). Actin filaments were stained with Alexa-488–conjugated phalloidin (green), and the nucleus was stained with DAPI (blue). Merged images are shown. Scale bar, 10 µm. (B) The cytoplasmic and nuclear proteins extracted from human PBMCs were analyzed by SDS-PAGE (12.5%), electrotransferred onto a polyvinylidene difluoride membrane, and blotted with the PT2 monoclonal antibody. The nuclear protein marker was stained with anti-lamin monoclonal antibody. (C) Endogenous phostensin binds to actin filaments. Crude proteins extracted from PBMCs or from HeLa cells were incubated with the PT2 monoclonal antibody and then precipitated protein G–Sepharose. After centrifugation, all components in the pellet were analyzed by Western blot using an anti-actin monoclonal antibody.
Immunohistochemistry
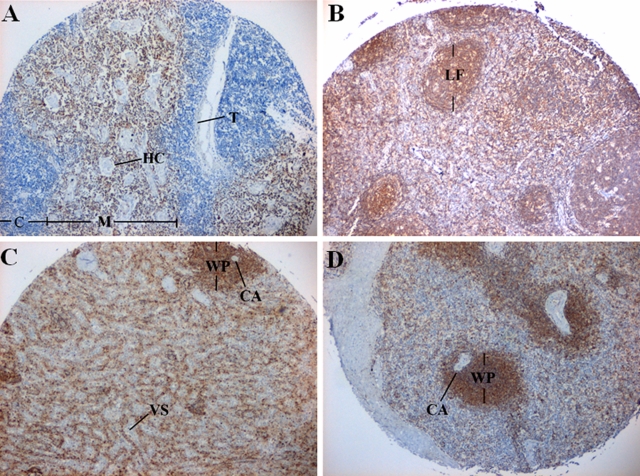
Immunohistochemical analysis with the PT2 monoclonal antibody was performed to examine phostensin expression in human lymphatic tissues, including the thymus, lymph nodes, and spleen. Phostensin immunoreactivity was detected in the medulla of thymus, which was stained in these experiments whereas the adjacent portion of the cortex was not (Figure 4A). This result indicates that phostensin is present in mature T lymphocytes but not in immature T cells.
Figure 4.
Phostensin is abundant in the thymus, lymph nodes, and spleen. Tissue sections were examined by immunohistochemistry analysis with the PT2 monoclonal antibody. (A) Thymus. C, cortex; HC, Hassall’s corpuscles; M, medulla; T, trabeculae. (B) Lymph node. LF, lymphoid follicle. (C) Spleen. CA, central artery; VS, venous sinusoid; WP, white pulp. (D) Spleen. WP, white pulp; CA, central artery.
In the lymph node tissues, the lymphoid follicle, paracortex, and medulla of the parenchymal compartment were stained intensely with PT2 (Figure 4B), suggesting that phostensin is expressed in the primary cell type of this compartment. After staining splenic sections with PT2, the cells attached to the wall of the venous sinusoids were stained, suggesting that phostensin is expressed in phagocytic macrophages (Figure 4C). The white pulp was also intensely stained with the PT2 antibody (Figure 4D), confirming that phostensin is present in lymphoid cells.
Discussion
We generated an anti-phostensin monoclonal antibody and demonstrated that this antibody recognizes residues 89–124 of phostensin. Phostensin is encoded by KIAA1949, and the open reading frame of this gene was predicted by the GENSCAN gene prediction program to encode a putative protein of 613 amino acids (Nagase et al. 2001). Examination by 5′-RACE, however, only identified a short transcript that encoded a small protein of 165 amino acids (Kao et al, 2007). The primary sequence of this small protein version was identical to the C-terminal region of the predicted larger protein. Western blot analysis with the PT2 antibody only identified a phostensin protein with an apparent molecular weight of 26 kDa in protein extracts from human PBMCs and leukemic cell lines. No proteins with molecular weights of more than 60 kDa were detected by this antibody.
Previous proteomic studies have analyzed the PP1-binding proteins in the nucleus of HeLa cells and indicated that phostensin is a nuclear PP1-binding protein in this cell type (Trinkle-Mulcahy et al. 2006). Interestingly, phostensin was not detected in HeLa cells by our Western blot analysis (Figure 3A). This difference may arise from the different sensitivities of the assay methods. Phostensin in the nucleus was observed in previous studies by indirect immunostaining analysis (Kao et al. 2007). Although the overexpressed phostensin-EGFP noticeably co-localized with actin filaments at the cell periphery in MDCK cells, some of the recombinant fusion protein was also present in the nucleus. Distribution of the overexpressed phostensin-EGFP in the cytoplasm and in the nucleus was also observed in 293T cells (data not shown). In this study, endogenous phostensin mainly co-localized with the actin cytoskeleton at the cell periphery of human PBMCs but was also observed in the nucleus to a lesser extent (Figure 3A). Analysis of the proteins extracted from the cytoplasm and the nucleus of PBMCs also confirmed that the majority of phostensin localizes to the cytoplasm rather than to the nucleus (Figure 3C). In the cytoplasm, phostensin binds to the pointed ends of F-actin and modulates actin dynamics (Lai et al. 2009); however, the biochemical function of phostensin in the nucleus is not known. Phostensin does not contain a perfect nuclear localization sequence. Therefore, the mechanism for transportation of phostensin from the cytoplasm to the nucleus remains unidentified.
Phostensin is abundantly distributed in many leukocyte populations. The compartments that contain leukocytes in lymphatic tissues were found to contain an abundance of phostensin by immunohistochemical analysis. The distribution of phostensin in the thymus is interesting. The thymus, which is the location for T-cell development, is composed of the cortex, which contains immature T-cells, and the medulla, which contains mature T-cells. Immunohistochemical analysis demonstrated that phostensin was present in the thymic medulla but not the thymic cortex. This result suggests that phostensin is only expressed in mature T-cells.
Recently, microarray analysis was used to screen 25,985 cDNAs for differences between MDA-MB-231, a tumorigenic and metastatic breast cancer cell line, and MDA/H6, the chromosome 6–mediated suppressed, non-tumorigenic, and non-metastatic derivative cell line. KIAA1949 was identified in this study as one of the differentially expressed genes (Su et al. 2010). In addition, microarray analysis demonstrated that the KIAA1949 transcript was downregulated in 86.7% of breast cancer lines and tumor tissues. Northern blot analysis also revealed that the KIAA1949 transcript was undetectable or significantly decreased in 10 breast cancer cell lines, suggesting that KIAA1949 is a potential breast cancer suppressor gene. These results strongly suggest that phostensin functions as a breast cancer suppressor. Analysis of the levels of phostensin in breast cancer cell lines and MDA/H6 will be critical to verify this notion. Biochemically, phostensin binds to the pointed ends of actin filaments and retards the elongation and depolymerization rates (Lai et al. 2009). The importance of this activity in cancer cell invasion and metastasis needs to be investigated. Moreover, phostensin targets PP1 to the actin cytoskeleton (Kao et al. 2007). PP1 is a major serine/threonine protein phosphatase that is capable of reversing the action of protein kinases (Cohen 1989; Cohen 2002; Shenolikar 1994; Shi 2009). Targeting of PP1 to the actin filament pointed ends via phostensin may modulate phosphorylation/dephosphorylation of the actin cytoskeleton network, and this activity may, in turn, affect cancer cell invasion and metastasis. In this study, we found that phostensin is only expressed in mature T-cells and that differentiation of promyelocytic leukemia cells (HL60) significantly increases the expression of phostensin. These data suggest that phostensin affects leukocyte differentiation, and the role of this protein on differentiation is currently under investigation.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Science Council, ROC (NSC 96-2320-B-194-005-MY3, NSC 99-2320-B194-003-MY3), and the DaLin Tzu Chi Buddhist Hospital (DTCRD95(2)-16 and DTCRD97-13).
References
- Birnie GD. 1988. The HL60 cell line: a model system for studying human myeloid cell differentiation. Br J Cancer Suppl. 9:41–45 [PMC free article] [PubMed] [Google Scholar]
- Cohen P. 1989. The structure and regulation of protein phosphatases. Annu Rev Biochem. 58:453–508 [DOI] [PubMed] [Google Scholar]
- Cohen PTW. 2002. Protein phosphatase 1-targeted in many directions. J Cell Sci. 15:241–256 [DOI] [PubMed] [Google Scholar]
- Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. 1979. Characterization of the continuous differentiation myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 54:713–733 [PubMed] [Google Scholar]
- Kao SC, Chen CY, Wang SL, Yang JJ, Hung WC, Chen YC, Lai NS, Liu HT, Huang HL, Chen HC, Lin TH, Huang HB. 2007. Identification of phostensin, a PP1 F-actin cytoskeleton targeting subunit. Biochem Biophys Res Commun. 356:594–598 [DOI] [PubMed] [Google Scholar]
- Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, Vanky F. 1976. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer. 18:421–431 [DOI] [PubMed] [Google Scholar]
- Lai NS, Wang TF, Wang SL, Chen CY, Yen JY, Huang HL, Li G, Huang KY, Liu SQ, Lin TH, Huang HB. 2009. Phostensin caps the pointed of actin filaments and modulates actin dynamics. Biochem Biophys Res Commun. 387:676–681 [DOI] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. 1975. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 45:321–334 [PubMed] [Google Scholar]
- Lu MC, Lai NS, Yu HC, Hsieh SC, Tung CH, Yu CL. 2008. Nifedipine suppresses Th1/Th2 cytokine production and increased apoptosis of anti-CD3 + anti-CD28-activated mononuclear cells from patients with systemic lupus erythematosus via calcineurin pathway. Clin Immunol. 129:462–470 [DOI] [PubMed] [Google Scholar]
- Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. 2010. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor α̣production. Arthritis Rheum. 62: 1213–1223 [DOI] [PubMed] [Google Scholar]
- Nagase T, Kikuno R, Ohara O. 2001. Predication of the coding sequences of unidentified human genes. XXII. The complete sequences of 50 new cDNA clones which code for large proteins. DNA Res. 8:319–327 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Hagiwara T, Ishigami A, Nagata S, Asaga H, Kuramoto M, Senshu T, Yamada M. 1999. Molecular characterization of peptidylarginine deiminase in HL60 cells induced by retinoic acid and 1a,25-dihydroxyvitamin D3. J Biol Chem. 274:27786–27792 [DOI] [PubMed] [Google Scholar]
- Shenolikar S. 1994. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu Rev Cell Biol. 10:55–86 [DOI] [PubMed] [Google Scholar]
- Shigenari A, Ando A, Renard C, Chardon P, Shiina T, Kulski JK, Yasue H, Inoko H. 2004. Nucleotide sequencing analysis of the swine 433-kb genomic segment located between the non-classical and classical SLA class I gene clusters. Immunogenetics. 55:695–705 [DOI] [PubMed] [Google Scholar]
- Shi Y. 2009. Serine/threonine phosphatases: mechanism through structure. Cell. 139:468–484 [DOI] [PubMed] [Google Scholar]
- Su YA, Yang J, Tao L, Nguyen H, Ping H. 2010. Undetectable and decreased expression of KIAA1949 (phostensin) encoded on chromosome 6p21.33 in human breast cancers revealed by transcriptome analysis. J Cancer. 1:38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Andersen J, Lam YW, Moorhead G, Mann M, Lamond AI. 2006. Repo-Man recruits PP1γ to chromatin and is essential for cell viability. J Cell Biol. 172:679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]