Abstract
To ensure the maintenance of tissues in mammals, cell loss must be balanced with cell production, the proliferative activity being different from tissue to tissue. In this article, the authors propose a new method for the quantification of the proliferative activity, defined as the S-phase fraction of actively cycling cells, by dual labeling with fluorescence and peroxidase immunohistochemistry using BrdU (marker of S-phase) and Ki67 antibodies (marker of G1-, S-, G2-, and M-phases) after a one-step antigen retrieval. In the generative cell zones of fundic and pyloric glandular stomachs, where the majority of cells were cycling, the authors measured a proliferative activity of 31%. In the epithelium of the forestomach and the skin, where cycling cells are intermingled with G0 and differentiated cells, proliferative activities were 21% and 13%, respectively. In the adrenal cortex, in which cycling cells were sparsely distributed, the proliferative activity reached 32%. During the regenerative process in the skin after a lesion, the proliferative activity increased in proximity to the wound. The present one-step dual-labeling method has revealed that the proliferative activity is different between tissues and depends on the physiological or pathological state.
Keywords: BrdU, Ki67, stomach, adrenal gland, skin, fluorescence immunohistochemistry, peroxidase immunohistochemistry
To maintain tissue homeostasis, the cell loss by apoptosis should be compensated by a steady supply of cells by proliferation. The balance between cell loss and production is strictly controlled in normal adult tissue because an excess of cell loss will result in atrophy and progressive tissue failure, whereas excessive cell production may cause hyperplasia and even tumors. Therefore, the understanding of how such a fine balance is achieved is particularly relevant for human health.
The proportion and the distribution of cells in the cell cycle are different from tissue to tissue. For detecting actually proliferating cells in the cell cycle, the nuclear protein Ki67 is frequently used because Ki67 is expressed in all phases of cell cycle, including G1, S, G2, and M, but is absent in resting cells (G0) and differentiated cells (Gerdes et al. 1982; Gerdes et al. 1984; Burger et al. 1986; Lellé et al. 1987). Ki67 immunohistochemistry, however, can only yield information about the proliferative state of the cells but not about the rate at which they are produced (Nakajima et al. 1999). To estimate the rate of cell proliferation, the labeling of S-phase by incorporation of [3H]thymidine or its nonradioactive pyrimidine analogue, 5-bromo-2′-deoxyuridine (BrdU; Gunduz 1985; Langer et al. 1985), is frequently used. These S-phase markers were previously used for analyzing the proliferative activity in tissues having distinct regions composed of proliferating cells—that is, the generative cell zones of the gastrointestinal tract (Lipkin et al. 1964; Hattori and Fujita 1976; Vries et al. 2010).
In most tissues and organs, however, the proliferating cells are not spatially accumulated like the generative cell zones of the intestinal crypt bottoms but are dispersed and intermingled with dormant G0 and/or differentiated cells. For example, in the cortex of the adrenal gland, proliferative cells are very sporadically distributed in the zona glomerulosa and outer half of the zona fasciculata (Ulrich-Lai et al. 2006). Previously, [3H]thymidine or BrdU was used, but they were only used for localizing S-phase cells in the adrenal gland (Schulte et al. 2007). In the present study, we developed a new method enabling the quantification of the S-phase fraction (S-phase cells/actively cycling cells) by dual-labeling fluorescence/peroxidase immunohistochemistry using BrdU and Ki67 antibodies.
Using this method, we accurately quantified the proliferative activity, here defined as the S-phase fraction of a proliferating cell population, in the forestomach, skin, and adrenal gland, where proliferating cells are sparse in the tissue. In addition, using the same method, we showed the effect of a skin lesion on the proliferative activity surrounding the wound.
Materials and Methods
Animals
Eight-week-old male C57BL/6 mice (Japan Lab Animals Co., Ltd., Osaka, Japan) were used in this study. BrdU (0.15 mg/g body weight; Sigma, St. Louis, MO) was injected intraperitoneally. After 30 min, animals were sacrificed by decapitation. Stomach, adrenal gland, and dorsal skin were excised. Sampled tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 48 hr. After fixation, the tissues were embedded in paraffin, and 3-µm-thick sections were prepared using a microtome. The sections were then mounted on glass slides. For the skin epidermis regeneration study, animals were anesthetized deeply with ether, and then a 3-mm2 area in the back skin was excised, containing the epidermis, dermis, and subcutaneous tissue. After 5 days following the lesion, animals were injected with BrdU and sampled as described above. All studies reported here were approved by the Animal Experimentation Committee of Kyoto University.
Immunohistochemistry
Paraffin-embedded, glass slide–mounted mouse tissue sections were deparaffinized and hydrated. For antigen retrieval, sections were then boiled under pressure in 10 mM Tris-HCl (pH 9.0) 1 mM EDTA for 2.5 min. After pressurizing was stopped, the cooker was left to cool at room temperature for 1 min, followed by cooling with tap water.
After rinsing in 0.01 M phosphate-buffered saline (PBS) for 5 min, the sections were incubated with the mixture of primary rat monoclonal anti-BrdU antibody (code no. BU1/75(ICR1); dilution 1:2000 with 0.01 M PBS; Abcam, Cambridge, UK) and rabbit anti-Ki67 antibody (Abcam; dilution 1:500) overnight at 4C. After rinsing with 0.01 M PBS containing 0.1% Tween-20, sections were incubated with the mixture of three species-specific secondary antibodies: donkey anti-rat Alexa 488–conjugated IgG (Molecular Probes, Eugene, OR; dilution 1:200), donkey anti-rat biotin-conjugated IgG (Jackson ImmunoResearch, West Grove, PA; dilution 1:1000), and donkey anti-rabbit Alexa 594–conjugated IgG (Molecular Probes; dilution 1:200) for 1 hr at room temperature.
Fluorescent images were acquisitioned with a fluorescence microscope (Zeiss, Oberkochen, Germany). After fluorescent picture acquisition, sections were rinsed and then treated with avidin/biotinylated horseradish peroxidase (Vector Labs, Burlingame, CA; dilution 1:200) for 1 hr at room temperature, then visualized with diaminobenzidine (DAB; Wako Pure Chemical, Osaka, Japan). Sections were counterstained with hematoxylin, dehydrated, and mounted with Entellan (Merck, Whitehouse Station, NJ). Sections were observed and photographed using a light microscope (Zeiss).
Count and Statistics
BrdU-positive cells exhibited both Alexa 488 green fluorophore and peroxidase-DAB brown chromogens. Ki67-positive cells showed Alexa 594 red fluorophore. BrdU/Ki67 double-labeled cells showed the three labels, Alexa 488, Alexa 596, and DAB. Cells from the glandular stomach (fundus and pylorus), the forestomach, the skin, and the adrenal cortex were counted. Cells with brown-stained granules without Alexa 488 fluorescence are melanin-storing cells and were excluded from BrdU counting. The incidence of Alexa 488–labeled BrdU-positive staining within the Alexa 594–labeled Ki67-positive cell population was measured by counting more than 100 Ki67-positive cells per animal (n = 4) and was expressed as a percentage of proliferative activity in each tissue. For statistical analysis, one-way analysis of variance (ANOVA) followed by Scheffé’s multiple comparisons was applied.
Results
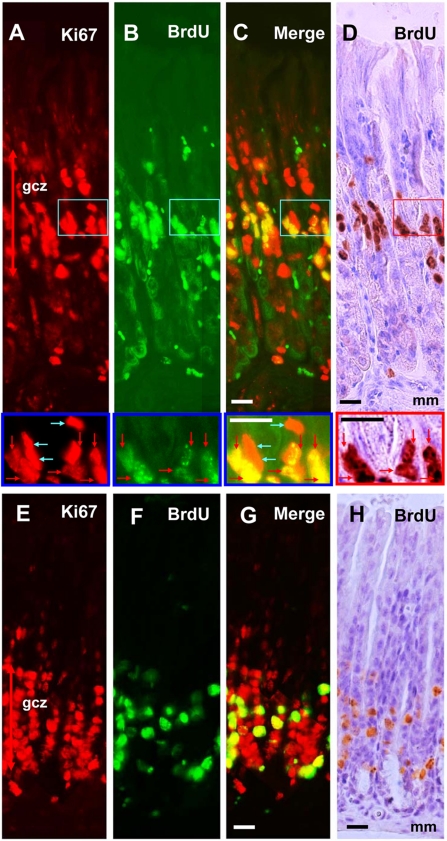
Ki67 and BrdU stainings were found in all tissues examined with characteristic distribution patterns. In the fundic mucosa of the glandular stomach, Ki67-positive cells were accumulated in the isthmus of the gland. We defined the generative cell zone as the innermost to outermost area delimited by Ki67-positive cells, which is an extension of the definition from previous reports using the mitotic index (Stevens and Leblond 1953) or [3H]thymidine incorporation (Lipkin et al. 1964; Hattori and Fujita 1976) (Fig. 1A). A single injection of BrdU labeled many cells in this zone (Fig. 1B,D). As expected, all BrdU-immunopositive cells were also immunoreactive to Ki67 (Fig. 1C), but there were some BrdU-negative Ki67-positive cells in the generative cell zone (blue arrows in high-magnification photos in Fig. 1A–D). In the pyloric mucosa of the glandular stomach, a Ki67-positive generative cell zone was observed near the bottom of the glandular epithelium just above the muscularis mucosae (Fig. 1E). Similar to the fundic mucosa, a number of BrdU-positive cells were observed after the BrdU loading (Fig. 1F–H).
Figure 1.
Dual fluorescence and peroxidase immunohisto-chemistry with BrdU and Ki67 antibodies in the glandular stomach. Panels A, B, C, E, F and G are assembled from adjacent fields taken at high resolution. Fundic gland mucosa (A–D) and pyloric gland mucosa (E–H) stained with Ki67–Alexa 594 (A, E), BrdU–Alexa 488 (B, F), merged images of Ki67–Alexa 594 and BrdU–Alexa 488 to see the colocalization (C, G), and BrdU-peroxidase-DAB counterstained with hematoxylin (D, H). Squared areas of A to D are magnified in the bottom of each photograph. Red arrows indicate S-phase cells (BrdU+/Ki67+ cells), and blue arrows indicate non-S-phase cells (BrdU−/Ki67+) in the mitotic cycle. gcz, generative cell zone; mm, muscularis mucosae. Bars = 20 µm.
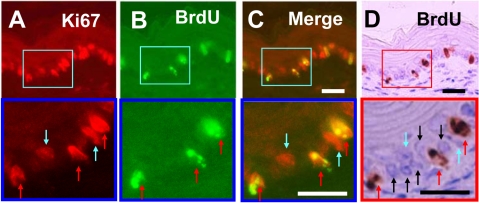
Double labeling of Ki67 and BrdU was also examined in the forestomach, where the epithelium is covered with stratified squamous epithelium (Fig. 2). In this tissue, Ki67-positive cells represented about 40% to 60% of total epithelial cells (Fig. 2A). After BrdU injection, many of the Ki67-positive cells were also immunoreactive to BrdU (Fig. 2B–D).
Figure 2.
Dual fluorescence and peroxidase immunohistochemistry in the forestomach covered with stratified squamous epithelium. Sections are stained with Ki67–Alexa 594 (A), BrdU–Alexa 488 (B), merge of photo A and photo B (C), and BrdU-peroxidase-DAB counterstained with hematoxylin (D). Squared areas of A to D are magnified at the bottom of each photograph. Note three types of cells: red arrows indicate S-phase cells (BrdU+/Ki67+ cells), blue arrows non-S-phase cells (BrdU−/Ki67+), and black arrows non-cycling cells. Bars = 20 µm.
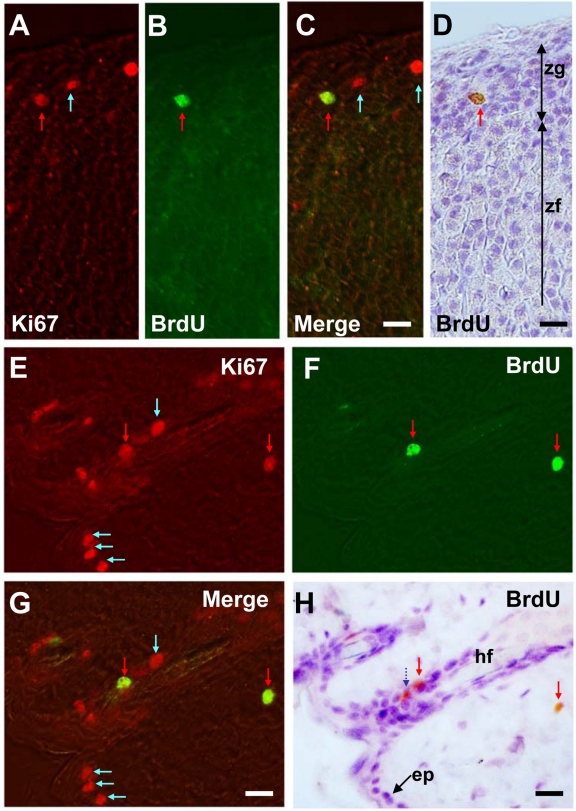
In the adrenal cortex, Ki67-positive cells were found mainly in the zona glomerulosa (Fig. 3A), and some were found in the outer half of the zona fasciculata (data not shown). The Ki67-positive cells represent 1% to 5% of total glomerulosa cells. After BrdU injection, about one-third of Ki67-positive cells were also immunoreactive for BrdU (Fig. 3B–D).
Figure 3.
Dual fluorescence and peroxidase immunohistochemistry in the adrenal cortex (A–D) and back skin (E–H). Sections are stained with Ki67–Alexa 594 (A, E), BrdU–Alexa 488 (B, F), merged images of Ki67–Alexa 594 and BrdU–Alexa 488 to see the colocalization (C, G), and BrdU-peroxidase-DAB counterstained with hematoxylin (D, H). Red arrows indicate S-phase cells (BrdU+/Ki67+ cells) and blue arrows non-S-phase cells (BrdU−/Ki67+). A dark blue dotted arrow indicates melanin-containing cells in the skin. ep, epithelial cells; hf, hair follicle; zg, zona glomerulosa; zf, zona fasciculata. Bars = 20 µm.
In the skin, Ki67-positive cells constitute about 20% to 40% of the one to two layered stratified epithelial cells (Fig. 3E). Ki67 immunoreactivity was also found in sebaceous cells nearby hair follicles. After BrdU injection, double-labeled cells were sporadically found (Fig. 3F–H). Cells with brown granules (melanin) but devoid of Alexa 488 signal were also observed. These cells were excluded from the analysis (dotted blue arrow in Fig. 3H).
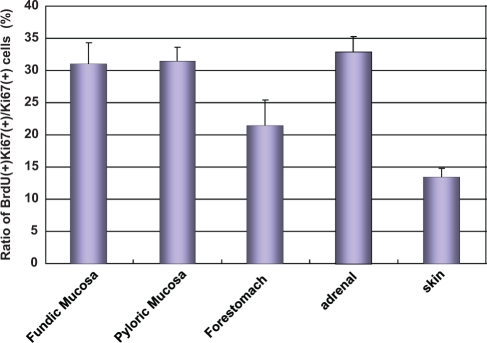
To quantify the proliferative activity, we calculated the ratio of BrdU-labeled cells among Ki67-immunopositive cells in each tissue (Fig. 4). In the generative cell zone of the glandular stomach, the proliferative activities of the fundic and pyloric glands were 30.92 ± 3.43% (mean ± SEM, n = 4) and 31.33 ± 2.12%, respectively. In the forestomach, the proliferative activity was 21.44 ± 3.95%. In the adrenal gland and skin, proliferative activities were 32.74 ± 2.47% and 13.30 ± 1.47%, respectively. The proliferative activity in the skin was significantly lower than that in the fundic and pyloric stomach and adrenal cortex (p < 0.05).
Figure 4.
Proliferative activity in the forestomach, fundic and pyrolic glandular stomach, adrenal cortex, and skin. Proliferative activity is shown as a ratio of BrdU labeling among Ki67-labeled cells. The values are expressed as mean ± SEM (n = 4).
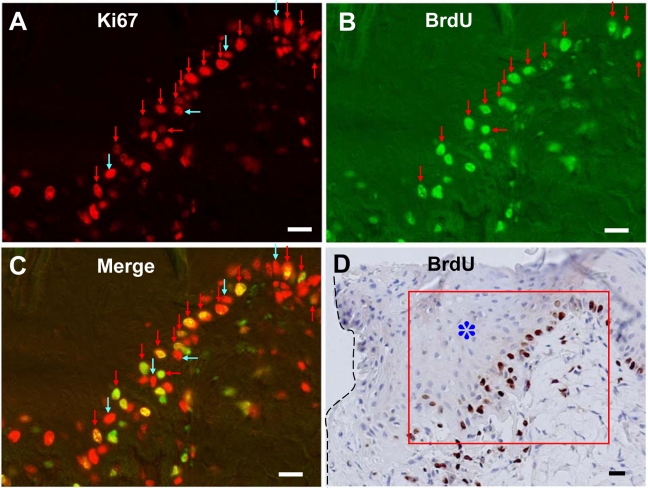
We also performed Ki67 and BrdU stainings in the regenerating skin after 5 days of skin excision. Neighboring skin near the wound edge became thicker to several layered squamous epithelium, but the Ki67 and/or BrdU stainings were only observed in the stratum basale (Fig. 5). The incidence of Ki67 immunoreactivity and BrdU incorporation in the epidermis near the wound was markedly increased. However, the skin further from the wound (1-cm distance) did not show any difference in morphology and in immunostainings for Ki67 and BrdU (data not shown). The proliferative activity of skin surrounding the lesion and of distal skin was 52.52 ± 4.35% (mean ± SEM, n = 3) and 12.88 ± 2.45%, respectively.
Figure 5.
Dual fluorescence and peroxidase immunohistochemistry in the regenerating skin marginal to the wound after 5 days of injury. Sections are stained with Ki67–Alexa 594 (A), BrdU–Alexa 488 (B), merged images of Ki67–Alexa 594 and BrdU–Alexa 488 to see the colocalization (C), and BrdU-peroxidase-DAB counterstained with hematoxylin (D). Red arrows indicate S-phase cells (BrdU+/Ki67+ cells) and blue arrows non-S-phase cells (BrdU−/Ki67+). Ki67 and/or BrdU stainings were observed only in the stratum basale and not in the thickened stratum spinosum (*: asterisk). Note the high incidence of S-phase cells (BrdU+/Ki67+ cells) in the stratum basale marginal to the wound. Margin of wound is shown in a broken line. Squared area of D shows the area of the photographed region of fluorescent data of A to C. Bars = 20 µm.
Discussion
By dual labeling with Ki67 and BrdU, we determined the proliferative activities in various tissues, in which the proportion and spatial distribution of proliferating cells diverged. In the generative zone of the glandular stomach, where the majority of cells are in the mitotic cycle, the proliferative activity was 30% to 35%, for both the fundus and the pyrolus. These values of proliferative activity are much higher than those of previous reports (Bykorez and Ivashchenko 1984) because we used Ki67-positive cells as a reference, whereas these authors have used an estimate of the total cells in the generative zone.
The present method allowed us to accurately quantify the proliferative activity, even in tissues in which actively cycling cells are sparsely distributed among many G0 and/or differentiated cells. In epithelia of the forestomach and the skin, where cycling cells comprise 30% to 60% of total cells, proliferative activities were 20% and 10% to 15%, respectively. In the zona glomerulosa of the adrenal cortex, where cycling cells are much less frequent than in the forestomach and skin, the proliferative activity, however, reached 35%. These findings suggest that the proliferative activity under physiological conditions, reflecting the ability of stem cells to provide the tissue with new cells, is different between tissues. Interestingly, we observed that the proliferative activity does not correlate with the amount of actively cycling cells in the whole tissue examined or with how localized the cycling cell population is. Indeed, 35% of the 1%–5% of cycling cells of the adrenal cortex were in the S-phase, compared with only ~20% of the 40%–60% of cycling cells in the forestomach. Moreover, the aggregated cells in the generative cell zones of the stomach and the dispersed cells in adrenal cortex both showed a proliferative rate of 30% to 35%.
Does the proliferative activity change under pathological conditions? To address this issue, we examined the dynamic changes of Ki67 expression, BrdU incorporation, and proliferative activity in the regenerating skin after a skin lesion. In the marginal skin region of the wound, not only the expression of Ki67 but also the incorporation of BrdU increased in the stratum basale, and the proliferative activity markedly increased in the regenerating epidermis. This suggests that the cell cycle duration of epidermal cells became shorter to allow accelerated regeneration (Clausen et al. 1986). The proliferative activity, as calculated here using the dual-labeling method, can be used as a marker to assess the cell regeneration ability in pathological conditions in vivo.
[3H]thymidine was first introduced in 1960s to detect cells in S-phase. Although the accuracy of this method has been proven, handling radioactive materials and time-consuming procedures are major drawbacks. BrdU has also been used extensively as an S-phase marker, but pretreatments necessary for visualization of BrdU in formaldehyde-fixed tissues such as hydrolysis by hydrochloride, denaturing by sodium hydroxide or formamide, and digestion by proteinase (Muskhelishvili et al. 2003) are not compatible with the detection of Ki67 protein (Sasaki et al. 1988). We succeeded in detecting both BrdU and Ki67 in paraformaldehyde-fixed specimens using a one-step heat retrieval in pH 9.0 buffer. This alkaline–heat retrieval method not only induces conformational change of covering proteins (Shi et al. 1996; Kajiya et al. 2009) but also denatures DNA permanently. Thus, our one-step alkaline–heat activation procedure constitutes a very simple and useful method for the double retrieval of BrdU and Ki67 antigens.
In the present study, we performed dual labeling against Ki67 and BrdU with species-specific secondary antibodies with specific fluorophores or chromogen markers. Using secondary antibodies labeled with specific fluorophores with distinct excitation–emission spectra or labeled with chromogen, we could omit sequential application of the antisera and could rapidly detect antigens without cross-reactivity. Moreover, the specificity of BrdU labeling was double-checked using Alexa 488 under fluorescent microscopy and DAB chromogen under light microscopy. In this way, we could discriminate brown DAB chromogen granules from melanin pigments present in the skin and other regions of the body of black-haired C57BL/6 mice. Light microscopic analysis of hematoxylin-counterstained sections improved histological examination on permanent specimens.
In conclusion, we have succeeded in measuring the proliferative activity in various tissues in the body, regardless of the amount of cells actively cycling and their distribution. This dual-labeling method could be applied to human tissues such as biopsy specimens and small surgical materials (Sasaki et al. 1988; Van Erp et al. 1989; Tsurusawa et al. 1995). Following preincubation with BrdU containing buffer, these tissues can be subject to our dual-labeling immunohistochemistry of Ki67 and BrdU to estimate the proliferative activity of tumors and that under various diseases such as injury and inflammation. By using this method, it would be possible to estimate kinetic parameters in tissue repair and tumor progression.
Acknowledgments
We thank Dr. Miki Tanioka for advice in the experiment of skin regeneration.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported in part by the Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Health Labour Sciences Research Grant; SRF; Uehara Memorial Foundation; and Takeda Science Foundation.
References
- Burger PC, Shibata T, Kleihues P. 1986. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol. 10:611–617 [DOI] [PubMed] [Google Scholar]
- Bykorez AI, Ivashchenko YuD. 1984. Gastrointestinal stem cells and their role in carcinogenesis. Int Rev Cytol. 90:309–373 [DOI] [PubMed] [Google Scholar]
- Clausen OP, Kirkhus B, Schjølberg AR. 1986. Cell cycle progression kinetics of regenerating mouse epidermal cells: an in vivo study combining DNA flow cytometry, cell sorting, and [3H]dThd autoradiography. J Invest Dermatol. 86:402–405 [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stern H. 1984. Cell cycle analysis of a cell proliferation–associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 133:1710–1715 [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemre H, Stein H. 1982. Production of a mouse monoclonal antibody reactive with a human-nuclear antigen associated with cell proliferation. Int J Cancer. 31:13–20 [DOI] [PubMed] [Google Scholar]
- Gunduz N. 1985. The use of FITC-conjugated monoclonal antibodies for determination of S-phase cells with fluorescence microscopy. Cytometry. 6:597–601 [DOI] [PubMed] [Google Scholar]
- Hattori T, Fujita S. 1976. Tritiated thymidine autoradiographic study of cell migration and renewal in the pyloric mucosa of golden hamsters. Cell Tiss Res. 175:49–57 [DOI] [PubMed] [Google Scholar]
- Kajiya H, Takekoshi S, Takei M, Egashira N, Miyakoshi T, Serizawa A, Teramoto A, Osamura RY. 2009. Selection of buffer pH by the isoelectric point of the antigen for the efficient heat-induced epitope retrieval: re-appraisal for nuclear protein pathobiology. Histochem Cell Biol. 132:659–667 [DOI] [PubMed] [Google Scholar]
- Langer EM, Rottgers HR, Schliermann MG, Meier EM, Miltenburger HG, Schumann J, Gohde W. 1985. Cycling S-phase cells in animal and spontaneous tumours: I. Comparison of the BrdUrd and 3H-thymidine techniques and flow cytometry for the estimation of S-phase frequency. Acta Radiol Oncol. 24:545–548 [DOI] [PubMed] [Google Scholar]
- Lellé RJ, Heidenreich W, Stauch G, Wecke I, Gerdes J. 1987. Determination of growth fractions in benign breast disease (BBD) with monoclonal antibody Ki-67. J Cancer Res Clin Oncol. 113:73–77 [DOI] [PubMed] [Google Scholar]
- Lipkin M, Sherlock P, Bell B. 1964. Cell proliferation kinetics in the gastrointestinal tract of man: II. Cell renewal in stomach, ileum, colon, and rectum. Gastroenterology. 45:405–417 [PubMed] [Google Scholar]
- Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. 2003. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 51:1681–1688 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kagawa K, Deguchi T, Kimura H, Kakusui M, Katagishi T, Mitsumoto Y, Okanoue T, Kashima K, Ashihara T. 1999. Novel formula for cell kinetics in xenograft model of hepatocellular carcinoma using histologically calculable parameters. Exp Cell Res. 246:412–420 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Matsumura K, Tsuji T, Shinozaki F, Takahashi M. 1988. Relationship between labeling indices of Ki-67 and BrdUrd in human malignant tumors. Cancer. 62:989–993 [DOI] [PubMed] [Google Scholar]
- Schulte DM, Shapiro I, Reincke M, Beuschlein F. 2007. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr Patterns. 7:72–81 [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Yang C, Chen C, Xu HJ, Benedict WF, Taylor CR. 1996. Development of an optimal protocol for antigen retrieval: a ‘test battery’ approach exemplified with reference to the staining of retinoblastoma protein (pRB) in formalin-fixed paraffin sections. J Pathol. 179:347–352 [DOI] [PubMed] [Google Scholar]
- Stevens CE, Leblond CP. 1953. Renewal of the mucous cells in the gastric mucosa of the rat. Anat Rec. 115:231–246 [DOI] [PubMed] [Google Scholar]
- Tsurusawa M, Aoyama M, Saeki K, Fujimoto T. 1995. Cell cycle kinetics in childhood acute leukemia studied with in vitro bromodeoxyuridine labeling, Ki67-reactivity, and flow cytometry. Leukemia. 9:1921–1925 [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 291:E965–E973 [DOI] [PubMed] [Google Scholar]
- Van Erp PE, De Mare S, Rijzewijk JJ, Van de Kerkhof PC, Bauer FW. 1989. A sequential double immunoenzymic staining procedure to obtain cell kinetic information in normal and hyperproliferative epidermis. Histochem J. 21:343–347 [DOI] [PubMed] [Google Scholar]
- Vries RG, Huch M, Clevers H. 2010. Stem cells and cancer of the stomach and intestine. Mol Oncol. 4:373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]