Abstract
Cutaneous lupus erythematosus and dermatomyositis (DM) are chronic inflammatory diseases of the skin with accumulated dermal mucin. Earlier work has shown chondroitin sulfate (CS) accumulation within the dermis of discoid lupus erythematosus (DLE), subacute cutaneous lupus erythematosus (SCLE), and DM lesions compared with control skin. Immunohistochemistry for C4S revealed a greater density in DLE and DM lesions, whereas SCLE lesions did not differ from controls. Scleredema and scleromyxedema are attributed to increased hyaluronic acid, and lesional samples from these diseases also demonstrated accumulated dermal C4S. Interferon-γ and interleukin-1α, but not interferon-α, treatment of cultured dermal fibroblasts induced mRNA expression of CHST-11, which attaches sulfates to the 4-position of unsulfated chondroitin. These studies on possible CS core proteins revealed that serglycin, known to have C6S side chains in endothelial cells, had greater density within DM dermal endothelia but not in DLE or SCLE, following the pattern of C6S overexpression reported previously. CD44 variants expand the CS binding repertoire of the glycoprotein; CD44v7 co-localized to the distribution of C4S in DLE lesions, a finding not observed in DM, SCLE lesions, or controls. Because C4S and C6S have immunologic effects, their dysregulation in cutaneous mucinoses may contribute to the pathogenesis of these disorders.
Keywords: chondroitin sulfate, glycosaminoglycans, lupus erythematosus, dermatomyositis, cutaneous autoimmune disease
Cutaneous lupus erythematosus (CLE) and dermatomyositis (DM) are autoimmune skin diseases whose pathogenesis is not completely understood (Krathen et al. 2008). Both conditions show increased Hale stain, which identifies mucin thought due to production of hyaluronic acid by immunologically activated dermal fibroblasts (Ackerman et al. 1997; Igarashi et al. 1985; del Pozo et al. 2001; Rongioletti and Rebora 2001). More recent work suggests the role of an activating serum factor in these patients, and in vitro treatment of dermal fibroblasts with various cytokines such as interleukin (IL)-1 has been documented to modulate glycosaminoglycan (GAG) synthesis (Campo et al. 2006; Duncan and Berman 1989; Edward et al. 2007; Postlethwaite et al. 1989).
Mucin is chemically composed of GAG chains, repeating disaccharide units of N-acetylgalactosamine and glucuronic acid that bind to core proteins to form proteoglycans. The functional roles of proteoglycans are broad and may be attributable to its core protein, the GAG side chains, or both. GAG chains typically exert these functions largely through interactions with proteins, and these interactions are governed by the fine structure of the GAG (Tiedemann et al. 2005). Accordingly, structural composition provides a basis for classification of GAG chains; chondroitin sulfate (CS) is one such negatively charged GAG chain naturally occurring within the extracellular matrix of a variety of human connective tissues.
CS-binding proteoglycans are an area of active investigation and include versican, decorin, biglycan, and serglycin, to name a few. Serglycin has a wide range of functions within hematopoietic and endothelial cells, including leukocyte migration and modulation of secretory vesicle activity (Kulseth et al. 1999; Schick et al. 2001). Recent studies have demonstrated that CS chains bind to the cell surface glycoprotein CD44, particularly in the setting of an expanded GAG-binding repertoire secondary to the strictly regulated, alternatively spliced inclusion of variant exons (Sleeman et al. 1997). CD44 has been implicated in a variety of allergic and autoimmune reactions for its role in adhesion-dependent cellular processes as well as cell signaling and activation, cell–cell and cell–matrix interactions, and metastasis (Herrlich et al. 1993; Knudson and Knudson 1993; Lesley et al. 1993; Seiter et al. 1998).
More recent work on CS has demonstrated a variety of immunologic functions in vitro, including blocking the effects of tumor necrosis factor (TNF)-α and IL-1β in cell culture and activating neutrophils, monocytes, and B-cells (Fioravanti and Collodel 2006; Rachmilewitz and Tykocinski 1998; Xiao et al. 2008; Xu et al. 2008).
Earlier work from our group has shown an accumulation of both hyaluronic acid and CS within the dermal matrix mucin found in CLE and DM lesions, which both stain blue on Hale stain (Chang et al. 2011). The CS in the dermis was not C6S and could be attributable to chondroitin sulfate A (C4S), E, or dermatan sulfate. Validated microarray of lesional CLE skin revealed an upregulation in the genes encoding two key enzymes involved in CS synthesis and/or assembly: CS synthase-1 (CSS1) and the carbohydrate sulfotransferase-11 (CHST11, also referred to as C4ST1), which attaches sulfates to the 4-position of unsulfated chondroitin. Because distinct structural features mediate specific functions of Chondroitin sulfate proteoglycans (CSPGs), the aim of the present study was to further characterize the accumulated CS and core proteins in CLE and DM lesional skin as well as evaluate potential mechanisms for the regulation of polysaccharides in these inflammatory conditions.
Materials and Methods
Skin Specimen Collection
Lesional skin biopsies from patients with subacute CLE (SCLE, n=11), discoid lupus erythematosus (DLE, n=11), dermatomyositis (DM, n=12), scleredema (n=3), and scleromyxedema (n=3) were obtained from patients in the dermatology clinic at the Hospital of the University of Pennsylvania. Twelve healthy control skin samples were provided by volunteer patients without history of an autoimmune skin disease at the Philadelphia VA Hospital. All biopsies were obtained according to approved protocol under the University of Pennsylvania institutional review board, following the Declaration of Helsinki protocols as well as written declaration of informed consent. Biopsies were fixed in 10% neutral buffered formalin overnight and embedded into paraffin blocks, and sections were cut.
Immunohistochemical Staining
Immunohistochemical staining was performed on human skin samples as described previously (Chang et al. 2011), using an anti-human monoclonal antibody recognizing an octasaccharide epitope containing a core GlcUA-GalNAc(4S)-GlcUA(2S)-GalNAc(6S) tetrasaccharide with a neighboring reducing structural element (Ito et al. 2005) (clone CS-56; Sigma, St. Louis, MO); an anti-human polyclonal antibody raised against a peptide mapping near the N-terminus of serglycin (clone N-13, Santa Cruz Biotechnology, Santa Cruz, CA); and anti-human monoclonal antibodies against a glutathione S transferase fusion protein corresponding to the variable domains (v3, v6, and v7) of human CD44 (AbD Serotec, Raleigh, NC).
Immunostaining of chondroitin-6-sulfate and chondroitin-4-sulfate was achieved by predigestion with chondroitinase AC II enzyme (Sigma, St. Louis, MO) followed by anti-proteoglycan ΔDi-6S or anti-proteoglycan ΔDi-4S monoclonal antibody; these antibodies recognize a terminal glucuronic acid residue adjacent to N-acetylgalactosamine-6- or 4-sulphate at the non-reducing end of chondroitin sulfate chains in proteoglycans produced after chondroitinase digestion of chondroitin sulphate chains (clones 2-B-6 and 3-B-3, Seikagaku Biobusiness Corporation, Tokyo, Japan). CD44 variant 6 required heat-induced antigen retrieval, whereby slides were incubated in ready-to-use Target Retrieval Solution (Dako, Carpinteria, CA) at 70C for 10 min prior to nonspecific blocking step. Briefly, paraffin-embedded tissue sections were baked overnight at 55C, deparaffinized in three changes of xylene, and rehydrated through graded ethanol solutions (75%, 90%, 100%). Specimens were blocked with 10% normal swine serum (Vector, Burlingame, CA) for 30 min and then endogenous peroxidase blocking reagent (Dako, Carpinteria CA) for 10 min, followed by an hour-long incubation in primary antibody at optimized concentrations. Slides were rinsed in three changes of PBS between all reagent steps. Sections were then incubated in biotinylated secondary antibody and streptavidin-horseradish peroxidase (Dako, Carpinteria, CA) for 30 min each. Vector NovaRED substrate kit was applied for 5 min following manufacturer’s protocol (Vector, Burlingame, CA), and specimens were dehydrated in graded ethanol solutions. Negative controls were processed in an identical fashion save the application of mouse IgG, k isotype antibody (Sigma, St. Louis, MO).
Systematic Quantitation of Immunohistochemical Staining
All high-power fields in three sections of each 3- to 5-mm skin biopsy were examined at ×10 magnification. Among all fields examined, one high-power field appearing most representative of all sections was selected from each biopsy to measure matrix intensity, and this field was photographed at ×10 magnification. An intensity channel was applied to photograph files using commercial software to isolate staining intensity (Image-Pro Plus v5.0). One-hundred 5-µm circular manual tags were selected by systematic uniform random sampling of the area of interest (e.g., within dermal vessels for serglycin or within the upper dermis for CD44v7) (Mayhew 2008). The mean intensity of staining within the total area selected by the manual tags is reported as the representative staining intensity value for each specimen.
Cell Culture and Cytokine Treatment
Normal human fibroblasts were obtained from the American Type Culture Collection (ATCC, Rockville MD) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. Passage 5 or 6 cells were plated in triplicate 100-mm diameter dishes and grown to confluence before cytokine addition. Fibroblasts were treated for 6 hr with human interferon (IFN)-β, 100 U/mL; interferon-α2, 100 U/ mL (PBL Interferon Source, Piscataway, NJ); recombinant human IL-1α, 20 ng/mL; TNF-α, 10 ng/mL; or IFN-γ, 10 ng/mL (R&D Systems, Minneapolis, MN) before lysis with RNAeasy mini kit lysate buffer and processing of mRNA according to manufacturer’s protocol (Qiagen, Valencia, CA). Experiments were performed in triplicate and repeated with reproducible results.
mRNA and Quantitative Real-Time RT-PCR
Total RNA was reverse transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) with random hexamer primers. Real-time PCR was performed using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA) specific for CSS-1 (Assay ID Hs00208704_m1) and CHST11 (Assay ID Hs00218229_m1). PPIA endogenous control (Assay ID Hs99999904_m1) was run for each cDNA sample, and all reactions were performed in triplicate. Real-time PCR was performed on an ABI 7000 Sequence Detection System using the following program: (step 1) 50C for 2 min; (step 2) 95C for 10 min; (step 3) 95C for 15 sec for 40 repetitions; (step 4) 60C for 1 min.
The ΔΔCt method was used to analyze the differential gene expression in cytokine-treated fibroblast cultures compared with controls, as described previously (Chang et al. 2011).
Statistical Analysis
Comparison of several groups simultaneously was performed by initially using analysis of variance (ANOVA) by commercial statistical software (GraphPad Prism v5.01). When the ANOVA indicated differences among the groups of data passing tests for normality, comparisons of each experimental group versus the control group were performed using the Dunnett q statistic posttest. Unless otherwise indicated, we display individual data points along with mean (wide horizontal bars) and SEM (short horizontal bars).
Results
Chondroitin-4-Sulfate Is Significantly Increased in the Dermal Matrix of Lesional DLE and Dermatomyositis Skin
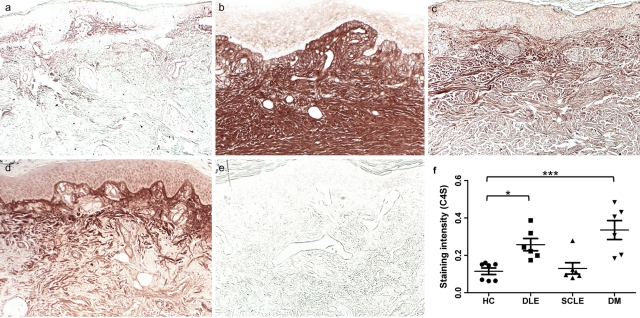
Consistent with prior reports (Sorrell et al. 1990), relatively low levels of C4S were found in the papillary dermis or localized within the dermal endothelia of normal skin (Fig. 1a). The papillary dermal matrix of all DLE (Fig. 1b) and DM (Fig. 1d]) lesional skin samples demonstrated a mean 2.2-fold (p<0.05) and 2.9-fold (p<0.0001) greater density of C4S compared with healthy control biopsies (Fig. 2), respectively, consistent with our prior finding that total CS is increased in lesional DLE and DM skin compared with controls (Chang et al. 2011). Prior work has shown that CD3+ cells are located in areas of dense CS staining in DLE lesions, although this staining did extend beyond the clusters of cells (Chang et al. 2011). DM lesions, however, did not display CD3+ cells in areas of dense CS. C4S expression extended into the reticular dermis of DLE skin, in contrast to an expression limited to the papillary dermis in all other samples. SCLE samples, which we previously found to contain normal amounts of total CS, did not differ in dermal C4S content from controls (Fig. 1c). Accordingly, DLE dermis contained 2.0-fold more C4S than did SCLE dermis (p<0.05). Of note, immunostaining of C4S stubs created by predigestion with chondroitinase AC-II invariably exceeded that of total CS chains identified by the CS-56 monoclonal antibody. However, the relative densities of C4S staining in healthy controls and DLE, SCLE, and DM lesional skin samples were proportional to that of total CS that we previously reported (Chang et al. 2011).
Figure 1.
Cutaneous lupus and dermatomyositis lesions express increased chondroitin-4-sulfate (C4S) in the dermal matrix. Formalin-fixed, paraffin-embedded skin sections of (a) healthy control skin, (b) DLE lesional skin, (c) SCLE lesional skin, and (d, e) DM lesional skin were stained with an antibody specific for δ-di-4S stubs (a-d) or isotype control antibody (e) following predigestion with chondroitinase AC II enzyme. Systematic quantitation of C4S (f) in DLE and DM lesional skin demonstrate a 2.2-fold and 2.9-fold greater density, respectively, compared with healthy control biopsies; C4S density in SCLE lesions did not differ from controls. p=0.0003 by ANOVA; *p<0.05; ***p<0.001 compared with control by the Dunnett test. Every data point is displayed; wide and short horizontal bars represent mean and SEM, respectively. HC, healthy controls; DLE, discoid lupus erythematosus; SCLE, subacute cutaneous lupus erythematosus; DM, dermatomyositis.
Figure 2.
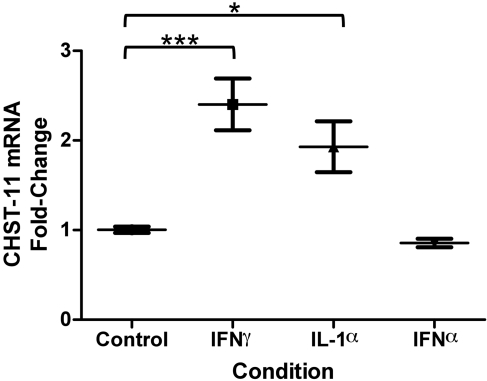
Effect of cytokine treatment on fibroblast CHST-11 mRNA expression. Treatment of cultured dermal fibroblasts with interferon (IFN-)-γ (10 ng/mL) induced a 2.4-fold mean increase in carbohydrate sulfotransferase-11 (CHST11) mRNA expression; interleukin (IL)-1α (20 ng/mL) treatment caused a 1.9-fold mean increase, whereas IFN-α had no effect. p=0.0018 by ANOVA; *p<0.05; ***p<0.001 compared with control by the Dunnett test. Wide and short horizontal bars represent mean and SEM, respectively, of experiments performed in triplicate.
Cytokines IFN-γ and IL-1α Upregulate CHST-11 mRNA Expression in Cultured Dermal Fibroblasts
Treatment of cultured dermal fibroblasts with five cytokines implicated in DLE and DM (TNF-α; IFN-α, β, and γ; IL-1α) failed to alter CSS-1 mRNA levels (data not shown). In contrast, CHST-11 mRNA demonstrated a 2.4-fold mean increase upon exposure of fibroblasts to IFN-γ (p<0.01) and a 1.9-fold mean increase with IL-1α (p<0.05) (Fig. 3). Induction of CHST-11 mRNA was specific to these two cytokines and was not affected by IFN-α, TNF-α, or IFN-β (data not shown).
Figure 3.
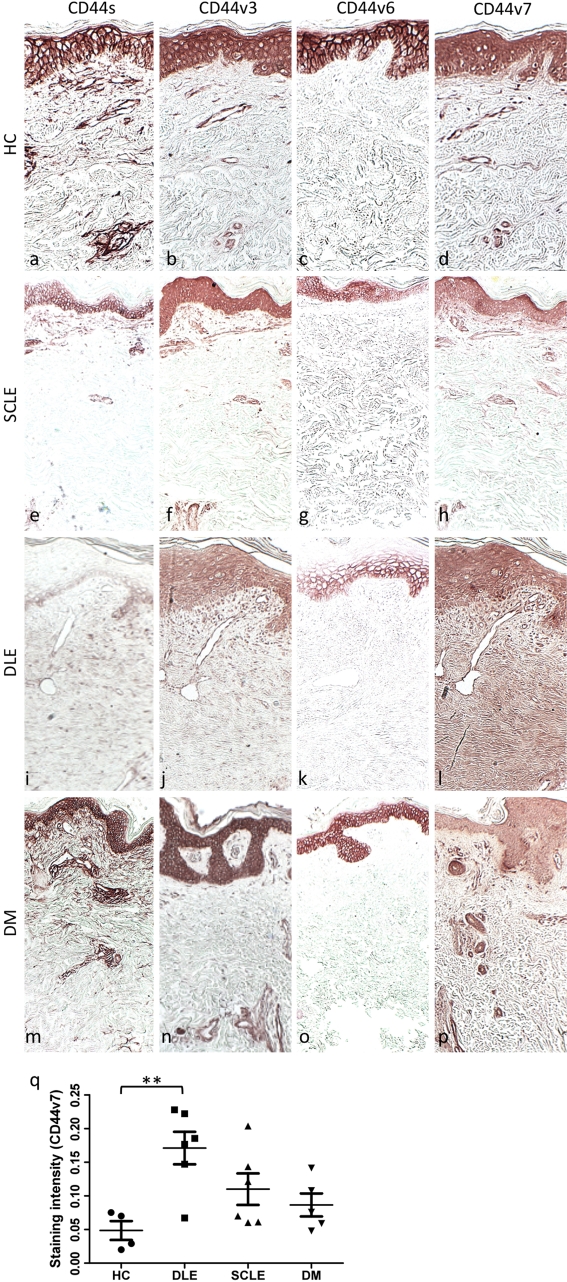
CD44 protein expression in CLE, DM, and healthy control skin; dermal matrix CD44v7 is significantly increased in DLE lesions. Formalin-fixed, paraffin-embedded skin sections of (a-d) healthy control skin, (e-h) SCLE lesion, (i-l) DLE lesion, and (m-p) DM lesion were stained with antibodies specific for CD44s (a, e, i, m), CD44v3 (b, f, j, n), CD44v6 (c, g, k, o), and CD44v7 (d, h, l, p). Systematic quantitation of CD44v7 immunostaining (q) within the papillary dermal matrix of all DLE lesions demonstrated a 3.5-fold greater mean density compared with healthy control biopsies. CD44v7 density in SCLE and DM lesions did not significantly differ from controls. p=0.0085 by ANOVA; **p<0.01 compared with control by the Dunnett test. HC, healthy controls; DLE, discoid lupus erythematosus; SCLE, subacute cutaneous lupus erythematosus; DM, dermatomyositis. Every data point is displayed; wide and short horizontal bars represent mean and SEM, respectively. (Original magnification ×5.)
CD44 Variants Are Differentially Expressed in SCLE, DLE, and DM Skin
Given the presence of C4S as the predominant CS in DLE and DM, we next wanted to look at molecules that are either covalently or noncovalently associated with C4S and that have been causally implicated in autoimmunity. CD44 was selected because of its associations with C4S and the association of several of its isoforms with autoimmunity (Keller et al. 2007; Wittig et al. 2000). Inclusion of alternatively spliced variant exons has been shown to expand the GAG binding repertoire of CD44 to include CS (Sleeman et al. 1997), leading us to investigate the expression of CD44 variant isoforms in our skin samples. In the rat pancreatic carcinoma cells studied by Sleeman et al. (1997), both CD44v6 and v7 were required to confer binding ability to chondroitin sulfate chains. CD44s, CD44v3, CD44v6, and CD44v7 expression in healthy control skin samples was consistent with that previously described (Leigh et al. 1996; Seiter et al. 1996, 1998) and displayed strong epidermal expression, particularly within the basal layer and stratum spinosum, with little dermal matrix expression beyond staining of infiltrating leukocytes (Fig. 4).
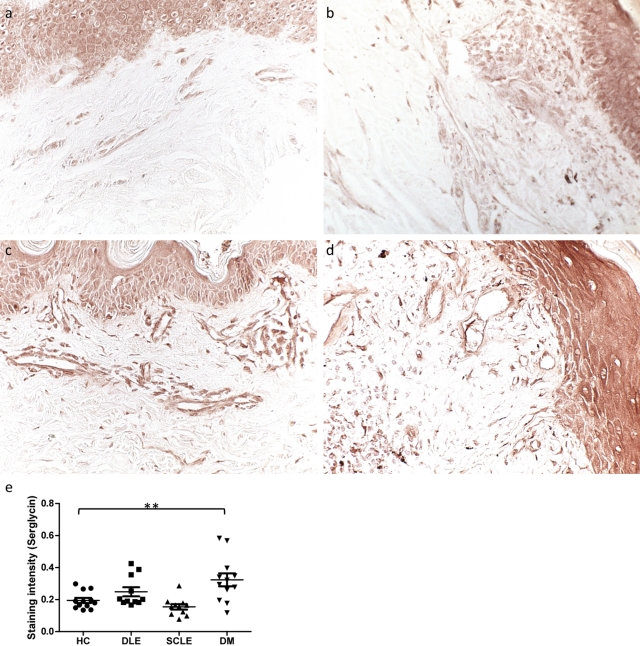
Figure 4.
Serglycin is significantly increased in the dermal vessels of DM lesions. Formalin-fixed, paraffin-embedded skin sections of (a) healthy control skin, (b) SCLE lesional skin, (c) DM lesional skin, and (d) DLE lesional skin were stained with an antibody specific for serglycin (original magnification ×20). (e) Systematic quantitation of serglycin immunostaining revealed a 1.7-fold greater expression in DM dermal vessels compared with controls. This significance was not reached in DLE or SCLE vessels. p=0.0007 by ANOVA; **p<0.01 compared with control by Dunnett test. Every data point is displayed; wide and short horizontal bars represent mean and SEM, respectively. HC, healthy controls; DLE, discoid lupus erythematosus; SCLE, subacute cutaneous lupus erythematosus; DM, dermatomyositis.
DM skin exhibited qualitatively stronger CD44s staining within vessels, whereas DLE and SCLE skin demonstrated weaker epidermal and dermal CD44s staining compared with controls. No further differences in CD44s staining pattern were observed in diseased samples compared with healthy controls. CD44v3 staining was increased in infiltrating leukocytes of DLE samples and within the dermal vessels of DM samples compared with controls; in contrast, SCLE skin had decreased epidermal CD44v3 expression. CD44v6 was limited to the epidermis in all specimens, was decreased in diseased samples compared with controls, and was most notably absent in the most superficial layers of DLE epidermis.
Immunostaining of both healthy control and SCLE skin revealed weak CD44v7 expression in the papillary dermal matrix. DLE skin, however, demonstrated relatively stronger staining in the dermal vessels and on the infiltrating immune cells compared with controls, as well as a robust increased dermal matrix density that extended throughout the entire dermis. DM lesional skin exhibited strong CD44v7 staining within vessels and on infiltrating inflammatory cells but not in the dermal matrix.
Because the C4S and CD44v7 expression patterns of diseased skin samples were most distinct from healthy controls in the papillary dermal matrix, CD44v7 immunostaining was systematically quantitated in this area in our samples to facilitate comparison. CD44v7 expression in SCLE and DM dermis was not found to be statistically different from controls, whereas papillary dermal matrix CD44v7 in DLE samples was a mean 3.5-fold that of controls (p<0.05) (Fig. 5).
Figure 5.
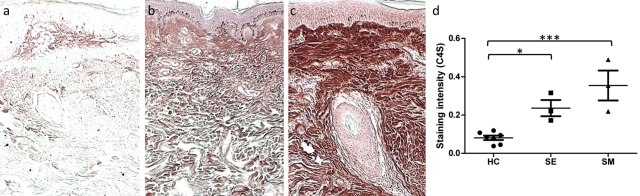
Chondroitin-4-sulfate is significantly increased in scleredema and scleromyxedema lesions. Formalin-fixed, paraffin-embedded skin sections of (a) healthy control skin, (b) scleredema, and (c) scleromyxedema were stained with an antibody specific for chondroitin-4-sulfate (C4S) following digestion with chondroitinase AC II enzyme. (Original magnification ×5.) (d) Systematic quantitation of C4S immunostaining within the dermal matrix of scleredema lesions demonstrated a 2.9-fold greater density compared with healthy control biopsies. Scleromyxedema lesions contained a 4.4-fold mean density. p=0.0008 by ANOVA; *p<0.05; ***p<0.001 compared with control by the Dunnett test. Every data point is displayed; wide and short horizontal bars represent mean and SEM, respectively. HC, healthy controls; SE, scleredema; SM, scleromyxedema.
Serglycin, Like C6S, Is Increased in the Dermal Endothelium of Dermatomyositis Skin
We have previously shown accumulation of C6S within blood vessels of DM and DLE lesions, with greater staining in the former (Chang et al. 2011). Because serglycin has C6S side-chains in endothelium (Schick et al. 2001; Valiyaveettil et al. 2004) and plays a role in adhesion of inflammatory cells, we used immunohistochemistry to characterize serglycin location and intensity. Increased staining was demonstrated in the vessels of DM and DLE lesional skin compared with controls, with more in DM than in DLE vessels (Fig. 4a-d). Systematic quantitation of serglycin immunostaining revealed a 1.7-fold greater expression in DM dermal vessels compared with controls (p<0.01); this significance was not reached in DLE or SCLE vessels (Fig. 4e).
Chondroitin-4-Sulfate, but Not CD44v7, Is Increased in the Dermal Matrix of Scleredema and Scleromyxedema
Prior to our studies, the mucin seen in DLE and DM was attributed predominantly to hyaluronic acid, based in part by positive staining with Alcian blue at pH 2.5 (Kobayashi et al. 1997). We thus investigated the involvement of these proteoglycan components in two other skin conditions known to have significant accumulation of mucin attributed previously to hyaluronic acid. There was a substantial accumulation of CS in the form of C4S in the dermal matrix of both lesional scleredema and scleromyxedema skin compared with healthy controls (Fig. 5a-c]. The expression of C4S mimicked the mucin pattern in the upper and mid-dermis as previously characterized by Rongioletti and Rebora (2001). Scleredema samples exhibited a 2.9-fold (p<0.05) increase in mean C4S density compared with controls, and scleromyxedema samples, 4.4-fold that of controls (p<0.001) (Fig. 5d). CD44v7 expression in scleromyxedema and scleredema did not differ from controls. When compared with scleromyxedematous skin, DLE lesions contained a mean 3.8-fold increased density of CD44v7 (p<0.05).
Discussion
Soluble GAG chains such as CS, attached to core proteins, may be secreted in sites of inflammation by activated monocytes/macrophages or fibroblasts or may accumulate as a byproduct of extracellular matrix degradation (Uhlin-Hansen et al. 1993). The potential functional roles of these CSPGs are diverse, and the literature accordingly reports both pro-inflammatory and anti-inflammatory properties (Campo et al. 2008, 2009; Rachmilewitz and Tykocinski 1998). The selective binding ability of the CSPG may be determined by the structural features of the chain; 4-sulfated CSA and CSB, for example, have expression patterns and functional roles distinct from CSC, which is primarily 6-sulfated. For example, although both forms have been shown to promote fibroblast proliferation, C4S has been shown to inhibit cell adhesion whereas C6S promotes it (Zou et al. 2009); CSA released from platelets increases CCL5 chemokine binding to endothelial cells and supports receptor internalization whereas CSB and CSC block CCL5 binding (Weingart et al. 2008); C4S-containing CSA has been shown to activate monocytes to secrete monokines whereas CSB activates B-cells to proliferate (Rachmilewitz and Tykocinski 1998).
We have shown that the previously reported accumulation of total CS within the papillary dermis of DLE and DM lesions is accounted for by an increase in the C4S species. Furthermore, this C4S accumulation is also observed in scleredema and scleromyxedema lesions pathophysiologically unrelated to DM or DLE. One study detected C4S in sclerotic lesions of generalized morphea (Akimoto et al. 1996). The patterns of C4S are different in DLE relative to DM. DLE lesions demonstrate C4S in a pattern that extends from the papillary to reticular dermal matrix; these lesions commonly have a deeper periadnexal and perivascular infiltrate in addition to that seen at the dermal–epidermal junction, and prior work has shown that CD3+ cells are present in these areas of dense CS staining (Chang et al. 2011; Costner and Jacobe 2000). Although an association with CS and an inflammatory infiltrate is suggested, whether the CS preceded or followed the T cells into the skin is unclear. DM lesions, which typically display a sparse perivascular lymphocytic infiltrate at the dermal–epidermal junction, demonstrate increased C4S density within the papillary dermis with sparing of the reticular dermis and have CD44v7 in a similar distribution to controls.
The observation that immunostaining of C4S stubs exceeded that of total CS is likely secondary to the well-characterized epitope specificity of the CS-56 antibody, which recognizes octasaccharides containing a specific tetrasaccharide sequence for binding (Deepa et al. 2007). The δ-di4S (clone 2B6) monoclonal antibody, in contrast, likely recognizes a neoepitope produced after chondroitinase digestion of chondroitin-4-sulphate glycosaminoglycan chains (e.g., chondroitinase ABC or ACII), that is, a δ-unsaturated glucuronic acid adjacent to N-acetylgalactosamine-4-sulphate in the non-reducing terminal disaccharide “stub” of 4-sulphated chondroitin sulfate. We have shown that the availability of this epitope is indeed increased in DM and DLE lesions compared with that in healthy controls. Based on the method of detection used in this study, it is difficult to definitively conclude what proportion, if any, of the CS chains detected are present in vivo as free GAG chains or bound to core proteins. However, in animals, all GAG chains with the exception of hyaluronic acid are covalently linked to a core protein to give a proteoglycan, and this is likely the case here (Gandhi and Mancera, 2008). Alternatively, hyaluronidases endogenously produced by dermal fibroblasts (Stair-Nawy et al. 1999) may be increased, as these enzymes are overexpressed in the inflammatory setting post UVB irradiation (Averbeck et al. 2007) and in wound repair (Dechert et al. 2006). Chondroitinase AC-II has some known activity against chondroitin sulfate E (CSE), and, accordingly, we cannot rule out the possibility that CSE is present and may contribute to the liberation of C4S stubs observed in our samples. However, at least one study reported partial resistance of CSE to the enzymatic action of chondroitinase, making it less likely to be a significant source of this C4S (Deepa et al. 2007).
Our previous work revealed a 6-fold upregulation of CHST-11 (C4ST1) in the total dermis of CLE lesions compared with healthy control skin samples on validated microarray (Chang et al. 2011). We hypothesized that the derangement of cytokine levels such as IFN-γ or IL-1α in the local matrix milieu of autoimmune skin lesions could induce upregulated mRNA expression of the CHST-11 gene, with a resultant increase in sulfotransferase and C4S production. The present study demonstrates that the regulation of this particular gene in dermal fibroblasts is indeed affected by at least two cytokines, IFN-γ and IL-1α, both implicated in the activated state of innate immunity. Of interest, IFN-α, a type I interferon linked to both CLE and DM (Baechler et al. 2003, 2007; Bennett et al. 2003; Crow et al. 2003; Greenberg et al. 2002, 2005; Raju and Dalakas 2005; Tezak et al. 2002), failed to induce increased CHST-11 expression, demonstrating differential effects of the type I and type II interferons on various components of the pathophysiological cascade of these conditions. Expression of the CS synthase gene CSS-1, in contrast, was found increased on CLE microarray, but the treatment of fibroblast cells with cytokines in this present study failed to affect mRNA expression of this gene; regulation of CSS-1 may be controlled at a different level.
As the CS-binding core protein itself can have a function, we also investigated within our samples the presence of proteins with both known binding ability to CS and association with autoimmunity. CD44 is a transmembrane glycoprotein mediating an abundance of cell–cell and cell–matrix interactions, including cell adhesion, migration, metastasis, lymphocyte recirculation, and wound healing (Haynes et al. 1991). Tightly regulated alternative splicing generates multiple CD44 isoforms, and the inclusion of specific variant 6 and 7 peptide sequences has been shown to expand the binding repertoire of CD44 to include CS (Keller et al. 2007; Sleeman et al. 1997). In addition, blocking or removing CD44v7 plays a key role in downregulating inflammation in a mouse colitis model (Witting et al. 2000). Our results show a co-localization of CD44v7 in the distribution of C4S in DLE lesions, extending from the papillary to reticular dermal matrix, a finding not observed in DM, SCLE lesions, or healthy controls. There are clear differences in the core protein for C4S in various autoimmune skin diseases. In addition, we report that this finding is specific to CD44v7, because CD44s, CD44v3, and CD44v6 failed to show a differential expression between lesions and healthy control skin. The proteoglycan core protein in DM that binds C4S is an area of ongoing investigation.
The matrix pattern of the CD44v7 in DLE lesions is an unusual finding for a cell surface molecule typically localized to the infiltrating inflammatory cell. One hypothesis for this pattern may be that cell surface CD44 is being cleaved and released in a soluble form, as this has been implicated in several pathological processes (Ahrens et al. 2001; Cichy and Pure 2004; Cichy et al. 2005; Knepper et al. 2002; Komura et al. 2002; Miller et al. 2007; Stamenkovic and Yu 2009). CD44 cleavage may be induced by a variety of molecules including hyaluronan oligosaccharides, chondroitin sulfate E, metalloproteases, protein kinase C, epidermal growth factor, or Ras oncoprotein (Murai et al. 2006; Nagano and Saya 2004; Sugahara et al. 2006, 2008).
Our study also investigated potential proteoglycan core proteins for C6S in the endothelium, given the increase of C6S in vessels of specific autoimmune skin diseases. Serglycin is traditionally described as an intracellular proteoglycan with a functional role in the formation of storage granules and is highly expressed in hematopoietic and endothelial cells. The side chains of serglycin vary depending on the cells type, with C4S, C6S, CSE, or CSB seen (Kolset and Gallagher 1990). Increased C6S was found in vessels of DM and to some extent DLE (Chang et al. 2011). The specificity of C6S side chains in endothelial serglycin led to examination of this core protein in DM. Given that serglycin mRNA expression is activated in endothelial cells by TNF-α and IL-1α in a time- and dose-dependent manner (Kulseth et al. 1999), we propose that the activation of the dermal vessels within DLE and DM lesions by circulating pro-inflammatory cytokines contributes to this upregulation of serglycin expression and functions as at least one of the core proteins for the C6S observed. In future work we will investigate the functional significance of these core proteins and their role in autoimmune cutaneous disease.
Last, we compared our findings in DLE and DM lesional skin with those of scleredema and scleromyxedematous skin, two pathophysiologically unrelated cutaneous mucinoses attributed to increased dermal hyaluronic acid. We report a significant accumulation of C4S in scleredema and scleromyxedema skin in a distribution mimicking that seen on Alcian blue at pH 2.5. Alcian blue is nonspecific at pH 2.5 and stains nonsulfated acid GAGs such as hyaluronic acid in addition to strongly acidic sulfated acid GAGs like chondroitin sulfate. In contrast, the same stain at pH 0.5 has greater specificity for chondroitin sulfate, because strongly acidic sulfated acid GAGs also stain at this lower pH whereas hyaluronic acid and nonsulfated acid GAGs do not (Elenitsas et al. 2008). Misinterpretation of Alcian blue staining at various pH conditions prior to the availability of more sophisticated immunohistochemical methods may have contributed to a misidentification of the GAG species in these conditions.
Overall, our results identify specific molecular components of the mucin that accumulates in DLE and DM lesions, and we implicate IFN-γ and IL-1α as key enhancers of CHST-11 expression by dermal fibroblasts. Because C4S and C6S have immunologic effects, their regulation within a complex matrix milieu in DLE and DM may contribute to the pathogenesis of these disorders.
Acknowledgments
Presented at the Society of Investigative Dermatology, 2010.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This material is based on work supported by the Lupus Foundation of America and in part by a Merit Review Grant from the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development).
References
- Ackerman AB, Chongchitnant N, Sanchez J, Guo Y, Bennin B, Reichel M. 1997. Histologic diagnosis of inflammatory skin diseases: an algorithmic method based on pattern analysis. Baltimore, MD: Lippincott Williams & Wilkins [Google Scholar]
- Ahrens T, Sleeman J, Schempp C, Howells N, Hofmann M, Ponta H, Herrlich P, Simon J. 2001. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 20:3399–3408 [DOI] [PubMed] [Google Scholar]
- Akimoto S, Ishikawa O, Yokoyama Y, Amano H, Miyachi Y. 1996. Generalized morphea with vascular involvement: a case report and disaccharide analysis of the skin glycosaminoglycans. Acta Derm Venereol. 76:141–143 [DOI] [PubMed] [Google Scholar]
- Averbeck M, Gebhardt C, Voigt S, Beilharz S, Anderegg U, Termeer C, Sleeman J, Simon J. 2007. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 127:687–697 [DOI] [PubMed] [Google Scholar]
- Baechler E, Batliwalla F, Karypis G, Gaffney P, Ortmann WA, Espe K, Shark K, Grande W, Hughes K, Kapur V, Gregersen P, Behrens T. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 100:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler E, Bauer J, Slattery C, Ortmann W, Espe K, Novitzke J, Ytterberg S, Gregersen P, Behrens T, Reed A. 2007. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med. 13:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L, Palucka A, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 197:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo G, Avenoso A, Campo S, D’Ascola A, Ferlazzo A, Calatroni A. 2006. TNF-α, IFN-γ, and IL-1β modulate hyaluron synthase expression in human skin fibroblasts: synergistic effect by concomitant treatment with FeSO4 plus ascorbate. Mol Cell Biochem. 292:169–178 [DOI] [PubMed] [Google Scholar]
- Campo G, Avenoso A, Campo S, D’Ascola A, Traina P, Calatroni A. 2008. Chondroitin-4-sulphate inhibits NF-kB translocation and caspase activation in collagen-induced arthritis in mice. Osteoarthritis Cartilage. 16:1474–1483 [DOI] [PubMed] [Google Scholar]
- Campo G, Avenoso A, Campo S, D’Ascola A, Traina P, Sama D, Calatroni A. 2009. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 106:83–92 [DOI] [PubMed] [Google Scholar]
- Chang LM, Maheshwari P, Werth S, Schaffer L, Head SR, Kovarik C, Werth VP. 2011. Identification and molecular analysis of glycosaminoglycans in cutaneous lupus erythematosus and dermatomyositis. J Histochem Cytochem 59:336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichy J, Kulig P, Pure E. 2005. Regulation of the release and function of tumor cell-derived soluble CD44. Biochim Biophys Acta. 1745:59–64 [DOI] [PubMed] [Google Scholar]
- Cichy J, Pure E. 2004. Cytokines regulate the affinity of soluble CD44 for hyaluronan. FEBS Lett. 556:69–74 [DOI] [PubMed] [Google Scholar]
- Costner M, Jacobe H. 2000. Dermatopathology of connective tissue diseases. Adv Dermatol. 16:323–360 [PubMed] [Google Scholar]
- Crow M, Kirou K, Wohlgemuth J. 2003. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 36:481–490 [DOI] [PubMed] [Google Scholar]
- Dechert T, Ducale A, Ward S, Yager D. 2006. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen. 14:252–258 [DOI] [PubMed] [Google Scholar]
- Deepa S, Kalayanamitra K, Ito Y, Kongtawelert P, Shigeyuki F, Yamada S, Mikami T, Sugahara K. 2007. Novel sulfated octa- and decasaccharides from squid cartilage chondroitin sulfate E: sequencing and application for determination of the epitope structure of the monoclonal antibody MO-225. Biochemistry. 46:2453–2465 [DOI] [PubMed] [Google Scholar]
- Deepa S, Yamada S, Fukui S, Sugahar K. 2007. Structural determination of novel sulfated octasaccharides isolated from chondroitin sulfate of shark cartilage and their application for characterizing monoclonal antibody epitopes. Glycobiology. 17:631–645 [DOI] [PubMed] [Google Scholar]
- del Pozo J, Almagro M, Martinez W, Yebra-Pimentel MT, Garcia-Silva J, Pena-Penabad C, Fonseca E. 2001. Dermatomyositis and mucinosis. Int J Dermatol. 40:120–124 [DOI] [PubMed] [Google Scholar]
- Duncan M, Berman B. 1989. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol. 92:699–706 [DOI] [PubMed] [Google Scholar]
- Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. 2007. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 156:473–479 [DOI] [PubMed] [Google Scholar]
- Elenitsas R, Nousari C, Ayli E, Seykora J. 2008. Laboratory methods. In Elder D, editor. Lever’s histopathology of the skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; p. 59–74. [Google Scholar]
- Fioravanti A, Collodel G. 2006. In vitro effects of chondroitin dulfate. Adv Pharmacol. 53:449–465 [DOI] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL. 2008. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 72:455–482 [DOI] [PubMed] [Google Scholar]
- Greenberg S, Pinkus J, Pinkus G, Burleson T, Sanoudou D, Tawil R, Barohn R, Saperstein D, Briemberg H, Ericsson M, Park P, Amato A. 2005. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 57:664–678 [DOI] [PubMed] [Google Scholar]
- Greenberg S, Sanoudou D, Haslett J, Kohane I, Kunkel L, Beggs A, Amato A. 2002. Molecular profiles of inflammatory myopathies. Neurology. 59:1170–1182 [DOI] [PubMed] [Google Scholar]
- Haynes B, Liao H, Patton K. 1991. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 3:347–350 [PubMed] [Google Scholar]
- Herrlich P, Zoller M, Pals ST, Ponta H. 1993. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 14:395–399 [DOI] [PubMed] [Google Scholar]
- Igarashi M, Aizawa H, Tokudome Y, Tagami H. 1985. Dermatomyositis with prominent mucinous skin change: histochemical and biochemical aspects of glycosaminoglycans. Dermatologica. 170:6–11 [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. 2005. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology 15:593–603 [DOI] [PubMed] [Google Scholar]
- Keller K, Kelley M, Acott T. 2007. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 48:1164–1172 [DOI] [PubMed] [Google Scholar]
- Knepper P, Mayanil C, Goossens W, Wertz R, Holgren C, Ritch R, Allingham R. 2002. Aqueous humor in primary open-angle glaucoma contains an increased level of CD44S. Invest Ophthalmol Vis Sci. 43:133–139 [PubMed] [Google Scholar]
- Knudson CB, Knudson W. 1993. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 7:1233–1241 [PubMed] [Google Scholar]
- Kobayashi T, Yamasaki Y, Watanabe T. 1997. Diabetic scleredema: a case report and biochemical analysis for glycosaminoglycans. J Dermatol. 24:100–103 [DOI] [PubMed] [Google Scholar]
- Kolset S, Gallagher J. 1990. Proteoglycans in haemopoietic cells. Biochim Biophys Acta. 1032:191–211 [DOI] [PubMed] [Google Scholar]
- Komura K, Sato S, Fujimoto M, Hasegawa M, Takehara K. 2002. Elevated levels of circulating CD44 in patients with systemic sclerosis: association with a milder subset. Rheumatology. 41:1149–1154 [DOI] [PubMed] [Google Scholar]
- Krathen M, Fiorentino D, Werth V. 2008. Dermatomyositis. Curr Dir Autoimmun. 10:313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulseth M, Kolset S, Ranheim T. 1999. Stimulation of serglycin and CD44 mRNA expression in endothelial cells exposed to TNF-α and IL-1α. Biochim Biophys Acta. 1428:225–232 [DOI] [PubMed] [Google Scholar]
- Leigh C, Palechek P, Knutson J, McCarthy J, Cohen M, Argenyi Z. 1996. CD44 expression in benign and malignant nevomelanocytic lesions. Human Pathol. 27:1288–1294 [DOI] [PubMed] [Google Scholar]
- Lesley J, Hyman R, Kincade PW. 1993. CD44 and its interaction with extracellular matrix. Adv Immunol. 54:271–335 [DOI] [PubMed] [Google Scholar]
- Mayhew TM. 2008.. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 29:1–14 [DOI] [PubMed] [Google Scholar]
- Miller A, Nolan M, Choi J, Koga T, Shen X, Yue B, Knepper P. 2007. Lactate treatment causes NF-kB activation and CD44 shedding in cultured trabecular meshwork cells. Invest Ophthalmol Vis Sci. 48:1615–1621 [DOI] [PubMed] [Google Scholar]
- Murai T, Miyauchi T, Yanagida T, Sako Y. 2006. Epidermal growth factor-regulated activation of Rac GTPase enhances CD44 cleavage by metalloproteinase disintegrin ADAM10. Biochem J. 395:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano O, Saya H. 2004. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 95:930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A, Smith G, Lachman L, Endres R, Poppleton H, Hasty K, Seyer J, Kang A. 1989. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. J Clin Invest. 83:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz J, Tykocinski M. 1998. Differential effects of chondroitin sulfates A and B on monocyte and B-cell activation: evidence for B-cell activation via a CD44-dependent pathway. Blood. 92:223–229 [PubMed] [Google Scholar]
- Raju R, Dalakas M. 2005. Gene expression profile in the muscles of patients with inflammatory myopathies: effect of therapy with IVIg and biological validation of clinically relevant genes. Brain. 128:1887–1896 [DOI] [PubMed] [Google Scholar]
- Rongioletti F, Rebora A. 2001. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 23:257–267 [DOI] [PubMed] [Google Scholar]
- Schick B, Gradowski J, San Antonio J. 2001. Synthesis, secretion, and subcellular localization of serglycin proteoglycan in human endothelial cells. Blood. 97:449–458 [DOI] [PubMed] [Google Scholar]
- Seiter S, Schadendorf D, Tilgen W, Zoller M. 1998. CD44 variant isoforms expression in a variety of skin-associated autoimmune diseases. Clin Immunol Immunopathol. 89:79–93 [DOI] [PubMed] [Google Scholar]
- Seiter S, Tilgen W, Herrmann K, Schadendorf D, Patzelt E, Moller P, Zoller M. 1996. Expression of CD44 splice variant in human skin and epidermal tumours. Virchows Arch. 428:141–149 [DOI] [PubMed] [Google Scholar]
- Sleeman J, Kondo K, Moll J, Ponta H, Herrlich P. 1997. Variant exons v6 and v7 together expand the repertoire of glycosaminoglycans bound by CD44. J Biol Chem. 272:31837–31844 [DOI] [PubMed] [Google Scholar]
- Sorrell M, Mahmoodian F, Schafer I, Davis B, Caterson B. 1990. Identification of monoclonal novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chain: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem. 38:393–402 [DOI] [PubMed] [Google Scholar]
- Stair-Nawy S, Csoka A, Stern R. 1999. Hyaluronidase expression in human skin fibroblasts. Biochem Biophys Res Comm. 266:268–273 [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Yu Q. 2009. Shedding light on proteolytic cleavage of CD44: the responsible sheddase and functional significance of shedding. J Invest Dermatol. 129:1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Hirata T, Hayasaka H, Stern R, Murai T, Miyasaka M. 2006. Tumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragments. J Biol Chem. 281:5861–5868 [DOI] [PubMed] [Google Scholar]
- Sugahara K, Hirata T, Tanaka T, Ogino S, Takeda M, Terasawa H, Shimada I, ten Dam G, van Kuppevelt T, Miyasaka M. 2008. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 68:7191–7199 [DOI] [PubMed] [Google Scholar]
- Tezak Z, Hoffman E, Lutz J, Fedczyna T, Stephan D, Bremer E, Krasnoselska-Riz I, Kumar A, Pachman L. 2002. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 168:4154–4163 [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, Malmstrom A. 2005. Regulation of the chondroitin/dermatan fine structure by transforming growth factor-1 through effects on polymer-modifying enzymes. Glycobiology. 15:1277–1285 [DOI] [PubMed] [Google Scholar]
- Uhlin-Hansen L, Wik T, Kjellen L, Berg E, Forsdahl F, Kolset S. 1993. Proteoglycan metabolism in normal and inflammatory human macrophages. Blood. 82:2880–2889 [PubMed] [Google Scholar]
- Valiyaveettil M, Achur R, Muthusamy A, Gowda D. 2004. Chondroitin sulfate proteoglycans of the endothelia of human umbilical vein and arteries and assessment for the adherence of Plasmodium faciparum-infected erythrocytes. Mol Biochem Parasitol. 134:115–126 [DOI] [PubMed] [Google Scholar]
- Weingart C, Nelson P, Kramer B, Mack M. 2008. Dose dependent effects of platelet derived chondroitin sulfate A on the binding of CCL5 to endothelial cells. BMC Immunol. 9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B, Johansson B, Zoller M, Schwarzler C, Gunthert U. 2000. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7). J Exp Med. 191:2053–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Visentin G, Dayananda K, Neelamegham S. 2008. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood. 112:1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Jin H, Chung Y, Shin J, Woo M, Lee K, Palmos G, Choi B, Cho M. 2008. Chondroitin sulfate extracted from the Styela clava tunic suppresses TN-α-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-jB signal in JB6 cells. Cancer Lett. 264:93–100 [DOI] [PubMed] [Google Scholar]
- Zou X, Jiang Y, Zhang G, Jin H, Nguyen T, Ouyang H. 2009. Specific interactions between human fibroblasts and particular chondroitin sulfate molecules for wound healing. Acta Biomater. 5:1588–1595 [DOI] [PubMed] [Google Scholar]