Abstract
Purpose of review
Chronic immune activation is a key factor driving the immunopathogenesis of AIDS. During pathogenic HIV/SIV infections, innate and adaptive antiviral immune responses contribute to the chronic immune activation. In contrast, non-pathogenic SIV infections of natural hosts such as sooty mangabeys (SMs) and African green monkeys (AGMs) are characterized by low immune activation despite similarly high viremia. This review focuses on the role of innate immune responses in SIV infection.
Recent findings
Several studies have examined the role of innate immune responses to SIV as potential drivers of immune activation. The key result of these studies is that both pathogenic SIV infection of macaques and non-pathogenic SIV infections of natural hosts are associated with strong innate immune responses to the virus, high production of type-I interferons by plasmacytoid dendritic cells, and up-regulation of interferon stimulated genes (ISGs). However, SIV-infected SMs and AGMs (but not SIV-infected macaques) rapidly down-modulate the interferon response within 4–6 weeks of infection, thus resulting in a state of limited immune activation during chronic infection.
Summary
Studies in nonhuman primates suggest that chronic innate/interferon responses may contribute to AIDS pathogenesis. Further, the ability of natural host species to resolve innate immune responses after infection provides a novel avenue for potential immunotherapy.
Keywords: Simian Immunodeficiency Virus, SIV, Chronic Immune Activation, Innate Immune Responses, Plasmacytoid Dendritic Cells, Type I Interferon, IFNa, Interferon Stimulated Genes (ISGs)
Introduction
The nonhuman primate models of Simian Immunodeficiency Virus (SIV) or Simian/Human Immunodeficiency Virus (SHIV) infection represent the most biologically relevant and widely used animal model for HIV infection and AIDS. There are two basic types of SIV/SHIV infections, the pathogenic infection of the experimental (i.e., non-natural) Asian macaque hosts and the nonpathogenic infection of African natural hosts such as sooty mangabeys (SMs), African green monkeys (AGMs), and numerous others. Experimental S I V infection of macaques results in a disease characterized by high level virus replication, progressive CD4+ T cell depletion, chronic immune activation, and establishment of a mucosal and systemic immunodeficiency that closely resembles human AIDS. For this reason, pathogenic SIV infection of macaques has been used in many studies of HIV transmission, pathogenesis, prevention, and therapy that have been extremely influential in the field. In contrast, SIV infection of natural hosts is typically non-pathogenic despite robust virus replication, and results in a substantial preservation of the immune system function, lack of chronic immune activation, and a lifespan similar to SIV uninfected individuals [1–2]. An exception to the rule that natural SIV infections are nonpathogenic is the relatively understudied natural SIVcpz infection of chimpanzees that can progress to AIDS, albeit at a lower frequency than HIV-1-infected humans or SIV-infected macaques [3]. Over the past decade, many studies have investigated the factors underlying the benign nature of natural SIV infections, with numerous mechanisms proposed including (i) lack of chronic immune activation, (ii) reduced infection of central memory CD4+ T cells, (iii) preserved or enhanced immune regeneration, (iv) absence of microbial translocation, and (v) ability to mediate T helper function by CD3+CD4−CD8− T cells [1–2]. In this review, we will discuss the role of chronic, generalized immune activation as a factor discriminating pathogenic and non-pathogenic SIV infections, and analyze in detail the similarities and differences between these two models in terms of innate immune responses to the virus.
Chronic immune activation in pathogenic HIV and SIV infections
Chronic immune activation was proposed as a key determinant of AIDS pathogenesis in the late '80s/early '90s [4–6]. Shortly thereafter, Giorgi and colleagues reported that the level of immune activation, as determined by the fraction of CD8+ T cells expressing CD38, is a very strong and independent correlate of disease progression [7]. Strong support for the crucial role of immune activation in AIDS pathogenesis was then provided by studies of non-pathogenic SIV infections of natural hosts, in which high levels of virus replication are associated with low levels of immune activation [8]. The HIV-associated chronic immune activation is characterized by a constellation of immunological signs including increased frequency of lymphocytes expressing activation markers, high levels of activation-induced apoptosis, accelerated T cell turnover as measured by direct labeling, and high plasma and tissue levels of pro-inflammatory mediators (Reviewed in [9]). Importantly, these signs of chronic immune activation are clearly present during pathogenic SIV infection of macaques, thus emphasizing the relevance of this model in terms of HIV immunopathogenesis [9]. While there is a broad consensus that chronic immune activation is a consistent feature of pathogenic HIV/SIV infections and plays an important role in the pathogenesis of AIDS, there is still controversy as to what causes the HIV-associated immune activation. Factors that have been proposed to contribute to this phenomenon include (i) the direct effect of specific virus proteins (Env, Nef, Tat, etc), (ii) the generation of innate and adaptive immune responses to the virus, (iii) an ineffective regulation of antiviral immune responses; (iv) a bystander activation of T and B lymphocytes caused by increased production of pro-inflammatory cytokines (e.g., TNF-α, I L-1, IL-6, and several others); (v) the translocation of microbial products from the intestinal lumen to the systemic circulation, where they can activate the immune system by binding to certain Toll-like receptors (TLRs), and (vi) the presence of clinical or sub-clinical co-infections [10]. More recently it was proposed that preferential infection of central-memory CD4+ T cells (as opposed to effector-memory CD4+ T cells) is a factor favoring the maintenance of chronic immune activation by concentrating the bulk of antigenic load in central lymphoid tissues [2]. Regardless of the mechanisms responsible for its establishment, chronic immune activation is thought to disrupt CD4+ T cell homeostasis and overall immune function by several mechanisms including: (i) increased number of available target cells for the virus (i.e., activated CD4+ T cells), (ii) exhaustion of antiviral CD8+ T cells, (iii) excessive lymphocyte apoptosis, (iv) suppression of T cell regeneration, (v) accelerated proliferative senescence of lymphocytes, and (vi) disruption of the lymphoid tissue niche necessary for the proper functioning of naive and central-memory T cells [10–11].
In the following sections of this article we will focus on the potential role of innate antiviral immune responses as key mediators of the chronic immune activation associated with pathogenic SIV infection of macaques, and how rapid regulation of these responses may be responsible for the low immune activation found in the chronic phase of infection of natural SIV hosts.
Innate immune responses in SIV-infected Asian macaques
The role of the innate immune response to HIV has been the subject of intense study over the past few years. In SIV-infected macaques, recent reports have examined the role of specific innate leukocyte subsets and/or effector molecules (macrophages, NKT cells, γδ T cells, defensins, etc). Here we will mainly discuss the contribution to AIDS pathogenesis of the axis of innate antiviral immunity represented by plasmacytoid dendritic cells (pDCs), type I interferon production, and up-regulation of interferon stimulated genes (ISGs). In both humans and non-human primates, pDCs represent ~0.2–0.5% of total peripheral blood mononuclear cells and are identified phenotypically as Lineage-HLADR+CD123+/BDCA2+ and functionally as producing type I IFNs (α,β) as well as a number of pro-inflammatory cytokines and chemokines (i.e., TNFα, IL-6, CXCL10, CCL4, and CCL5) [12]. It should be noted that BDCA2 is not commonly used to define pDCs in macaques due to poor cross-reactivity of reagents, but has been used successfully in other NHP species. Importantly, pDCs express high levels of two key Toll-like receptors, TLR7 and TLR9 [13–15], that mediate the response to viral ssRNA and DNA, respectively, with the production of type I IFNs in response to TLR-7/9 stimulation being dependent on the phosphorylation and nuclear translocation of the transcription factor IRF7 [16]. Upon activation in vivo, pDCs upregulate CCR7 and CD62L, migrate to lymph nodes, and mature into typical antigen-presenting cells [17–18]. While pDCs are not the only cell type capable of producing type I IFNs, most studies indicate that they are the key cells that dictate the tempo and magnitude of the in vivo type I IFN response to a viral infection.
In HIV-infected humans, the observations that pDC are both depleted and dysfunctional when compared to uninfected controls led to the formulation of a model in which pDCs contribute to the immune control of HIV via their ability to induce antiviral responses and augment CTL and NK responses [19]. The longitudinal kinetics of pDC levels were more rigorously defined in SIV-infected macaques, in which these cells increase rapidly in blood during the first few days of infection, then rapidly decline to levels ~50% of baseline by the time of peak viremia (days 10–14 post infection) and remain reduced throughout the chronic phase of SIV infection [20–24]. Of note, the SIV-associated decline of circulating pDCs is associated with increased levels of these cells in lymph nodes [22–25]; however, it remains unclear whether this increased pDC homing to lymph nodes persists during the chronic phase of SIV infection [21–24]. Collectively, these studies of pDCs in SIV-infected macaques appeared to support the model in which these cells play a protective role against disease progression.
Several recent findings, however, have suggested that pDC activation and type I interferon responses may not be wholly beneficial to the HIV/SIV infected host and, in fact, may be detrimental by promoting immune activation and/or bystander apoptosis. First, the observation that ISGs are upregulated during chronic SIV infection of macaques indicated that even though pDCs are partially depleted from the blood and/or lymph nodes, they (or complementing cell types) are able to promote strong type I interferon responses [26–28]. Second, in vitro and ex vivo studies have shown that HIV-activated pDCs may acquire the ability to induce apoptosis of CD4+ T cells via a TRAIL/DR5 dependent mechanism [29–30]. Third, stimulation of human pDCs with HIV RNA induces the production of indoleamine 2,3 dioxygenase (IDO), thereby promoting the differentiation of T regulatory cells, thus potentially suppressing a number of antiviral immune responses [31]. Fourth, acute SIV infection is associated with significant migration of pDCs to the intestinal mucosa, where these cells persist throughout the chronic phase of infection in association with high levels of local immune activation [32–34]. A recent report has suggested that during SIV infection pDCs are depleted to near absolute levels in SIV infected macaques and, therefore, could not contribute to immunopathogenesis [35]. In this regard, the observations that pDCs localize in the gut after infection are particularly important because they indicate that (i) the depletion of pDCs from peripheral blood during SIV infection is likely due to mucosal retention, rather than to a net loss of pDCs due to cell death, and (ii) pDCs remain in the gut well into chronic infection and cannot be ruled out as candidate drivers of immune activation. Finally, as discussed in detail in the next section, natural SIV hosts display robust pDC activation and interferon production during acute SIV infection that is rapidly resolved upon the transition to chronic infection (reviewed in [36]). In light of these data, and in combination with recent human studies demonstrating chronic activation and dysregulation of pDCs in HIV infection [37–39], an alternative model has been proposed by several investigators in which pDCs and/or the type I interferon response may represent a major cause of immune activation and immunopathogenesis during pathogenic HIV and SIV infections [40–41].
Innate immunity in non-pathogenic SIV infection of African natural hosts
The role of innate immune responses to SIV during non-pathogenic infections of SMs and AGMs has been the subject of several recent studies aimed at testing the hypothesis that attenuated innate immunity to the virus is responsible for, or at least associated to the low levels of immune activation observed during the chronic phase of these infections. In this regard, all published works indicate that, despite robust virus replication, the key markers of innate immune activation, such as production of type I interferons by pDCs, up-regulation of ISGs, and activation of macrophages and natural killer cells, are not elevated during chronic SIV infection of natural hosts [25,42–47]. A number of hypotheses have been proposed to explain this intriguing phenomenon, including an intrinsic defect in the ability of pDCs to respond to Toll-like receptor (TLR)-7 and 9 signaling, the presence of active inhibitory mechanisms (acting either at the level of pDCs or through cross-talk with other cell types), and changes in the maturation and/or trafficking of these cells that limit their interaction with viral products (reviewed in [10]).
The possibility that innate immune responses to SIV are intrinsically deficient in natural SIV hosts was proposed by Mandl et al. based on a comparative in vitro study of pDC function in SMs and macaques [25]. This study described a reduced production of IFNα protein levels in response to TLR-7 and -9 stimulation of SM pDCs when compared to the same cells of macaques [25]. These authors concluded that divergent TLR-7 and -9 signaling was present in macaques compared to SMs, even though a similarly strong upregulation of type I IFN mRNAs in response to inactivated SIV was observed in both species [25]. More recently, several independent in vivo studies conducted using different groups of animals have more reliably assessed the dynamics of innate immune responses to SIV in natural hosts using various combinations of high-throughput genomic analysis, multiparametric flow cytometry, detection of plasma levels of cytokines, and immunohistochemistry [42–47]. These studies have conclusively shown that, in both pathogenic infections of macaques and non-pathogenic infections of SMs and AGMs, the acute phase of SIV infection is associated with a robust innate immune response to the virus. This response involves major changes in the transcriptome consistent with activation of the innate immune system, including a massive up-regulation of ISGs in both peripheral blood and lymphoid tissues [42–45]. Consistent with these observations is the fact that high levels of IFN-I-producing pDCs can be detected by immunohistochemistry in the lymph nodes of SIV-infected SMs and AGMs during acute infection [46]. This large set of in vivo studies indicates that an intrinsic deficiency of pDCs to stimulate a strong type-I IFN-mediated response to the virus is neither a feature of natural SIV infections nor a requirement to reach a state of non-pathogenicity.
Importantly, a key difference between pathogenic SIV infection of macaques and non-pathogenic SIV infection of natural hosts is that the latter resolves the widespread expression of innate effector molecules during the post-acute phase of infection (i.e., by day 30–45 post-infection) [42–43], reviewed in [48]. The observed similarities in the overall pattern of SIV-induced transcriptional changes in SMs and AGMs suggest a common evolutionary pathway of disease resistance in natural hosts that relies on the active regulation of an otherwise normal innate immune response to the virus [36]. In stark contrast, SIV-infected macaques maintain their elevated expression of innate immune response genes, including ISGs, throughout the chronic phase of infection [42–43]. The observation of chronically high levels of ISGs in SIV-infected macaques is consistent with the finding of high levels of ISG mRNAs during chronic HIV infection in humans [49–50]. In addition, recent work characterizing the transcriptomes of a rare population of chronically HIV-infected humans that do not exhibit CD4+ T cell depletion despite plasma viremia exceeding 100,000 RNA copies/ml demonstrated remarkable overlap with those of sooty mangabeys, including a reduced ISG response compared to patients with a rapid progressor phenotype [51]. Collectively, these comparative studies of innate immune responses during pathogenic and non-pathogenic SIV infections delineate a model in which natural SIV infections are characterized by a robust and extensive innate immune response to the virus that—in contrast to pathogenic infections—resolves during the transition from acute to chronic infection despite ongoing virus replication (Figure 1). This model of immune resolution also suggests that active immune regulatory mechanisms are responsible for the low immune activation of chronically SIV-infected SMs and AGMs. While resolution of the pDC/interferon response was reported by multiple groups, the mechanism by which natural hosts arrest IFNα production despite persistent virus replication remains a key unanswered question. Several recent studies detailing virus/DC interplay at the cellular level provide intriguing potential mechanisms by which natural hosts may halt the interferon response (summarized in Figure 2) including: (i) active immunoregulatory mechanisms; (ii) restriction of infection of DCs [57]; (iii) reduced DC trafficking to mucosal tissues [32–33]; (iv) desensitization/maturation of pDCs [39]. Consistent with a mechanism of immune regulation is the observation that the return to baseline expression levels of innate immunity genes in SIV-infected SMs is temporally coincident with a marked up-regulation of immune regulatory genes such as IDO and ADAR [42]. An intriguing, non-mutually exclusive possibility is that the rapid regulation of pDC- and type I IFN-mediated responses in SIV-infected SMs is related to the low levels of virus replication in the central-memory CD4+ T cells of these animals [58]. Further studies will hopefully elucidate the role of these and other immunomodulatory factors in determining the low immune activation state of chronically SIV-infected SMs and AGMs.
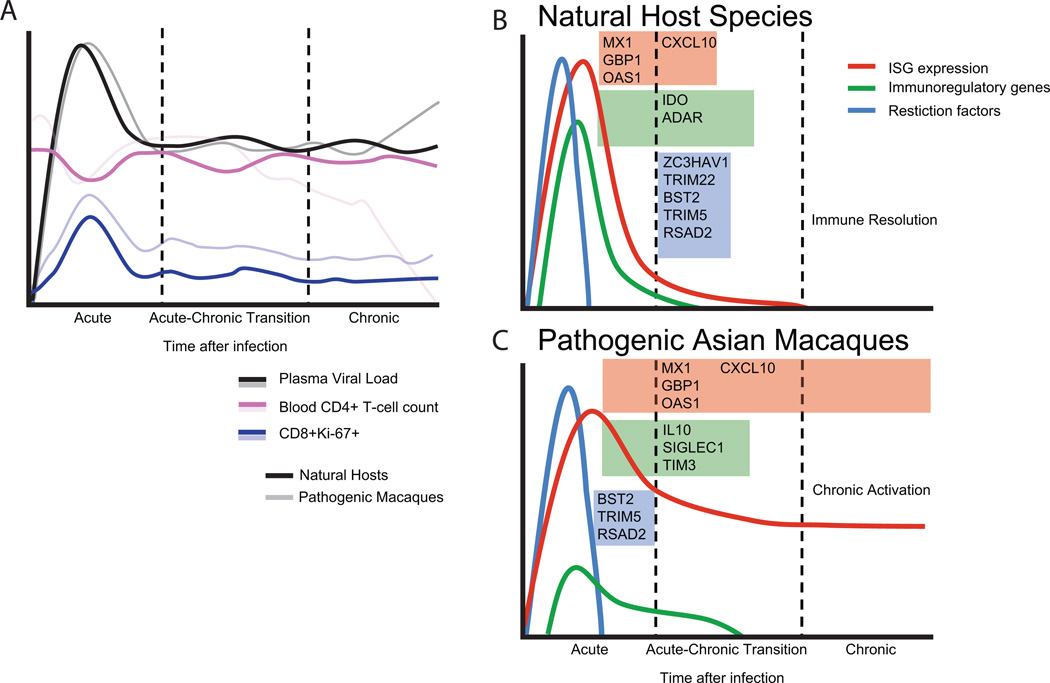
Figure 1. Transcriptional profiles of pathogenic and nonpathogenic SIV infection.
(A) Virological and immunological parameters of prototypical SIV infection in natural host species contrasted with pathogenic infections. Solid lines denote natural hosts, transparent lines depict macaque species. Kinetics of viral loads are similar between species. In general, natural hosts do not undergo rapid peripheral CD4+ T lymphocyte depletion as observed in macaques; it should be noted that in a handful of natural hosts, CD4+ depletion has been observed in the absence of disease [52–53], and that long term SIV infection of sooty mangabeys is associated with a gradual, although clinically benign, decline of blood CD4+ T cells [54]. Immune activation as assessed by peripheral blood CD8+Ki67+ levels occurs in both animals during acute SIV infection [55–56], but returns to near-baseline levels after infection, in contrast to macaque species, which remain elevated. Transcriptional regulation in (B) natural host and (C) macaque species during SIV infection. Both species have massive upregulation of interferon stimulated genes (ISGs) during acute infection (depicted by red lines); during the transition to chronic phase the ISG response resolves in natural hosts but is maintained indefinitely in macaques. mRNAs for multiple restriction factors (blue lines) are present in blood during acute infection of both species but decline rapidly. Genes associated with negative regulation of the immune response (green lines) are expressed differentially between species.
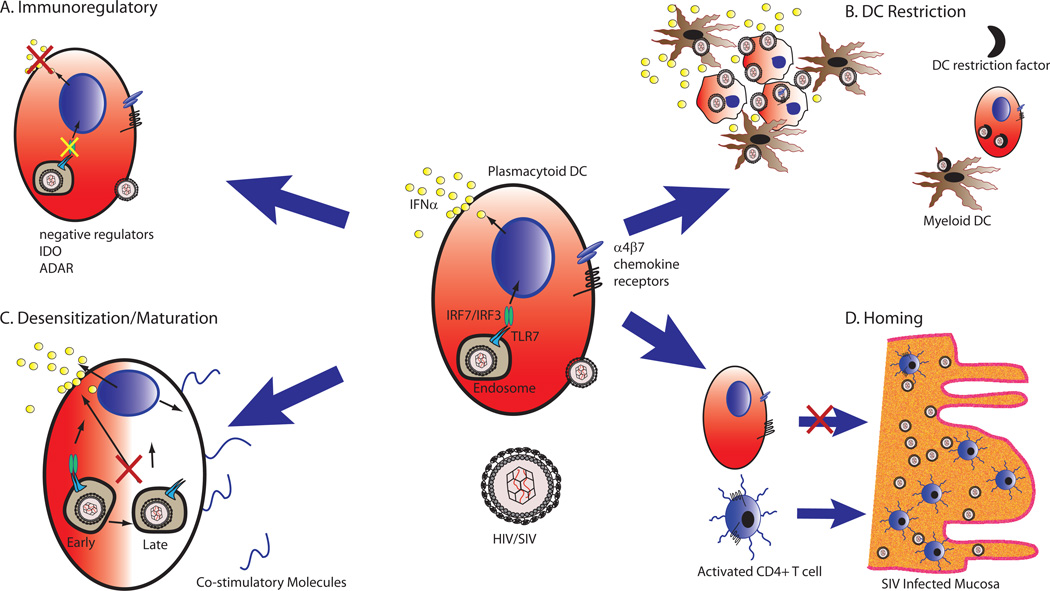
Figure 2. Potential mechanisms of resolution of ISG response in natural hosts during SIV infection.
CD123+ Plasmacytoid DCs are the primary producers of IFNα in LNs of SIV-infected RMs, AGMs and SMs [46]. During chronic infection, pDCs in natural host species stop producing IFNα, concurrent with the resolution to baseline of ISG expression, despite continued high level viremia. Possible mechanisms by which the resolution occurs are (A) Immunoregulatory: transcript profiling data demonstrated elevated expression of immunomodulatory proteins such as ADAR and IDO in SMs; alternatively negative regulation of IFNα production in pDCs by ITAM mediated pathways has been demonstrated in mice, but data are lacking in the context of pDC/HIV interactions. (B) DC-specific HIV Restriction: Recent data has demonstrated that productive infection of DCs by HIV is blocked by an uncharacterized restriction factor [57]; circumvention of DC restriction yields a high level of virus production and secretion of Type I IFNs. During acute infection there is widespread induction of restriction factors, and inhibition of DC infection may remove the nidus for IFNα production. (C) Reduced mucosal homing - pDCs in natural hosts have attenuated recruitment to vaginal and rectal mucosa in comparison with macaques during chronic infection. Lowered gut homing may reduce pDC exposure to sites of high level virus replication, and limit their potential for driving immune activation in mucosal lymphoid tissue. (D) Dysregulated pDC Desensitization/Maturation - Recent work has shown that HIV, unlike other viruses recognized by pDC TLRs, signal predominately in early endosomes, resulting in partially mature pDCs that have a continual ability to make IFNα and poor expression of costimulatory molecules[39]. pDCs in natural hosts may have altered interactions with SIV that allow for full maturation and cessation of interferon production.
Conclusion
The role of innate immunity and chronic immune activation in the immunopathogenesis of AIDS has been recently investigated by comparative studies of experimental, pathogenic SIV infection of Asian macaques and natural, non-pathogenic SIV infection of African non-human primates. These studies have shown that both pathogenic and non-pathogenic SIV infections are characterized by a robust innate immune response to the virus. However, this response is transient in natural hosts, but persistent in Asian macaques, thus suggesting that this down-regulation of innate immunity may protect SMs and AGMs from chronic immune activation and progression to AIDS. Importantly, it is hoped that further elucidation of the genes and molecular pathways involved in maintenance of a state of low immune activation in chronically SIV-infected SMs and AGMs will identify targets for therapeutic interventions aimed at limiting or abrogating the aberrant immune activation that is associated with HIV infection in humans.
Key points.
The role of innate immunity and chronic immune activation in AIDS pathogenesis has been recently investigated by comparative studies of pathogenic SIV infection of macaques and non-pathogenic SIC infection of sooty mangabeys and African green monkeys.
During pathogenic SIV infection, innate immune responses to the virus may favor the immune-mediated control of virus replication, but also act as immunopathogenic mechanisms by mediating the chronic immune activation/inflammation that characterizes this infection.
Both pathogenic and non-pathogenic SIV infections are associated with robust innate immune responses, significant plasmacytoid dendritic cell activation, and massive type I IFN stimulated genes (ISG) up-regulation during the acute phase of infection.
In natural, non-pathogenic SIV infections the innate immune response to the virus is dramatically reduced during the transition between the acute and the chronic phase of infection.
Acknowledgements
The authors would like to thank Michaela Müller-Trutwin, Rama Amara, Sue-Fen Kwa and Ann Chahroudi for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
References
- 1.Paiardini M, Pandrea I, Apetrei C, et al. Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med. 2009;60:485–495. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keele BF, Jones JH, Terio KA, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascher MS, Sheppard HW. AIDS as immune system activation: a model for pathogenesis. Clin Exp Immunol. 1988;73:165–167. [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman Z, Bentwich Z, Herberman RB. From HIV infection to AIDS: are the manifestations of effective immune resistance misinterpreted? Clin Immunol Immunopathol. 1993;69:123–135. doi: 10.1006/clin.1993.1160. [DOI] [PubMed] [Google Scholar]
- 6.Grossman Z, Herberman RB. T-cell homeostasis in HIV infection is neither failing nor blind: modified cell counts reflect an adaptive response of the host. Nat Med. 1997;3:486–490. doi: 10.1038/nm0597-486. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 9.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 10.Sodora DL, Allan JS, Apetrei C, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Jarrossay D, Napolitani G, Colonna M, et al. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 17.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 18.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 19.Soumelis V, Scott I, Liu YJ, et al. Natural type 1 interferon producing cells in HIV infection. Hum Immunol. 2002;63:1206–1212. doi: 10.1016/s0198-8859(02)00760-7. [DOI] [PubMed] [Google Scholar]
- 20.Reeves RK, Fultz PN. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology. 2007;365:356–368. doi: 10.1016/j.virol.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 22.Malleret B, Karlsson I, Maneglier B, et al. Effect of SIVmac infection on plasmacytoid and CD1c+ myeloid dendritic cells in cynomolgus macaques. Immunology. 2008;124:223–233. doi: 10.1111/j.1365-2567.2007.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malleret B, Maneglier B, Karlsson I, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 24.Brown KN, Wijewardana V, Liu X, et al. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandl JN, Barry AP, Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 26.Abel K, Alegria-Hartman MJ, Rothaeusler K, et al. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosinger SE, Hosiawa KA, Cameron MJ, et al. Gene expression profiling of host response in models of acute HIV infection. J Immunol. 2004;173:6858–6863. doi: 10.4049/jimmunol.173.11.6858. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer TM, Fuller CL, Basu S, et al. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect. 2006;8:1839–1850. doi: 10.1016/j.micinf.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Hardy AW, Graham DR, Shearer GM, et al. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stary G, Klein I, Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 31.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwa S, Kannanganat S, Nigam P, et al. Plasmacytoid dendritic cells express β7-integrin and are recruited to the colorectum during pathogenic SIV infection. Abstract# P09.10. AIDS Vaccine 2010. Sept 28 – Oct 1 Atlanta GA USA. 2010 This study comprehensively the recruitment of pDCs to the gut during acute and chronic HIV/SIV infection in rhesus macaques, sooty mangabey and humans. They demonstrated that pDC drive an interferon response in the gut that correlates with immune activation measured by CD8+Ki67+ levels, which was abrogated by blockade of mucosal homing. They also demonstrated a lack of mucosal homing by pDCs in SIV+ sooty mangabeys.
- 33. Reeves RK, Evans T, Gillis J, et al. SIV Infection Induces Increased Trafficking of pDC to Gut Mucosa. Abstract# 23. 18th Conference on Retroviruses and Opportunistic Infections; Feb 27 – Mar 2; Boston MA USA. 2011. This work demonstrated the mucosal recruitment of pDCs to the gut, and demonstrated that pDCs are not depleted during SIV infection.
- 34. Ansari AA, Reimann KA, Mayne AE, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. This study utilized in vivo antibody mediated blockade of a4b7 to abrogate gut homing of multiple leukocyte subsets in SIV infection, including pDCs, during SIV infection demonstrating remarkable decreases in viremia, and lowered ISG responses in gut.
- 35.Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39:235–242. doi: 10.1111/j.1600-0684.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mir KD, Gasper MA, Sundaravaradan V, et al. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect. 2011;13:14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabado RL, O'Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. This study characterized pDC responses in primary HIV infection, and demonstrated, in contrast to classical human studies, that pDCs had an elevated, not attenuated, IFNα response to viral stimulation.
- 39. O'Brien M, Manches O, Sabado RL, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. This study demonstrated that pDC activation by HIV did not become refractory to secondary stimulation, in contrast to influenza or Sendai virus, suggesting a mechanism by which HIV has the unique ability to continually stimulate pDCs and potentially perpetuate immune activation via Type I Interferon.
- 40.Altfeld M, Fadda L, Frleta D, et al. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boasso A. HIV and DC: hate at first sight. Blood. 2010;116:3687–3689. doi: 10.1182/blood-2010-08-302331. [DOI] [PubMed] [Google Scholar]
- 42. Bosinger SE, Li Q, Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. This study was the first report of high-throughput transcript profiling of SIVsmm-infected sooty mangabeys and demonstrated that SMs have a massive innate response to SIV in acute infection that subsequently resolves before chronic infection, in striking contrast to control rhesus macaques infected with SIVsmm or SIVmac239, in which the ISG response persisted indefinitely. A companion paper by Jacquelin and colleagues demonstrated parallel findings in African Green Monkeys.
- 43. Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. This study comprehensively characterized genome-wide expression in several tissues in SIV-infected African Green monkeys and observed a marked induction in innate responses shortly after infection, but returned to baseline within two month postinfection; these results mirrored findings in a companion paper by Bosinger et al in sooty mangabeys.
- 44.Lederer S, Favre D, Walters KA, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris LD, Tabb B, Sodora DL, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. This study definitively demonstrates the production of IFNα in situ in CD123+ cells in the lymph nodes of acutely infected SMs and AGMs.
- 47. Campillo-Gimenez L, Laforge M, Fay M, et al. Nonpathogenesis of simian immunodeficiency virus infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol. 2010;84:1838–1846. doi: 10.1128/JVI.01496-09. This work definitively demonstrates the in vivo production of IFNα in SIV-infected AGMs and confirms their ability to respond to TLR7/8 ligands.
- 48.Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009;119:3512–3515. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedaghat AR, German J, Teslovich TM, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol. 2008;82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyrcza MD, Kovacs C, Loutfy M, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol. 2007;81:3477–3486. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rotger M, Dalmau J, Rauch A, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011 doi: 10.1172/JCI45235. This study is among the first to characterize the viremic nonprogressor (VNP) human phenotype, in which immune activation is absent despite long-term, high-level viremia (HIV RNA copies >100,000 per ml plasma). Extensive genomic characterization was performed and demonstrated that transcriptomes of VNPs shared characteristics of that of SIV+ sooty mangabeys, including reduced ISG expression (compared to rapid progressors) despite increased viral load.
- 52.Milush JM, Reeves JD, Gordon SN, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 53.Milush JM, Mir KD, Sundaravaradan V, et al. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest. 2011;121:1102–1110. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taaffe J, Chahroudi A, Engram J, et al. A five-year longitudinal analysis of sooty mangabeys naturally infected with simian immunodeficiency virus reveals a slow but progressive decline in CD4+ T-cell count whose magnitude is not predicted by viral load or immune activation. J Virol. 2010;84:5476–5484. doi: 10.1128/JVI.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monceaux V, Ho Tsong Fang R, Cumont MC, et al. Distinct cycling CD4(+)- and CD8(+)-T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J Virol. 2003;77:10047–10059. doi: 10.1128/JVI.77.18.10047-10059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon SN, Klatt NR, Bosinger SE, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manel N, Hogstad B, Wang Y, et al. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paiardini M, Cervasi B, Reyes-Aviles E, et al. Low levels of SIV infection in sooty mangabey central-memory CD4+ T-cells is associated with limited CCR5 expression. Nat Med. 2011 doi: 10.1038/nm.2395. Accepted May 9/11. [DOI] [PMC free article] [PubMed] [Google Scholar]