Abstract
Objectives
To determine the neuropathological load in the living brain of nondemented adults with Down syndrome using positron emission tomography with 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-napthyl}ethylidene) malononitrile ([18F]FDDNP) and to assess the influence of age and cognitive and behavioral functioning. For reference, [18F]FDDNP binding values and patterns were compared with those from patients with Alzheimer disease and cognitively intact control participants.
Design
Cross-sectional clinical study.
Participants
Volunteer sample of 19 persons with Down syndrome without dementia (mean age, 36.7 years), 10 patients with Alzheimer disease (mean age, 66.5 years), and 10 controls (mean age, 43.8 years).
Main Outcome Measures
Binding of [18F]FDDNP in brain regions of interest, including the parietal, medial temporal, lateral temporal, and frontal lobes and posterior cingulate gyrus, and the average of all regions (global binding).
Results
The [18F]FDDNP binding values were higher in all brain regions in the Down syndrome group than in controls. Compared with the Alzheimer disease group, the Down syndrome group had higher [18F]FDDNP binding values in the parietal and frontal regions, whereas binding levels in other regions were comparable. Within the Down syndrome group, age correlated with [18F]FDDNP binding values in all regions except the posterior cingulate, and several measures of behavioral dysfunction showed positive correlations with global, frontal, parietal, and posterior cingulate [18F]FDDNP binding.
Conclusions
Consistent with neuropathological findings from postmortem studies, [18F]FDDNP positron emission tomography shows high binding levels in Down syndrome comparable to Alzheimer disease and greater levels than in members of a control group. The positive associations between [18F]FDDNP binding levels and age as well as behavioral dysfunction in Down syndrome are consistent with the age-related progression of Alzheimer-type neuropathological findings in this population.
In patients with down syndrome, age is associated with neuropathological changes, and a large proportion of those older than 45 years show cognitive and behavioral symptoms suggestive of dementia.1–4 Down syndrome neuropathological changes include cerebral cortical deposits of amyloid senile plaques (SPs), composed mainly of fibrillar aggregates of β-amyloid protein, and tau neurofibrillary tangles (NFTs), composed mainly of fibrillar aggregates of hyperphosphorylated microtubule-associated protein tau. These proteinaceous deposits are indistinguishable from those observed in the cerebral cortices of patients with Alzheimer disease.2 Although the pattern of NFT formation in Down syndrome follows the hierarchical pattern observed in Alzheimer disease (first layer II of the entorhinal cortex, followed by the hippocampus, and then the neocortex), there is a clear difference in the pattern and time course of SP formation.2–12 Postmortem studies have established that SP deposition may be detected initially in individuals with Down syndrome in adolescence, and NFTs appear later as the person ages.5–10 It is the appearance of NFT deposits that induces Alzheimer-type dementia symptoms despite the early presence of SP deposits resulting from a third copy of chromosome 21, which includes the gene encoding the amyloid precursor protein.9
During the past decade, in vivo methods for identifying the neuropathology of Alzheimer disease have been developed. These methods use positron emission tomography (PET) with molecular probes designed to bind to SPs and NFTs (eg, 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-naphthyl}ethylidene) malononitrile [(18F)FDDNP])13–18 or SPs alone (eg, carbon 11–labeled Pittsburgh Compound-C [(11C)6-OH-BTA-1 or (11C)PIB]).19 Of the molecular probes currently available, only [18F]FDDNP PET has been shown to detect both SPs and NFTs in vivo.13–18 The feasibility of using molecular probes as a means of early detection of Alzheimer disease has been reported in studies of presymptomatic individuals (ie, individuals at risk for conversion to Alzheimer disease), patients with probable Alzheimer disease, and healthy control participants.13,14,16–20
Although people with trisomy 21 Down syndrome would be an interesting group to study with molecular imaging, to date an in vivo assessment of neuropathological deposition in the brain has not been performed in this population. To address this knowledge gap, we used [18F]FDDNP PET in volunteers with Down syndrome, compared their binding values with those of cognitively intact controls and patients with Alzheimer disease, and determined the influence of age and cognitive and behavioral function on these values.
METHODS
PARTICIPANTS
Study participants were all English speaking and excluded if they had any clinically significant medical or neurological condition or a diagnosis of a major psychiatric disorder during the 3 months preceding study participation. Volunteers were also excluded if they were taking medications known to have cognitive or neurological adverse effects, including central nervous system depressants or over-the-counter medications known to cause drowsiness (eg, sleep aids and cold and allergy medications). Individuals taking nonsteroidal anti-inflammatory drugs were also excluded because some of these drugs (eg, ibuprofen and naproxen) bind to SPs and can thus affect [18F]FDDNP binding values.21
All clinical assessments were performed within 4 weeks of the scanning procedures, and the clinicians were blinded to the scan results. Ten cognitively intact healthy controls (age range, 29–52 years; mean [SD] age, 43.8 [6.9] years; 4 men and 6 women) and 10 patients with Alzheimer disease (age range, 57–75 years; mean [SD] age, 66.5 [6.2] years; 5 men and 5 women) were part of a larger study17 and were included in this population for comparison with the Down syndrome group. Because the patients with Down syndrome were generally younger, we chose the 10 youngest controls available and patients with Alzheimer disease who were younger than 75 years. Participants were originally recruited through study advertisements, media coverage, and referrals from physicians and families. The Mini-Mental State Examination22 was used to determine the level of cognitive functioning in the Alzheimer disease and control groups. The mean (SD) Mini-Mental State Examination scores were 29.8 (0.42) for the control group and 19.0 (17.1) for the probable Alzheimer disease group. The mean (SD) educational level was 16.1 (2.2) years for the control group and 16.2 (3.0) for the Alzheimer disease group.
Patients with Alzheimer disease met the standard diagnostic criteria of memory impairment and impairment in at least one other cognitive domain, gradual onset, progressive decline, and impaired occupational and/or social functioning.23 Control participants were cognitively normal for their age and did not meet criteria for mild cognitive impairment or dementia.23–25
The Down syndrome group (n=19) included 7 men and 12 women with trisomy 21 (mean (SD) age, 36.7 [10.0] years; range, 21–60 years). Fourteen of these participants resided with their family of origin, whereas the remaining participants lived in a group home. Thirteen participants were employed in a sheltered workshop, where they performed primarily manual labor tasks. Eighteen participants had an educational level of 12 to14 years (high school equivalent), and the remainder attained a level of 7 years. The mean (SD) overall educational level was 11.7 (2.7) years. The Wechsler Abbreviated Scale of Intelligence26 was used to determine cognitive ability or intellectual level in the volunteers with Down syndrome; their mean (SD) full-scale or overall IQ was 57.1 (4.9), about 3 SDs below the normative mean for the test standardization sample.
The Dementia Questionnaire for Mentally Retarded Persons (DMR),27,28 a standardized test designed to measure symptoms of dementia in patients with Down syndrome, was administered to the caregivers of the Down syndrome group participants. The DMR provides scores from a cognitive scale, a social scale, and overall test results. Because of the relatively small sample size and the challenges in accurately diagnosing dementia in patients with Down syndrome, we did not use the DMR scale to stratify our sample into a demented and nondemented group; instead, we analyzed the DMR scale scores as continuous variables. Previous studies of Down syndrome have used the DMR scale scores as continuous variables.29–31 Moreover, all but 2 of the Down syndrome subjects were 50 years or younger, an age group with a reported dementia prevalence of less than 10%.32,33 The Neuropsychology Behavior and Affect Profile–Dementia (NBAP-D),34–38 a standardized test designed to assess emotional and behavioral functioning of adults with brain injuries or other neurological disorders, also was administered to the caregivers of the participants with Down syndrome. The NBAP-D contains 5 clinical scales measuring inappropriateness, pragnosia (ie, pragmatics of communication style or social discourse), indifference, depression, and mania. Age-corrected NBAP-D scale scores were used to examine the relationship between behavior function and levels of [18F]FDDNP binding. Participants with Down syndrome were assessed by having a family/caregiver informant who had known the subject for at least 5 years complete both tests. Table 1 provides mean scores from the DMR and NBAP-D scales for the Down syndrome group.
Table 1.
Neuropsychological Test Scores for Study Participants With Down Syndrome
| Test | Score, Mean (SD) |
|---|---|
| DMR scales | |
| Total | 10.8 (8.3) |
| Cognitive scale | 4.9 (5.8) |
| Social scale | 5.9 (4.1) |
| NBAP-D scalesa | |
| Inappropriateness | 30.0 (26.5) |
| Pragnosia | 25.8 (20.3) |
| Indifference | 9.2 (11.2) |
| Depression | 8.5 (14.1) |
| Mania | 9.6 (13.6) |
Abbreviations: DMR, Dementia Questionnaire for Mentally Retarded Persons; NBAP-D, Neuropsychology Behavior and Affect Profile–Dementia.
Scores are reported as percentages of the total raw score.
Written informed consent was obtained in accordance with the University of California, Los Angeles, Office of Protection of Research Subjects. Cumulative radiation dosimetry for all scans was below the mandated maximum annual dose and in compliance with state and federal regulations.
SCANNING AND IMAGE ANALYSIS PROCEDURES
As described elsewhere,39 [18F]FDDNP was prepared with very high specific activities (>37 GBq/μmol). Positron emission tomography was performed using a commercially available device (ECAT HR+ camera; Siemens/CTI, Knoxville, Tennessee). Dynamic scans were obtained for 65 minutes after injection of 370 MBq of [18F]FDDNP. All PET images were reconstructed using filtered back-projection with measured attenuation correction and were corrected for scatter. Dynamic images were reconstructed with a Hann filter (0.3 Nyquist cutoff) resulting in an in-plane resolution of 5.5-mm full-width half-maximum. The obtained images contained 63 contiguous sections with a plane-to-plane separation of 2.42 mm. Quantification of [18F]FDDNP binding data was performed using Logan graphical analysis with the cerebellum as the reference region.13,20 The [18F]FDDNP relative distribution volume (binding) parametric images were generated and analyzed using regions of interest drawn for the left and right parietal, medial temporal (limbic regions, including the hippocampus, parahippocampal areas, and entorhinal cortex), lateral temporal, and frontal regions and the posterior cingulate gyrus, as previously described.13 Each regional relative distribution volume or binding value was expressed as an average of left and right regions, and the global region was defined as the average of these. Owing to possible head movement during the PET procedure, a statistical correction was performed on PET data to account for potential motion artifacts.40
STATISTICAL ANALYSIS
The [18F]FDDNP binding values were compared among the 3 participant groups using nonparametric analyses of covariance, controlling for age. We conducted post hoc analyses to determine pairwise significant differences. Within the Down syndrome group, Spearman rank correlations were used to determine whether regional [18F]FDDNP binding levels were associated with age. Partial Spearman correlations, controlling for age, were used to examine whether [18F]FDDNP binding levels were associated with the cognitive and social scales of the DMR and the 5 NBAP-D. First, the 5 behavioral indices were correlated with the global [18F]FDDNP binding levels. If a significant association was found for global binding, follow-up regional analyses were conducted to determine which regions contributed to the global finding. All tests were 2-tailed, and a significance level of .05 was used for all inferences.
RESULTS
Nonparametric analyses of covariance revealed significant group differences in the [18F]FDDNP binding values in the parietal, medial and lateral temporal, posterior cingulate, and frontal regions and globally, controlling for age. Post hoc analyses indicated significantly higher binding levels in the Down syndrome group in all brain regions of interest compared with controls. Compared with the Alzheimer disease group, the Down syndrome group also showed significantly higher binding levels globally and in the parietal and frontal lobes (Table 2). In the posterior cingulate, medial temporal, and lateral temporal regions, there were no significant differences in binding levels between the Down syndrome and Alzheimer disease groups.
Table 2.
Age-Adjusted Mean [18F]FDDNP Binding Levels by Brain Region and Participant Group
| Brain Region | Groupa |
Group Comparisons
|
|||||
|---|---|---|---|---|---|---|---|
| Down Syndrome (n=19) | Alzheimer Disease (n=10) | Control (n=10) | Down Syndrome vs Alzheimer Disease, t35 Value | P Value | Down Syndrome vs Control, t35 Value | P Value | |
| Parietal | 1.18 (0.06) | 1.10 (0.02) | 1.06 (0.03) | 2.46 | .02 | 6.90 | <.001 |
| Medial temporal | 1.13 (0.03) | 1.17 (0.03) | 1.09 (0.03) | NS | 2.80 | .008 | |
| Lateral temporal | 1.14 (0.05) | 1.12 (0.03) | 1.07 (0.03) | NS | 4.94 | <.001 | |
| Posterior cingulate | 1.19 (0.05) | 1.17 (0.03) | 1.07 (0.04) | NS | 6.10 | <.001 | |
| Frontal | 1.14 (0.05) | 1.08 (0.02) | 1.02 (0.04) | 1.99 | .05 | 6.52 | <.001 |
| Global | 1.16 (0.04) | 1.13 (0.01) | 1.06 (0.02) | 2.19 | .04 | 7.74 | <.001 |
Abbreviations: [18F]FDDNP, 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-napthyl}ethylidene) malononitrile; NS, nonsignificant.
Reported as age-adjusted mean (SD) values.
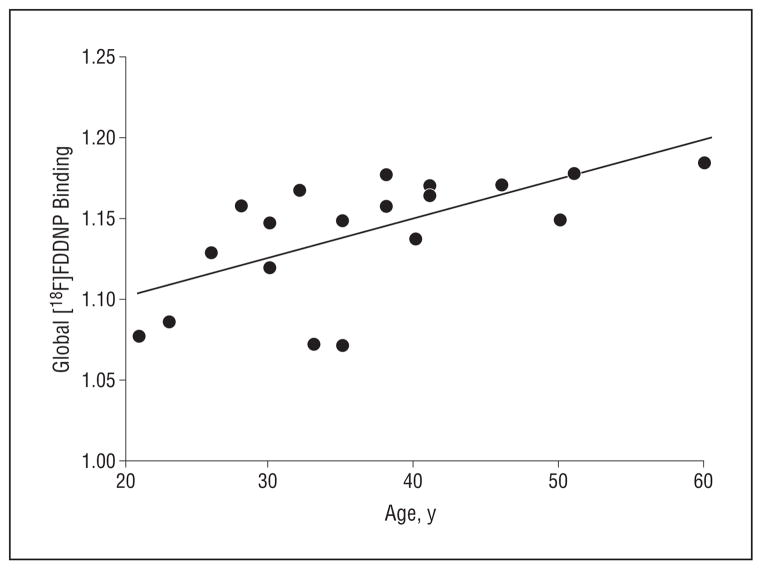
In the Down syndrome group, we found significant associations between age and [18F]FDDNP binding values in the parietal (r=0.51; P=.03), lateral temporal (r=0.69; P=.001), and frontal (r=0.44; P=.05) regions and globally (r=0.68; P=.001) (Figure 1). Figure 2 illustrates [18F]FDDNP binding patterns in representative participants from each of the subject groups, including a younger and an older participant from the Down syndrome group.
Figure 1.
Spearman correlation in Down syndrome group between global 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-napthyl}ethylidene) malononitrile ([18F]FDDNP) binding levels and age (r=0.68; P=.001). Binding or relative distribution volume values are expressed as an average of left and right parietal, medial temporal, lateral temporal, posterior cingulate, and frontal regions.
Figure 2.
Representative 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-napthyl}ethylidene) malononitrile ([18F]FDDNP) positron emission tomography (PET) images of a control participant, 2 study participants with Down syndrome (DS), and a participant with Alzheimer disease. Parietal regions are depicted in the top row; temporal regions, the bottom row. The control participant shows minimal [18F]FDDNP PET binding compared with the other participants. The older participant with DS shows higher binding levels than the younger one. Yellow and green correspond to higher [18F]FDDNP binding values; blue, to lower binding values. DVR indicates relative distribution volume (binding value).
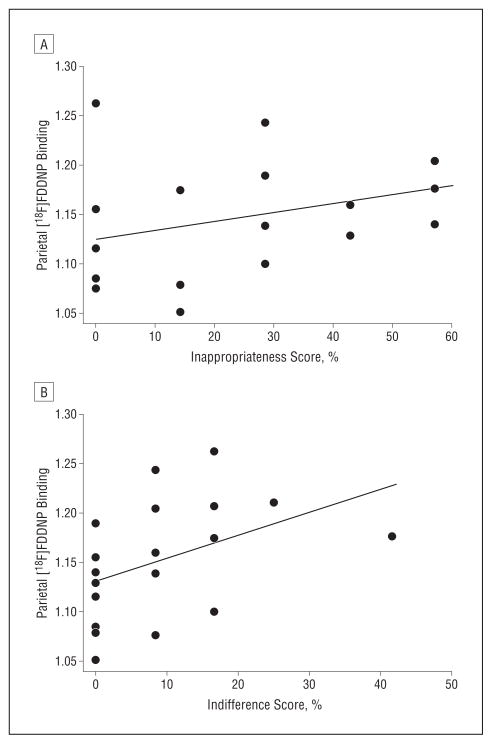
There were no significant associations between the cognitive and social scales of the DMR and regional [18F]FDDNP binding levels, controlling for age. Several of the NBAP-D scores demonstrated positive associations with [18F]FDDNP binding, controlling for age. The inappropriateness scale score correlated with parietal (r=0.65; P=.003), posterior cingulate (r=0.68; P=.002), frontal (r=0.53; P=.03), and global (r=0.57; P=.01) binding (Figure 3). The pragnosia scale score correlated with globalbinding(r=0.51; P=.03). The indifference scale score correlated with parietal (r=0.62; P=.005), posterior cingulate (r=0.64; P=.005), and global (r=0.58; P=.01) binding.
Figure 3.
Spearman correlations in the Down syndrome group between parietal 2-(1-{6-[(2-fluorine 18–labeled fluoroethyl)methylamino]-2-napthyl}ethylidene) malononitrile ([18F]FDDNP) binding levels and Neuropsychology Behavior and Affect Profile–Dementia scores. A, Inappropriateness scale score (r=0.65; P=.003, controlling for age). B, Indifference scale score (r=0.62; P=.005, controlling for age).
COMMENT
This work demonstrates the previously unrealized visualization of neuropathological aggregates in the living brains of a Down syndrome group. The observed [18F]FDDNP regional binding patterns were consistent with SP and NFT accumulation patterns previously reported in autopsy determinations in brains of persons with Down syndrome.4,5,11 Mann2 reported the presence of such abnormalities in 8% of subjects in the group aged 10 to 19 years, 16% in the group aged 20 to 29 years, 80% in the group aged 30 to 39 years, and 100% in the group older than 60 years. Wisniewski and colleagues3 reported similar increases in such abnormalities according to age. Thus, the age-based increase of [18F]FDDNP binding levels observed in the Down syndrome group was completely consistent with the increase in SP and NFT depositions seen at autopsy.2–6,11,12 Moreover, the regional distribution of [18F]FDDNP binding also corresponded with patterns reported in autopsy studies.2–7
Although [18F]FDDNP PET images cannot differentiate SPs from NFTs, follow-up autopsy neuropathological studies of regional patterns are elucidating the relative contributions of these 2 forms of abnormality in different brain areas. For example, previous follow-up immunohistochemistry studies of brain tissue from a patient with Alzheimer disease have confirmed our prediction that high medial temporal [18F]FDDNP binding indicates a preponderance of NFT accumulation, whereas high lateral temporal binding reflects relatively greater SP accumulation.13
Of interest was the observation that nondemented subjects with Down syndrome showed higher [18F]FDDNP binding globally and in the parietal and frontal lobes compared with the Alzheimer disease group. This observation could reflect the early and extensive accumulation of SP and NFT deposits in Down syndrome. Hof and colleagues4 have determined that, even in nondemented subjects with Down syndrome younger than 40 years, SPs can be found in densities comparable to those observed in individuals with Alzheimer disease. Neuropathological studies2–6,11,12 of the brains of persons with Down syndrome have reported SP deposition as early as the second and third decade before obvious dementia symptoms emerge and, by 50 years of age, nearly all subjects show SP and NFT deposition.41,42 In patients with Down syndrome, SPs are generally observed initially followed by NFTs in the limbic areas of the temporal lobe, and then NFT and SP deposition spreads to the prefrontal and other neocortical regions.4 By contrast, patients from the general population who develop Alzheimer disease show entorhinal and medial temporal NFT deposition before extensive cortical SP deposition, and this difference is also seen in the pattern of [18F]FDDNP brain distribution.13 Although Down syndrome and Alzheimer disease share the same type of pathological changes, people with Down syndrome have initial SPs earlier in the limbic and cortical regions. Dementia in Down syndrome is associated with development of abundant NFTs in the limbic regions followed by the cortical areas.
As people with Down syndrome age, they typically develop behavioral and cognitive changes suggestive of cellular neurodegeneration as a result of SP and NFT pathological changes, and accumulation of cortical NFTs appears to precede Alzheimer disease–like symptoms of cognitive decline.1,6,7 In the present study, DMR cognitive measures did not correlate with [18F]FDDNP binding levels, but this finding is consistent with previous studies indicating limitations of such measures in adults with mental retardation.34,43 Cognitive skills in people with Down syndrome can vary considerably, and their language impairment can compromise cognitive testing,29 which might explain the lack of association observed between such cognitive measures and PET binding values. Another explanation is that some of the earliest age-related changes in cognitive functioning in adults with Down syndrome are primarily spatial, and the DMR assesses memory but not spatial functions in people with intellectual disabilities.27
The cognitive and behavioral tests used for the study participants with Down syndrome were indirect methods of assessment (ie, completed by the caregivers or someone other than the participant). Although indirect assessment methods are typically more reliable than direct test administration in people with intellectual decline,44,45 direct cognitive assessment, including spatial learning and spatial memory functioning, would be of interest. Studies are currently under way that take into consideration direct test methods that allow for the high prevalence of communication disorders common to this aging population.
Although cognitive measures were not associated with [18F]FDDNP binding values in the Down syndrome group, we did find several positive correlations between [18F]FDDNP binding and behavioral abnormalities, including measures of indifference and inappropriateness and pragmatics of communication style or social discourse. Such findings suggest that behavioral abnormalities may be better indicators of underlying pathological changes than cognitive measures in adults with mental retardation. Greater severity of these behavioral changes, which are consistent with frontallobe dysfunction, correlated with higher [18F]FDDNP binding in frontal and global regions. The NBAP-D subscales of inappropriateness and indifference correlated with [18F]FDDNP binding in the parietal region in the Down syndrome group (Figure 3). Studies have demonstrated that certain parietal regions are involved in similar emotional functioning; depression and anxiety have been associated with the entire parietal lobe,46 and the mirror neuron system (empathy) has been associated with the inferior parietal lobule.47–49
The diagnosis of Alzheimer-type dementia in Down syndrome can be challenging because of the large intra-individual variability in cognitive functioning, the different diagnostic and methodological procedures currently used, and the difficulty in obtaining baseline levels of cognitive functioning in this population.50 Previously, PET brain glucose metabolic measurements with 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) have demonstrated significant decreases in parietal and lateral temporal regions in older people with Down syndrome, and such deficits are associated with reduced language, visuospatial, and attention abilities.51,52 Moreover, [18F]FDG PET has been used to assist in the diagnosis of Alzheimer-type dementia in Down syndrome and in patients with dementia in the general population. Given the considerable challenges in diagnosing dementia in people with Down syndrome using cognitive and behavioral measures, neuroimaging may offer added clinical value. Future studies and analyses will determine the relative benefits of different forms of PET imaging using various ligands, such as [18F]FDG, [18F]FDDNP, or [11C]PIB.
As in many clinical imaging studies, methodological limitations could affect our results and their interpretation, including partial volume effects53 and error introduced from head motion during scanning.40 In addition, the small number of study participants limited our power for direct comparisons of the Down syndrome and Alzheimer disease groups in comparable age ranges. Future studies that directly compare people with Down syndrome and patients with presenile dementias would offer an opportunity to contrast study groups in comparable age ranges. Previous [11C]PIB studies of patients with presenile-1 mutations have demonstrated high striatal binding.54,55 Because individuals with dementia who have presenile mutations were not included in the present study, we did not include the striatum as a region of interest.
Despite such limitations, our results indicate that [18F]FDDNP binding values in Down syndrome are significantly higher than those in cognitively intact controls and are closely associated with increasing age, consistent with previous autopsy studies of SP and NFT deposition. Further work is under way to determine [18F]FDDNP PET binding progression in the same study participants as an early predictor of future cognitive and behavioral decline in these individuals.
Acknowledgments
Funding/Support: This study was supported by grants R01 AG033015, P01-AG025831, AG13308, P50 AG 16570, and M01-RR00865 from the National Institutes of Health; contract DE-FC03-87-ER60615 from the Department of Energy; the UCLA Academic Senate; the Elizabeth and Thomas Plott Chair Endowment in Gerontology (Dr Barrio); the Parlow-Solomon Professorship (Dr Small); and the National Down Syndrome Society (Dr Nelson).
Footnotes
Author Contributions: Study concept and design: Nelson, Kepe, and Small. Acquisition of data: Nelson, Kepe, Scheibel, and Small. Analysis and interpretation of data: Siddarth, Kepe, Scheibel, Huang, Barrio, and Small. Drafting of the manuscript: Nelson, Siddarth, Kepe, Scheibel, and Small. Critical revision of the manuscript for important intellectual content: Nelson, Siddarth, Kepe, Scheibel, Huang, Barrio, and Small. Statistical analysis: Siddarth. Obtained funding: Nelson, Barrio, and Small. Administrative, technical, and material support: Scheibel, Huang, Barrio, and Small. Study supervision: Nelson, Scheibel, Barrio, and Small.
Financial Disclosure: The University of California, Los Angeles (UCLA), owns US patent 6 274 119 titled “Methods for Labeling β-Amyloid Plaques and Neurofibrillary Tangles,” which uses the approach outlined in this article. Drs Small, Huang, and Barrio are among the inventors, have received royalties, and are expected to receive royalties on future sales. Dr Small has served as a consultant and/or received lecture fees from Dakim, Eisai, Forest, Medivation, Novartis, and Pfizer and has received stock options from Dakim. Dr Huang has received lecture fees from GlaxoSmithKline. Dr Barrio has served as a consultant and received lecture fees from Nihon Medi-Physics Co, Bristol-Meyers Squibb, PETNet Pharmaceuticals, and Siemens.
Additional Contributions: From our UCLA research team, Matt Gee, Christine Hwang, Reza Rajaee, Maryam Sattari-Lenz, Eric Sun Kim, Samantha Wu, Andrea Kaplan, and Deborah Dorsey, RN, MBA, assisted in subject recruitment, data acquisition, and management; Lawrence Pang, Jennie Kusnadi, and Richard Eugene assisted in scanning procedures; and Nagichettiar Satyamurthy, PhD, and J. Liu, PhD, performed the synthesis of [18F]FDDNP. John M. Ringman, MD, assisted with clinical evaluations of subjects.
References
- 1.Margallo-Lana ML, Moore PB, Kay DW, et al. Fifteen-year follow-up of 92 hospitalized adults with Down’s syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51(pt 6):463–477. doi: 10.1111/j.1365-2788.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 2.Mann DM. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43(2):99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 3.Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM. Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology. 1985;35 (7):957–961. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- 4.Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome: quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol. 1995;52(4):379–391. doi: 10.1001/archneur.1995.00540280065020. [DOI] [PubMed] [Google Scholar]
- 5.Mann DM, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci. 1989;89(2–3):169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 7.Mann DM, Yates PO, Marcyniuk B. Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol Appl Neurobiol. 1984;10(3):185–207. doi: 10.1111/j.1365-2990.1984.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Mann DM, Yates PO, Marcyniuk B. Dopaminergic neurotransmitter systems in Alzheimer’s disease and in Down’s syndrome at middle age. J Neurol Neurosurg Psychiatry. 1987;50(3):341–344. doi: 10.1136/jnnp.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong RA. Differences in β-amyloid (β/A4) deposition in human patients with Down’s syndrome and sporadic Alzheimer’s disease. Neurosci Lett. 1994;169(1–2):133–136. doi: 10.1016/0304-3940(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 10.Vetrivel KS, Thinakaran G. Amyloidogenic processing of β-amyloid precursor protein in intracellular compartments. Neurology. 2006;66(2 suppl 1):S69–S73. doi: 10.1212/01.wnl.0000192107.17175.39. [DOI] [PubMed] [Google Scholar]
- 11.Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp Neurol. 1998;150(2):296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- 12.Prasher VP, Filer A. Behavioural disturbance in people with Down’s syndrome and dementia. J Intellect Disabil Res. 1995;39(pt 5):432–436. doi: 10.1111/j.1365-2788.1995.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 13.Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355(25):2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 14.Shoghi-Jadid K, Small GW, Agdeppa ED, et al. Localization of neurofibrillary tangles and β-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed] [Google Scholar]
- 15.Smid LM, Vovko TD, Popovic M, et al. The 2,6-disubstituted naphthalene derivative FDDNP labeling reliably predicts Congo red birefringence of protein deposits in brain sections of selected human neurodegenerative diseases. Brain Pathol. 2006;16(2):124–130. doi: 10.1111/j.1750-3639.2006.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boxer AL, Rabinovici GD, Kepe V, et al. Amyloid imaging in distinguishing atypical prion disease from Alzheimer disease. Neurology. 2007;69(3):283–290. doi: 10.1212/01.wnl.0000265815.38958.b6. [DOI] [PubMed] [Google Scholar]
- 17.Small GW, Siddarth P, Burggren AC, et al. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Arch Gen Psychiatry. 2009;66(1):81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braskie MN, Klunder AD, Hayashi KM, et al. Plaque and tangle imaging and cognition in normal aging and Alzheimer’s disease. Neurobiol Aging. 2010;31(10):1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 20.Kepe V, Huang SC, Small GW, Satyamurthy N, Barrio JR. Visualizing pathology deposits in the living brain of patients with Alzheimer’s disease. Methods Enzymol. 2006;412:144–160. doi: 10.1016/S0076-6879(06)12010-8. [DOI] [PubMed] [Google Scholar]
- 21.Agdeppa ED, Kepe V, Petri A, et al. In vitro detection of (S)-naproxen and ibuprofen binding to plaques in the Alzheimer’s brain using the positron emission tomography molecular imaging probe 2-(1-{6-[(2-[18F]fluoroethyl) (methyl)amino]-2-naphthyl}ethylidene)malononitrile. Neuroscience. 2003;117 (3):723–730. doi: 10.1016/s0306-4522(02)00907-7. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 26.Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace & Co; 1999. [Google Scholar]
- 27.Evenhuis HM. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. J Intellect Disabil Res. 1992;36(pt 4):337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 28.Evenhuis HM. Manual of the Dementia Questionnaire 5 for Persons With Mental Retardation. 2. Zwammerdam, the Netherlands: Hooge Burch Institute for Mentally Retarded People; 1995. [Google Scholar]
- 29.Nelson L, Johnson JK, Freedman M, et al. Learning and memory as a function of age in Down syndrome: a study using animal-based tasks. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(3):443–453. doi: 10.1016/j.pnpbp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Kirk LJ, Hick R, Laraway A. Assessing dementia in people with learning disabilities: the relationship between two screening measures. J Intellect Disabil. 2006;10(4):357–364. doi: 10.1177/1744629506070053. [DOI] [PubMed] [Google Scholar]
- 31.Prasher VP, Huxley A, Haque MS Down Syndrome Ageing Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Down syndrome and Alzheimer’s disease—pilot study. Int J Geriatr Psychiatry. 2002;17(3):270–278. doi: 10.1002/gps.587. [DOI] [PubMed] [Google Scholar]
- 32.Coppus A, Evenhuis H, Verberne G-J, et al. Dementia and mortality in persons with Down’s syndrome. J Intellect Disabil Res. 2006;50(pt 10):768–777. doi: 10.1111/j.1365-2788.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 33.Tyrrell J, Cosgrave M, McCarron M, et al. Dementia in people with Down’s syndrome. Int J Geriatr Psychiatry. 2001;16(12):1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- 34.Shultz J, Aman M, Kelbley T, et al. Evaluation of screening tools for dementia in older adults with mental retardation. Am J Ment Retard. 2004;109(2):98–110. doi: 10.1352/0895-8017(2004)109<98:EOSTFD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Nelson LD, Satz P, Mitrushina M, et al. Development and validation of the neuropsychology behavior and affect profile. Psychol Assess. 1989;1(4):266–272. [Google Scholar]
- 36.Nelson LD, Cichetti D, Satz P, Sowa M, Mitrushina M. Emotional sequelae of stroke: a longitudinal perspective. J Clin Exp Neuropsychol. 1994;16(5):796–806. doi: 10.1080/01688639408402693. [DOI] [PubMed] [Google Scholar]
- 37.Nelson LD, Mitrushina M, Satz P, Sowa M, Cohen S. Cross-validation of the Neuropsychology Behavior and Affect Profile in stroke patients. Psychol Assess. 1993;5(3):374–376. [Google Scholar]
- 38.Nelson LD, Scheibel KE, Ringman JM, Sayre JW. An experimental approach to detecting dementia in Down syndrome: a paradigm for Alzheimer’s disease. Brain Cogn. 2007;64(1):92–103. doi: 10.1016/j.bandc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Kepe V, Žabjek A, et al. High-yield, automated radiosynthesis of 2-(1-6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthylethylidene)malononitrile ([18F]FDDNP) ready for animal or human administration. Mol Imaging Biol. 2007;9(1):6–16. doi: 10.1007/s11307-006-0061-4. [DOI] [PubMed] [Google Scholar]
- 40.Wardak M, Wong KP, Shao W, et al. Movement correction method for human brain PET images: application to quantitative analysis of dynamic 18F-FDDNP scans. J Nucl Med. 2010;51(2):210–218. doi: 10.2967/jnumed.109.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 42.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 43.Deb S, Braganza J. Comparison of rating scales for the diagnosis of dementia in adults with Down’s syndrome. J Intellect Disabil Res. 1999;43(pt 5):400–407. doi: 10.1046/j.1365-2788.1999.043005400.x. [DOI] [PubMed] [Google Scholar]
- 44.Burt DB, Primeaux-Hart S, Loveland KA, et al. Comparing dementia diagnostic methods used with people with intellectual disabilities. J Policy Pract Intell Disabil. 2005;2(2):94–115. [Google Scholar]
- 45.Ball SL, Holland AJ, Huppert FA, Treppner P, Watson P, Hon J. The modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with Down’s syndrome. J Intellect Disabil Res. 2004;48(pt 6):611–620. doi: 10.1111/j.1365-2788.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 46.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 47.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 48.Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- 49.Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9(2):228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 50.Nieuwenhuis-Mark RE. Diagnosing Alzheimer’s dementia in Down syndrome: problems and possible solutions. Res Dev Disabil. 2009;30(5):827–838. doi: 10.1016/j.ridd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Schapiro MB, Haxby JV, Grady CL, et al. Decline in cerebral glucose utilisation and cognitive function with aging in Down’s syndrome. J Neurol Neurosurg Psychiatry. 1987;50(6):766–774. doi: 10.1136/jnnp.50.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schapiro MB, Haxby JV, Grady CL. Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging. 1992;13(6):723–734. doi: 10.1016/0197-4580(92)90096-g. [DOI] [PubMed] [Google Scholar]
- 53.Protas HD, Huang S-C, Kepe V, et al. FDDNP binding using MR derived cortical surface maps. Neuroimage. 2010;49(1):240–248. doi: 10.1016/j.neuroimage.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koivunen J, Verkkoniemi A, Aalto S, et al. PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer’s disease. Brain. 2008;131(pt 7):1845–1853. doi: 10.1093/brain/awn107. [DOI] [PubMed] [Google Scholar]
- 55.Villemagne VL, Ataka S, Mizuno T, et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66(12):1537–1544. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]